Сахарный диабет (СД) – группа метаболических (обменных) заболеваний, характеризующихся хронической гипергликемией, которая является результатом нарушения секреции инсулина, действия инсулина или обоих этих факторов. Хроническая гипергликемия при СД сопровождается повреждением, дисфункцией и недостаточностью различных органов, особенно глаз, почек, нервов, сердца и кровеносных сосудов [1].
В 2000 г. общее число пациентов с СД составило 151 млн человек. К 2009 г. этот показатель вырос на 88% – до 285 млн. На сегодня известно, что 9,3% взрослых в возрасте 20–79 лет, а это уже 463 млн человек, живут с СД [2].
В России также отмечается значимый рост распространенности заболевания. По данным федерального регистра СД, в нашей стране на окончание 2018 г. на диспансерном учете состояло 4 584 575 пациентов (3,1% населения), из них 92% (4 238 503) с СД 2 типа, 6% (256 202) – с СД 1 типа и 2% (89 870) – с другими типами СД, включая 8006 женщин с гестационным СД [1]. Однако мы понимаем, что эти данные не отображают реальное количество больных, ввиду того что учитывают только выявленные и зарегистрированные случаи заболевания. По результатам масштабного российского эпидемиологического исследования (NATION) было подтверждено, что в реальной практике выявляется лишь 54% случаев СД 2 типа.
Таким образом, реальная численность пациентов с СД в России составляет не менее 9 млн человек (около 6% населения); это серьезная проблема, поскольку значительная доля пациентов остается не диагностированной, а значит, не получает лечения и имеет высокий риск развития осложнений [3]. Именно из-за развития осложнений СД 2 типа у пациентов значительно снижаются качество и продолжительность жизни. Для того чтобы этого избежать, необходимо незамедлительно начинать подобранную индивидуально для каждого пациента терапию. Первым шагом в лечении является выбор индивидуального целевого уровня гликированного гемоглобина (HbA1c) для каждого пациента (табл. 1) [1].
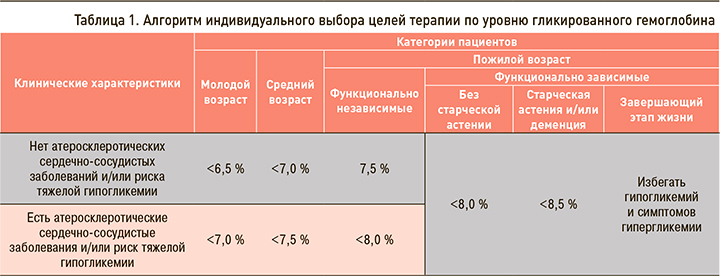
В соответствии с алгоритмами специализированной медицинской помощи больным СД исходный показатель HbA1c, превышающий индивидуальный целевой уровень более чем на 2,5%, характеризуется наличием выраженной глюкозотоксичности. Для уменьшения глюкозотоксичности, достижения целевых показателей углеводного обмена рекомендовано начинать лечение с инсулинотерапии или комбинации инсулина с другими сахароснижающими препаратами, в дальнейшем возможна отмена инсулинотерапии. При использовании комбинированной терапии важно помнить о необходимости рациональной комбинации различных сахароснижающих средств [1].
К одной из рациональных комбинаций сахароснижающих препаратов относится совместное применение базального инсулина и агонистов рецепторов глюкагоноподобного пептида-1 (аГПП-1), в том числе ликсисенатида. Важная особенность этого аГПП-1 – глюкозозависимый механизм действия, обеспечивающий поддержание целевых уровней гликемии с минимальным количеством гипогликемических реакций. Ликсисенатид способствует стимуляции секреции инсулина бета-клетками островков Лангерганса поджелудочной железы в ответ на гипергликемию. При снижении концентрации глюкозы в крови до нормальных значений стимуляция секреции инсулина прекращается. При гипергликемии ликсисенатид одновременно подавляет секрецию контринсулярных гормонов, однако при этом сохраняется секреция глюкагона в ответ на гипогликемию. Также препарат способствует снижению постпрандиального уровня глюкозы за счет замедления опорожнения желудка [4].
