Спондилоартриты (СпА) – группа воспалительных заболеваний суставов, имеющих общие клинические, генетические и рентгенологические особенности [1]. В зависимости от первичной локализации очага поражения выделяют аксиальную и периферическую формы СпА. Для СпА характерны частое вовлечение в воспалительный процесс крестцово-подвздошных сочленений, позвоночника, наличие периферического моно- или олигоартрита, серонегативность по ревматоидному фактору (РФ), отсутствие ревматоидных узелков, семейные случаи заболевания, ассоциация с носительством HLA-B27-антигена, нередкое развитие энтезитов, а также типичные внесуставные проявления (увеит, поражение кожи и слизистых оболочек, воспалительные заболевания кишечника и т.д.) [2]. Основными заболеваниями, относящимися к СпА, являются анкилозирующий спондилит (АС, или болезнь Бехтерева), псориатический артрит (ПсА), реактивный артрит; артрит, ассоциированный с воспалительными заболеваниями кишечника (ВЗК, болезнь Крона, язвенный колит); и недифференцированный СпА [2]. Наиболее распространенное и наиболее изученное заболевания этой группы – АС [3, 4].
В настоящее время СпА причисляют к иммуноопосредованным заболеваниям [5]. Последние достижения молекулярной биологии и иммунологии доказали, что ключевую роль в патогенезе этих заболеваний может играть нарушение регуляции выработки цитокинов, опосредующих нормальное функционирование иммунной системы человека. Нарушение баланса цитокинов лежит в основе многих как острых, так и хронических воспалительных заболеваний. В настоящее время проведено много исследований, убедительно свидетельствующих о важной роли интерлейкина-17A (ИЛ-17А) в иммунопатогенезе как АС, так и ПсА. Это послужило мощным стимулом для разработки новых генно-инженерных биологических препаратов (ГИБП), механизм действия которых основан на блокировании патологических эффектов ИЛ-17А. Особенно это важно для терапии пациентов с аксиальным спондилоартритом, так как это достаточно сложная задача для врача, в первую очередь из-за малого числа альтернативных возможностей лечения.
 Первым ингибитором ИЛ-17А, зарегистрированным в России, стал секукинумаб (СЕК). Результаты проведенных исследований показали, что СЕК эффективен для лечения АС и ПсА. Он обладает низкой иммуногенностью, а его профиль безопасности практически не отличается от такового плацебо [6, 7]. В 2013 г. для СпА, включая АС и ПсА, группой экспертов Европейской антиревматической лиги (EULAR) была предложена концепция Treat-to-Target (T2T), или «Лечение до достижения цели», согласно которой основной целью терапии следует считать достижение ремиссии или в качестве альтернативы минимальную активность заболевания [8, 9]. Концепция «Лечение до достижения цели» основана на необходимости его изменения в случае, если, по данным регулярного мониторинга, ремиссия или минимальная активность СпА не достигнута.
Первым ингибитором ИЛ-17А, зарегистрированным в России, стал секукинумаб (СЕК). Результаты проведенных исследований показали, что СЕК эффективен для лечения АС и ПсА. Он обладает низкой иммуногенностью, а его профиль безопасности практически не отличается от такового плацебо [6, 7]. В 2013 г. для СпА, включая АС и ПсА, группой экспертов Европейской антиревматической лиги (EULAR) была предложена концепция Treat-to-Target (T2T), или «Лечение до достижения цели», согласно которой основной целью терапии следует считать достижение ремиссии или в качестве альтернативы минимальную активность заболевания [8, 9]. Концепция «Лечение до достижения цели» основана на необходимости его изменения в случае, если, по данным регулярного мониторинга, ремиссия или минимальная активность СпА не достигнута.
В настоящее время первой линией терапии АС являются нестероидные противовоспалительные препараты (НПВП) [10] и лечебная физкультура. При неадекватном ответе на НПВП и нефармакологическое лечение рекомендована терапия ингибиторами фактора некроза опухоли α (ФНОα) [11].
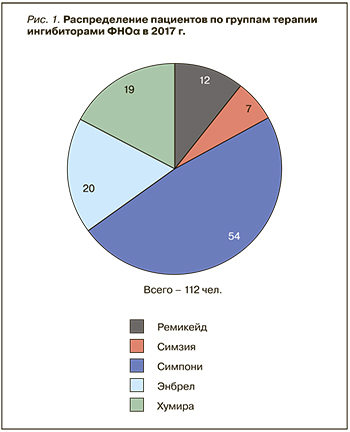
В НМХЦ им. Пирогова активная терапия АС и ПсА с применением ГИБП была начата в 2005 г. Тогда единственным представителем этого класса препаратов был инфликсимаб (Ремикейд) – первый ингибитор ФНОα, зарегистрированный для лечения широкого круга нозологий. Спектр используемых препаратов для биологической терапии СпА с каждым годом расширялся по мере появления новых ингибиторов ФНОα. В 2017 г. количество пациентов, получающих терапию этим классом препаратов, достигло 112 (рис.1).
