Ингаляционная терапия заняла прочное место в лечении респираторных заболеваний, что непосредственно связано с таргетной доставкой лекарственных препаратов и ограничением их неблагоприятных побочных эффектов. Практика показала, что технологии ингаляционных устройств чрезвычайно важны как для больных бронхиальной астмой (БА), так и хронической обструктивной болезнью легких (ХОБЛ), поскольку эффективность терапии определяется не только химическим составом лекарственных средств, входящих в ингалируемые растворы или порошки, но и способами их доставки.
Если раньше считалось, что ключевым фактором лечения является доставленная доза, т.е. то количество препарата, которое проникло в нижние дыхательные пути, а не осело в верхних, то сейчас все больше внимания уделяется удобству применения ингалятора. Это обусловлено изменениями в комплаенсе пациентов и наличием так называемых критических ошибок.
Выяснилось, что достаточно большое количество людей неправильно используют ингалятор даже после прочтения инструкции и консультации врача [1]. Это снижает приверженность пациентов к лечению, и традиционно считавшийся хорошим комплаенс у больных БА на деле не столь хорош; сегодня основная проблема в лечении этого заболевания – отсутствие контроля над его течением. Так, в специальном исследовании было показано, что только у 45% пациентов при правильном назначении препаратов обеспечивается контроль БА [2].
Относительно недавно было проведено серьезное исследование, анализирующее смертность от БА. В результате выяснилось, что, хотя в целом этот показатель невелик, в то же время смертность преобладает у лиц молодого возраста и связана с бесконтрольным применением короткодействующих ингаляторов [3].
На самом деле пациентам с БА, конечно, удобнее использовать препараты, дающие немедленное облегчение симптомов; около 50% из них считают, что использование базисной терапии не столь актуально, а применять ее нужно только тогда, когда есть симптомы заболевания [4, 5]. Эти наблюдения привели к серьезному пересмотру позиций по ведению больных БА. Дело в том, что чрезмерное неконтролируемое использование короткодействующих бронхолитиков приводит не только к возрастанию риска обострений астмы и повышению количества госпитализаций, не только к повышению потребности в системных глюкокортикостероидных препаратах, но и к таким осложнениям, как фатальные нарушения ритма сердца, повышение артериального давления и ряду других изменений.
Короткодействующие β2-андреномиметики (КДБА) в течение почти 50 лет были первой линией терапии, однако с 2019 г. экспертами GINA лечение только этими препаратами было признано нецелесообразым (GINA 2019–2020). Такие критерии, как выдача более 3 короткодействующих ингаляторов в год, значительно повышают риск обострения БА и смерти пациента [6]. Применение ≥12 ингаляторов за год связано с повышенным риском смерти по причине БА [7].
В настоящее время, согласно рекомендациям GINA и Российского респираторного общества, монотерапия КДБА более не рекомендуется [6, 8–12]. Всем взрослым и подросткам с 12 лет с БА рекомендовано применять противовоспалительную терапию, в частности низкие дозы ингаляционных кортикостероидов (ИГКС). Существует специальный валидированный опросник BMQ – тест по оценке приверженности терапии [13]. Им можно пользоваться при назначении и контроле за использованием скоропомощных короткодействующих ингаляторов.
В лечении БА широко используется комбинированный препарат Симбикорт® Турбухалер®, который содержит формотерол, сопоставимый по скорости наступления бронхолитического эффекта с КДБА, и будесонид – ИГКС с быстрым началом противовоспалительного действия. Благодаря синергизму активных веществ такая фиксированная комбинация очень хорошо себя зарекомендовала: оказалось, что ее можно использовать в том числе и для купирования приступов БА, если полный контроль заболевания не достигнут. Появилась концепция «противовоспалительного бронхолитика», означаюшая применение комбинированного препарата Симбикорт® Турбухалер® 160/4,5 мкг/доза, который и купирует бронхоспазм, и уменьшает воспаление, снижая риск и частоту обострений БА любой степени тяжести (от легкой до тяжелой).
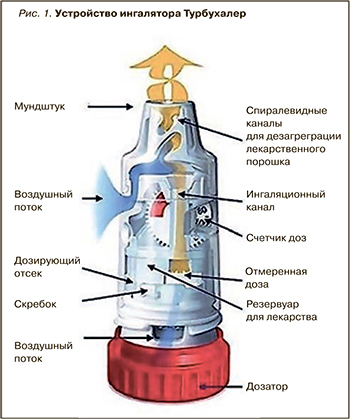 В случае с препаратом Симбикорт® Турбухалер® очевидные достоинства присущи не только составу его действующих веществ, но и самому ингаляционному устройству Турбухалер® (рис. 1): хорошая дисперсия с преобладанием респирабельной фракции, монодисперсность аэрозоля, обеспечивающая достаточно высокую доставленную дозу, простота использования. Симбикорт® Турбухалер® 160/4,5 мкг/доза включен в новую редакцию рекомендаций GINA как препарат для использования на всех ступенях терапии БА [11].