К наиболее важным преимуществам комбинированного препарата ликсисенатид + инсулин гларгин относится разнонаправленность действия его компонентов: инсулин регулирует уровень гликемии натощак, тогда как лексисенатид – прандиальную гликемию [5].
Фиксированная комбинация инсулина гларгин и ликсисенатида была зарегистрирована в России в 2018 г. Соответствующий препарат представляет из себя раствор для подкожного введения 1 раз/ сут в течение 1 ч перед любым приемом пищи. Для удобства подбора дозы он выпускается в двух шприц-ручках. Первый вариант: 1 единица препарата в шприц-ручке содержит 1 ЕД инсулина гларгин и 0,5 мкг ликсисенатида, максимальная суточная доза 40 ЕД. Такая форма выпуска препарата актуальна для пациентов, которые ранее находились на пероральной сахароснижающей терапии или получали инсулин гларгин в суточной дозе менее 30 ЕД. Во втором варианте шприц-ручки в 1 единице препарата содержатся 1 ЕД инсулина гларгин и 0,33 мкг ликсисенатида, максимальная суточная доза 60 ЕД. Эта форма рекомендована к применению пациентам, ранее получавшим терапию инсулином глагрин в суточной дозе от 30 до 60 ЕД/сут. Доза фиксированного комбинированного препарата подбирается индивидуально и титруется, исходя из потребности пациента в инсулине. Доза ликсисенатида титруется вместе с дозой инсулина гларгин [6].
КЛИНИЧЕСКАЯ ЭФФЕКТИВНОСТЬ: ДАННЫЕ ИССЛЕДОВАНИЙ
Эффективность и безопасность применения комбинированного препарата инсулин гларгин + ликсисенатид были изучены в трех рандомизированных, контролируемых клинических исследованиях (РКИ) с активным контролем у пациентов с СД 2 типа.
Исследование LixiLan-O представляло собой открытое, многонациональное, многоцентровое РКИ в параллельных группах, которое проводилось с 2014 по 2015 г. Средний возраст испытуемых составил 58 лет, они были преимущественно европеоидной расы (≈90%), имели избыточный вес или ожирение (ИМТ ≈32 кг/м2), а средняя продолжительность диабета у них составляла около 9 лет. 30-недельное исследование проводилось у пациентов с СД 2 типа, не получавших ранее терапию инсулином и с недостаточным гликемическим контролем при применении пероральных сахароснижающих препаратов. Эффективность и безопасность комбинированного препарата инсулин гларгин + ликсисенатид (n=468) в исследовании сравнивалась с инсулином гларгин (n=466) и ликсисенатидом (n=233) по отдельности. По результатам этого РКИ при добавлении к лечению фиксированной комбинации 74% (n=345) пациентов к 30-й неделе достигли значений HbA1c <7 %, тогда как в группе инсулина гларгин аналогичный показатель составил 59% (n=277), а в группе ликсисенатида – 33% (n=77). Средние значения HbA1с у пациентов, получавших инсулин гларгин + ликсисенатид, снизились на 1,6 %, только инсулин гларгин – на 1,3%, только ликсисенатид – на 0,9%, средние показатели концентрации глюкозы в плазме крови натощак уменьшились на 3,46, 3,27 и 1,5 ммоль/л соответственно, а средние величины постпрандиальной концентрации глюкозы в крови (через 2 ч после приема пищи) – на 5,68; 3,31 и 4,58 ммоль/л соответственно. К концу 30-недельного периода наблюдения среднее значение массы тела у пациентов из группы инсулина гларгин + ликсисенатид снизилось на 0,3 кг, из группы ликсисенатида – на 2,3 кг, тогда как в группе инсулина гларгин этот показатель возрос на 1,1 кг [7].