Несмотря на значительную эффективность ингибиторов ФНОα в лечении CпА [12–17], у них имеются определенные ограничения и недостатки. Так, у 20–40% больных наблюдается неадекватный ответ, отсутствие ответа на лечение или непереносимость этой группы препаратов. Кроме того, через 6–12 мес после прекращения терапии ингибиторами ФНОα почти у всех пациентов наблюдается обострение заболевания [18].
Первичная неэффективность ГИБП при СпА, по мнению экспертов, почти не встречается в реальной практике. Хороший эффект имеет место у подавляющего большинства пациентов. Тем не менее ряд больных приходится «переключать» на другие ГИБП вследствие развития вторичной неэффективности, нежелательных явлений (НЯ) и административных причин [19]. До недавнего времени при ускользании эффекта одного ингибитора ФНОα или развитии нежелательных реакций ревматологи были ограничены возможностью смены терапии только на другой препарат этой же группы. Однако с регистрацией препарата секукинумаб (СЕК) для лечения АС и ПсА появилась возможность «переключения» на препарат с другим механизмом действия.
В НМХЦ им. Н.И. Пирогова СЕК стали применять с 2017 г., и в этой статье обобщены первые результаты его применения. В анализ включено 15 пациентов (13 – с АС, 2 – с ПсА): 10 мужчин и 5 женщин. Средний возраст составил 39,9 лет. Ранее не получали биологическую терапию 4 пациента. С учетом высокой активности заболевания и риска структурного прогрессирования было решено применять СЕК в качестве первого ГИБП. Решение было основано на результатах анализа данных рандомизированного плацебо-контролируемого клинического исследования III фазы MEASURE 1, в котором СЕК показал эффективность в отношении торможения структурного прогрессирования заболевания у пациентов с АС [20]. Было принято во внимание также заключение совета экспертов «Вопросы “выживаемости“» генно-инженерных биологических препаратов и возможности переключения на СЕК при АС в реальной клинической практике» 2017 г. [21], где эксперты рассматривали вероятность назначения СЕК в качестве первой линии терапии. Остальные 11 пациентов имели предшествующий неудачный опыт терапии ингибиторами ФНОα. Из них пятеро ранее получали по одному ингибитору ФНОα, еще 5 – по два и 1 – три. Терапия СЕК назначалась в дозировках в строгом соответствии с инструкцией по медицинскому применению препарата с нагрузочным периодом на старте терапии. Все пациенты, наблюдаемые в нашем отделении, получают терапию СЕК более 4 мес.
Эффективность терапии оценивали по динамике клинических и лабораторных показателей, отражающих активность АС и ПсА: показатели СОЭ, СРБ, индексы BASDAI и ASDAS-CРБ. Изменения функционального статуса фиксировали по сдвигу индекса BASFI. Оценку безопасности терапии СЕК проводили путем регистрации возможных нежелательных явлений, связанных с приемом препарата, а также на основании результатов стандартных лабораторных тестов (развернутого общего анализа крови, общего анализа мочи, биохимических показателей крови).
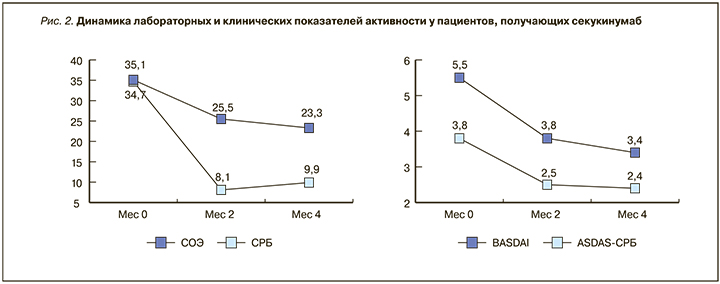
У 13 (86,6%) пациентов индекс BASDAI составлял более 4 баллов, оценка варьировала от 3,01 до 9,04 балла (в среднем 5,51±2,1 балла). Активность по критерию ASDAS CРБ составила в среднем 3,81±1,5 (от 0,51 до 5,93 балла), причем у 10 пациентов из 15 (66,6%) она была оценена как очень высокая (ASDAS CРБ (>3,5)). Индекс BASFI колебался в пределах от 0,51 до 8,12 (среднее значение 5,42±3,6 балла), и у 80% пациентов функциональные нарушения были выраженными.
Результаты мониторирования клинических и лабораторных показателей на протяжении 4 мес лечения представлены на рис. 2. Уже через 2 мес терапии СЕК наблюдалась выраженная положительная динамика клинических и лабораторных показателей.
Обращает на себя внимание, что, несмотря на значительное уменьшение лабораторной активности, значения ASDAS СРБ снижались не столь быстро и через 4 мес лечения еще находились в диапазоне высокой активности. Количество пациентов с очень высокой активностью сократилось на 60% (с 10 до 1 пациента).