В случае с препаратом Симбикорт® Турбухалер® очевидные достоинства присущи не только составу его действующих веществ, но и самому ингаляционному устройству Турбухалер® (рис. 1): хорошая дисперсия с преобладанием респирабельной фракции, монодисперсность аэрозоля, обеспечивающая достаточно высокую доставленную дозу, простота использования. Симбикорт® Турбухалер® 160/4,5 мкг/доза включен в новую редакцию рекомендаций GINA как препарат для использования на всех ступенях терапии БА [11].
Тем не менее в реальной практике ингаляционного лечения БА сохраняются некоторые проблемы, которые заставляют разработчиков лекарственных препаратов искать дополнительные способы оптимизации этой процедуры с учетом индивидуальных потребностей пациентов. С чем они связаны? Как раз не столько с удобством использования препарата (нужно признать, что Турбухалер® достаточно удобное средство доставки), сколько потребностью некоторых пациентов чувствовать обратную связь с ингаляционным устройством. Под обратной связью в данном случае подразумевается, что при использовании ингалятора больному очень важно точно контролировать процесс:
- чувствовать;
- слышать;
- видеть.
Поэтому различные производители современных ингаляционных устройств как раз идут по пути четкой визуализации и строгого контроля над исполнением процедуры ингаляции. При тяжелой обструкции дыхательных путей и формировании легочной гиперинфляции как результата «воздушной ловушки» существенно снижается не только емкость, но и скорость вдоха, что значительно снижает эффективность инициируемого вдохом порошкового ингалятора.
В связи с этим нужно сказать несколько слов о преимуществах и недостатках дозированных аэрозольных и порошковых ингаляторов.
Дозированные аэрозольные ингаляторы (ДАИ) преобладают среди препаратов для лечения БА и ХОБЛ: на них приходится более 50% всех используемых средств и до 80% ИГКС [14–16]. У них есть определенные достоинства: они портативные, мультидозовые, широко распространены, привычны в использовании для большинства пациентов. В то же время при использовании ДАИ существуют и определенные проблемы, например, необходимость координации вдоха с активацией ингалятора, что актуально для ряда категорий пациентов, чаще женщин, детей и пожилых. К сожалению, некоторые больные используют ДАИ неправильно, что часто ассоциировано с низкой легочной депозицией и значительным осаждением лекарственного препарата в ротоглотке.
Порошковые ингаляторы (ДПИ) характеризуются простотой использования, они портативные, мультидозовые, активируются вдохом (т.е. нет необходимости в пропелленте). Но также у них есть и свои недостатки, например, требуется минимальная скорость вдоха пациента (>30 л/мин) для того, чтобы произошла нормальная дисперсия аэрозоли и была обеспечена нормальная доставленная доза. К сожалению, и в этом случае некоторые пациенты используют ингалятор неправильно. Глубокий вдох с усилием, необходимый для дезагрегации частиц, зачастую невозможен у лиц с тяжелой обструкцией и сниженной емкостью вдоха, в частности у больных ХОБЛ, когда развивается гиперинфляция, значительно падает емкость вдоха. В результате использование порошкового ингалятора становится затруднительным и/или малоэффективным (табл.).
Конечно, всем пациентам для ингаляций можно использовать небулайзер. Но это не всегда удобно, особенно при регулярном применении. Кроме того, аэрозольный ингалятор можно дополнить спейсером, который решит проблему координации вдоха и ингаляции.
У какой категории пациентов чаще всего встречается ограничение скорости вдоха и кому больше других подходят ДАИ?
Это:
- дети младшего возраста от 6 до 11 лет;
- пожилые пациенты;
- пациенты с сопутствующим ожирением;
- пациенты с тяжелым течением БА и ХОБЛ, когда емкость вдоха падает и аэрозольные ингаляторы становятся предпочтительнее.
Для аэрозольных ингаляторов характерна интуитивная обратная связь, т.е. звук, перемещение счетчика (если ингалятор им оснащен), ощущение струи воздуха в верхних дыхательных путях.
Важным фактором лечения БА и ХОБЛ является и контроль количества оставшихся доз в в аэрозольном ингаляторе. Проведенный среди более 500 пациентов опрос показал, что 82% респондентов, применяя препараты без счетчика доз, порой не знали, что ингалятор уже пустой [18].
Относительно недавно был разработан ингаляционный препарат с новой системой доставки лекарственных средств – Симбикорт® Рапихалер. Он представляет собой ингалятор, содержащий будесонид и формотерол, в форме ДАИ со счетчиком доз (рис. 2).

У данной системы доставки есть несколько положительных и важных инноваций.
- Защита от случайного высвобождения дозы, которое возможно где угодно: в сумке, кармане и т.д. В данном случае специальный колпачок блокирует любое непроизвольное нажатие, тем самым способствуя контролю за расходом препарата.
- Большой и наглядный счетчик доз с цветовым выделением 10 и 5 последних доз.