В 30-недельном открытом многонациональном многоцентровом РКИ LixiLan-L (переход с базального инсулина), проводившемся с 27 января 2014 г. по 9 июля 2015 г., оценивалась эффективность и безопасность комбинации инсулин гларгин + ликсисенатид в сравнении с инсулином гларгин. В исследование вошли 736 пациентов в возрасте от 18 лет с СД 2 типа, диагностированным по крайней мере за 1 год до скрининга, у которых наблюдался недостаточный гликемический контроль при терапии пероральными гипогликемическими препаратами в комбинации с базальным инсулином. Пациенты должны были получать базальный инсулин в течение не менее 6 мес до скрининга со стабильным режимом дозирования на протяжении не менее 3 мес. При применении комбинации инсулин гларгин + ликсисенатид 54,9% пациентов (n=201) к 30-й неделе достигли HbA1c <7%, в то время как в группе лечения только инсулином гларгин данный показатель составил 29,6% (n=108). Средние значения HbA1c у пациентов, получавших комбинированный препарат, снизились на 1,1%, инсулин гларгин – на 0,6 %, средние показатели концентрации глюкозы в плазме крови натощак уменьшились на 0,35 и 0,47 ммоль/л соответственно, средние величины постпрандиальной концентрации глюкозы в крови (через 2 ч после приема пищи) – на 4,72 и 1,39 ммоль/л соответственно. К концу 30-недельного периода наблюдения среднее изменение массы тела у пациентов, применявших инсулин гларгин + ликсисенатид, составило –0,7 кг против +0,7 кг в группе сравнения. Таким образом, лечение инсулином гларгин + ликсисенатидом вызывало клинически и статистически значимое улучшение уровня HbA1c; при этом достижение более низких значений и большее снижение этого показателя при применении комбинации инсулин гларгин + ликсисенатид не увеличивало частоту развития гипогликемии по сравнению с монотерапией инсулином гларгин [8].
В 26-недельном открытом многоцентровом РКИ LixiLan-G (переход с терапии аГПП-1), проводившемся с 6 июля 2016 г. по 25 мая 2018 г., были включены взрослые больные СД 2 типа, диагностированным не менее чем за 1 год до скрининга, с HbA1c 7–9%, которые получали максимально переносимую дозу аГПП-1 в сочетании с пероральными сахароснижающими препаратами. В нем оценивалась эффективность и безопасность комбинированного препарата инсулин гларгин + ликсисенатид в сравнении с аГПП-1. К концу исследования уровня HbA1c <7% достигли 61,9% пациентов (n=156), использовавших комбинированный препарат, против с 25,7% пациентов (n=65), лечившихся только аГПП-1. Средние значения HbA1C у пациентов, получавших препарат инсулин гларгин + ликсисенатид, снизились на 1,0%, а у пациентов, получавших только аГПП- 1, – на 0,4%. Концентрация глюкозы в плазме крови натощак в основной группе в среднем снизилась на 2,28 ммоль/л, в контрольной – на 0,60 ммоль/л, а постпрандиальный уровень глюкозы в крови (через 2 ч после приема пищи) – на 4,0 и 1,1 ммоль/л соответственно [9].
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ С ПРИМЕНЕНИЕМ ФИКСИРОВАННОЙ КОМБИНАЦИИ ИНСУЛИН ГЛАРГИН + ЛИКСИСЕНАТИД
Пациентка Г., 68 лет, госпитализирована в терапевтическое отделение ГКБ № 1 им. Н.И. Пирогова в плановом порядке.
Жалобы: на сухость во рту, жажда, повышение уровня гликемии до 10–11,0 ммоль/л, боли и онемение в стопах преимущественно в ночные часы.