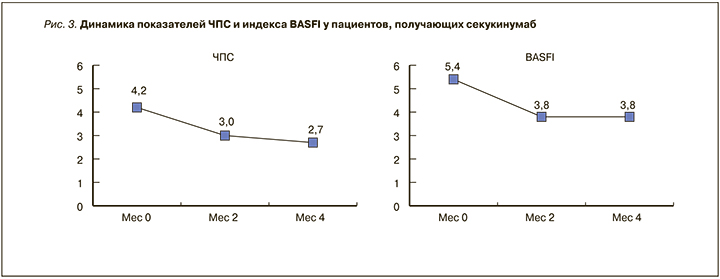
Как показывают полученные нами результаты, уменьшение выраженности суставного синдрома отмечалось уже через 2 мес терапии и имело тенденцию к дальнейшему снижению. Несмотря на это, функциональный статус пациентов (индекс BASFI) после первоначального улучшения ко 2 мес в дальнейшем оставался на прежнем уровне, и почти у половины пациентов (7 из 15) нарушения оценивались как выраженные (рис. 3). По нашему опыту эффективность СЕК зависит от состояния пациента. Пациенты с периферическими артритами и предшествующей неудачной терапией ингибиторами ФНОα отвечали на терапию более медленно. Чем больше ФНОα было назначено пациенту до старта терапии СЕК, тем медленнее была реакция на лечение. Очевидно, для оценки полного терапевтического воздействия СЕК на уменьшение воспаления периферических суставов требуется более длительное наблюдение. И, наоборот, пациенты, у которых СЕК был первым биологическим препаратом, реагировали на терапию достаточно быстро. Кроме того, представляет интерес оценка торможения рентгенологического прогрессирования аксиальных поражений, что также возможно в долгосрочном периоде.
Профиль безопасности СЕК был благоприятным. Серьезные нежелательные явления не зарегистрированы ни у одного из пациентов. Все контролируемые общепринятые лабораторные показатели оставались в пределах нормы. Эти результаты демонстрируют хорошую переносимость препарата.
Лечение CЕК может привести к снижению активности АС при неэффективности терапии БПВП, предыдущей терапии 2 ГИБП, что иллюстрирует следующее наблюдение.
КЛИНИЧЕСКОЕ НАБЛЮДЕНИЕ
Больной П., 37 лет, инвалид 2 группы. С 2000 г. страдает псориазом кожи рецидивирующего течения. С 2014 г. страдает АС с развитием анкилоза дугоотростчатых суставов С2-С3, Th11-Th12-L1, периферических артритов коленных, голеностопных суставов, деформации крупных и мелких суставов. В первые годы болезни проводилась терапия различными НПВП в сочетании с метотрексатом – эффект был недостаточным. В связи с сохранением высокой клинической и лабораторной активности заболевания в декабре 2016 г. принято решение о назначении адалимумаба в дозе 40 мг подкожно с интервалом введения 2 нед в комбинации с НПВП (нимесулид 200 мг сут), метотрексата 10,0 мг/нед (в связи с рецидивирующей инфекцией мочевыводящих путей доза не повышалась).
Несмотря на регулярный прием препаратов в течение 11 мес, ремиссии АС достичь не удалось. В ноябре 2016 г. назначен голимумаб в дозе 50 мг подкожно с интервалом введения 4 нед. На фоне терапии сохранялась высокая клиническая и лабораторная активность заболевания (артриты голеностопных, коленных суставов, продолжительная утренняя скованность, ускорение СОЭ до 58 мм/ч, повышение СРБ 33,8 мг/л, BASDAI=7,8; ASDAS-CРБ=3,52; по данным МРТ коленных суставов – асептические изменения надколенника, УЗИ-признаки синовита обоих голеностопных суставов, в большей степени выраженного справа, ретроахиллового бурсита справа). В связи с неэффективностью 2 ингибиторов ФНОα назначен СЕК в дозе 300 мг подкожно по традиционной схеме. Через 4 мес терапии достигнуто снижение активности заболевания (уменьшение СОЭ до 37 мм/ч, нормализация уровня СРБ 4,7, индекс BASDAI =3,6, ASDAS-CРБ=1,62). По данным УЗИ установлена положительная динамика в виде отсутствия признаков ретроахиллового бурсита правого голеностопного сустава, уменьшения степени выраженности синовита обоих голеностопных суставов при удовлетворительной переносимости лечения. К настоящему времени терапия продолжается.
Таким образом, данные нашего анализа свидетельствуют, что биологическая терапия с включением СЕК позволяет существенно снизить активность АС и ПсА, улучшить функциональное состояние у пациентов с резистентным течением заболевания, не повышая риск НЯ. Выраженное и быстрое снижение лабораторных показателей воспаления, уменьшение суставного синдрома у пациентов, ранее получавших ингибиторы ФНОα, дает основание рассматривать этот препарат как перспективный для переключения на биологический агент с другим механизмом действия.
Можно предположить, что по мере накопления клинического опыта биологической терапии ГИБП с различными механизмами действия будут улучшены не только результаты лечения «тяжелых» пациентов, но и получены новые данные, позволяющие сделать терапию иммуновоспалительных заболеваний более персонифицированной.