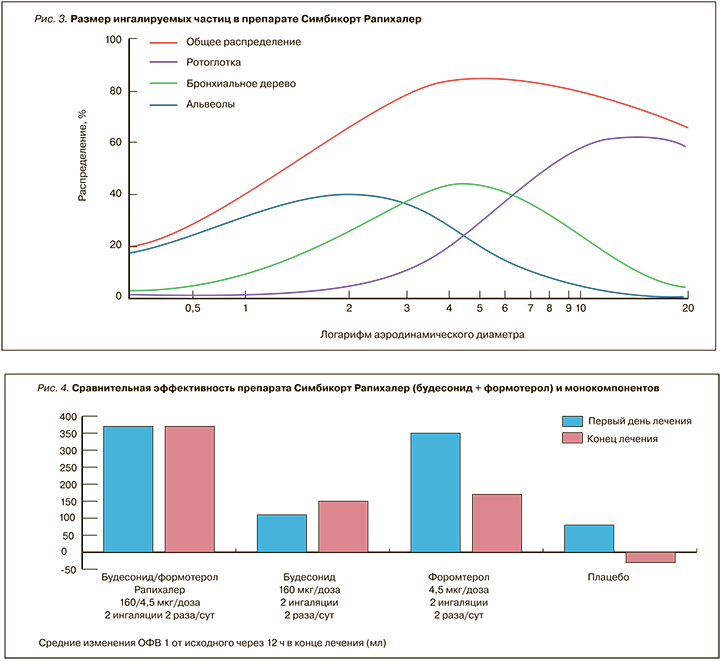
- Ингалятор обеспечивает хорошую респирабельную фракцию. Оптимальным считается размер частиц от 2 до 5 мкм. В Симбикорт® Рапихалер средний размер частиц будесонида – 3,6 микрон, формотерола – 3,3, что является очень хорошим показателем (рис. 3) [19].
Проведенное специальное исследование продемонстрировало одинаковую терапевтическую эффективность препарата Симбикорт® в ингаляторах Турбухалер® и Рапихалер в эквивалентных дозах. При этом показана значительно большая эффективность фиксированной комбинации будесонида и формотерола (ИГКС + ДДБА) в форме Рапихалер по сравнению с монокомпонентами (рис. 4) [20].
Также проведены исследования препарата Симбикорт® Рапихалер по сравнению с аналогичными препаратами, в частности с фиксированной комбинацией салметерол + флутиказона пропионат в ДАИ. Они показали, что Симбикорт® Рапихалер практически в два раза эффективнее в отношении снижения частоты обострений БА [21].
Наряду с БА ключевой проблема пульмонологии остается и ХОБЛ. По данным ВОЗ, сейчас эта болезнь является третьей причиной смертности в мире, причем, как показали исследования последних десятилетий, наиболее значимый предиктор летальности от ХОБЛ – обострение [22]. Поэтому предупреждение обострений – крайне важная задача терапии.
Всегда стоит вопрос о целесообразности применения ИКГС при ХОБЛ. Какие ключевые положения по этому вопросу можно выделить в современных рекомендациях GOLD (глобальная инициатива по ХОБЛ), российских рекомендациях, а также опираясь на предыдущие данные? Наибольшая эффективность ИКГС отмечается, конечно, при бронхитическом фенотипе ХОБЛ. При преобладающем эмфизематозном фенотипе эффективность применения ИГКС ниже и сопряжена с повышением риска развития пневмонии.
Очень важным критерием включения ИГКС в лечение ХОБЛ служит эозинофилия крови. Если количество эозинофилов в крови >300 кл/мкл, то применение комбинаций ИГКС+ ДДБА патогенетически обосновано [23, 24]. Кроме того, такая комбинация показана, если уровень эозинофилов >100 кл/мкл, но при этом есть более 2 в год среднетяжелых обострений ХОБЛ или отмечено тяжелое обострение, требующее госпитализации, т.е. налицо ситуация плохого контроля над заболеванием [24].
В связи с этим нужно отметить, что применение препарата Симбикорт® Рапихалер, по показаниям, снижало частоту обострений больных ХОБЛ на 35% эффективнее, чем использование монотерапии формотеролом. Кроме того, увеличилось время до первого обострения по сравнению с такой же монотерапией [25].
Недавно появившийся препарат Симбикорт® Рапихалер показан в следующих случаях:
- пациентам, предпочитающим использовать дозированные аэрозольные ингаляторы;
- пациентам со сниженной силой вдоха, т.е. слабым респираторным потоком (как правило, это дети 6–11 лет, пациенты с ожирением, пожилые больные с тяжелой БА и ХОБЛ);
- пациентам, которым принципиально важна интуитивная обратная связь («ощущения»).
ЗАКЛЮЧЕНИЕ
Таким образом, благодаря новой концепции лечения БА – использованию препарата Симбикорт® Турбухалер® 160/4,5 мкг/доза в качестве «противовоспалительного бронхолитика» для купирования приступов у пациентов с любой степенью тяжести астмы (с поддерживающей терапией или без нее) – у врачей есть возможность значительно снизить риски обострений, связанных с чрезмерным использованием короткодействующих бронхолитиков больными БА. Симбикорт® Рапихалер в форме ДАИ дает врачу больше шансов адаптировать лечение и устройство к потребностям пациентов. Расширение линейки не только препаратов, но и средств доставки существенно улучшит результаты лечения и уровень контроля у больных БА и ХОБЛ.