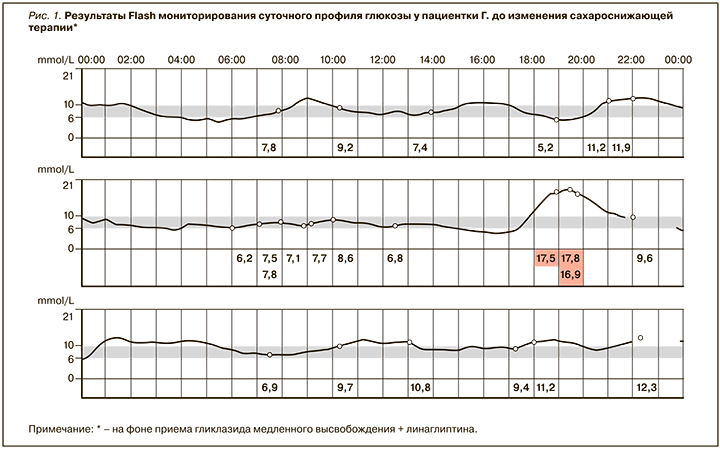
Из анамнеза: болеет СД 2 типа с 2014 г. С декабря 2018 г. принимает гликлазид медленного высвобождения по 60 мг утром и линаглиптин по 5 мг утром. На фоне этой терапии гликемия при самостоятельном измерении гликемия натощак составляла 10–11,9 ммоль/л, в течение дня пациентка гликемию не контролировала. Боли и онемение в стопах преимущественно в ночные часы беспокоят около двух лет. Длительное время отмечает повышение артериального давления (АД): максимальные значения 200/100 мм рт.ст., адаптирована к 110–120/80 мм рт.ст. Страдает ишемической болезнью сердца (ИБС), в 2016 г. больной были выполнены коронарная ангиография (КАГ) и стентирование передней межжелудочковой артерии (ПЖА).
Объективно: состояние удовлетворительное. Рост 158 см, вес 72 кг, индекс массы тела 28,8 кг/м2. Кожный покров теплый, нормальной влажности, пастозность стоп. Дыхание ровное, ритмичное. Частота дыхания 15/мин. Тоны сердца приглушены, ритмичны. Частота сердечных сокращений (ЧСС) 92/мин. АД 150/90 мм рт.ст. Живот мягкий, при пальпации безболезненный. Мочеиспускание самостоятельное, безболезненное.
Результаты лабораторного исследования. Клинический анализ крови: лейкоциты – 8,8×109/л; эритроциты – 4,53×1012/л; гемоглобин – 130,0 г/л; тромбоциты – 212×109/л; скорость оседания эритроцитов (СОЭ) 13 мм/ч.
Биохимический анализ крови: общий белок – 74,61 (60–83) г/л; креатинин – 114,9 (35–52) мкмоль/л; холестерин – 5,61 (0–6,2) ммоль/л; липопротеиды низкой плотности – 3,39 (2,07– 5,31) ммоль/л; триглицериды – 1,73 (<2,3) ммоль/л; липопротеиды высокой плотности – 1,43 (1–1,6) ммоль/л; глюкоза – 16,05 ммоль/л; гликированный гемоглобин (HbA1с) – 11,39 %; тиреотропный гормон – 3,6 мЕд/л.
Общий анализ мочи: лейкоциты – 250,00 лей/ мкл; кетоны – 0,00 ммоль/л; лейкоциты – 31 в п/зр; эритроциты – 1 в п/зр; глюкоза – 28,00 [+3 (28 ммоль/л)]; белок – 0,10 г/л; относительная плотность – 1,017 г/мл.
 Показатели гликемического профиля пациентки представлены в таблице 2.
Показатели гликемического профиля пациентки представлены в таблице 2.
Результаты инструментального исследования: данные Flash-мониторирования суточного профиля глюкозы – рисунок 1.
Электрокардиограмма (ЭКГ): ритм синусовый. Частота сокращений 93/мин; гипертрофия левого желудочка (ЛЖ).
Суточное мониторирование АД: среднее систолическое АД (САД) 147 мм рт.ст., диастолическое АД (ДАД) 88 мм рт.ст., максимальное САД 164 мм рт.ст., максимальное ДАД 108 мм рт.ст.
Суточное мониторирование ЭКГ по Холтеру: в течение всей записи регистрировался синусовый ритм. Средняя ЧСС 95 уд/мин, максимальная – 111 уд/мин.
Ультразвуковое исследование органов брюшной полости и забрюшинного пространства: без патологии.
Эхокардиография с допплеровским анализом: полости не расширены. Нарушений локальной сократимости не выявлено. Систолическая функция ЛЖ удовлетворительная, диастолическая нарушена (I тип). Незначительная гипертрофия миокарда ЛЖ. Дегенеративные изменения митрального, аортального клапанов. Незначительная недостаточность митрального, трикуспидального клапанов, клапана легочной артерии.
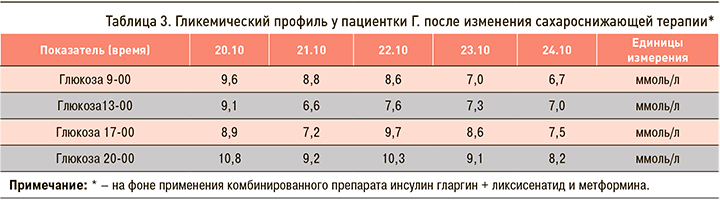
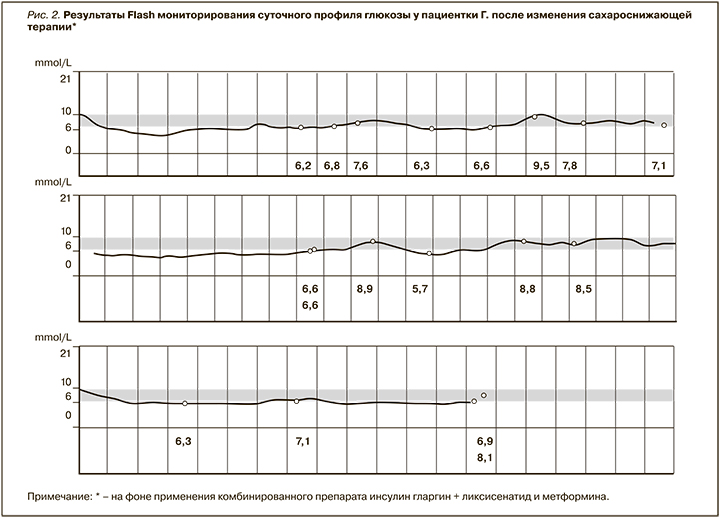
При госпитализации: в связи с недостижением целевых значений гликемии, превышением уровня HbA1c (целевой уровень для данной пациентки <7,5 %) более чем на 2,5% было принято решение отменить принимаемые пациенткой пероральные сахароснижающие препараты (гликлазид медленного высвобождения, линаглиптин) и заменить их на комбинированную терапию: инсулин гларгин + ликсисенатид в начальной дозировке 10 ЕД препарата с последующим прибавлением 2 ЕД 1 раз в трое суток до достижения целевых значений гликемии и метформин по 1000 мг 2 раза/ сут. Изменение гликемического профиля пациентки после смены терапии приведена в таблице 3. Данные Flash-мониторирования суточного профиля глюкозы после назначения инсулина гларгин + ликсисенатида и метформина показаны на рис. 2.
Клинический диагноз:
- основной: СД 2 типа. Целевой индивидуальный уровень HbA1c <7,5%;
- осложнения: диабетическая микроангиопатия: хроническая болезнь почек С3а (скорость клубочковой фильтрации 43,45 мл/мин/1,73 м2). Диабетическая дистальная симметричная полинейропатия. Диабетическая макроангиопатия: ИБС: стенокардия напряжения II функционального класса. КАГ со стентированием ПМЖА стентом Biomime (3,5×13 мм) от 30.05.2016;
- сопутствующие заболевания: гипертоническая болезнь III стадии, 3 степень, риск сердечно-сосудистых осложнений 4.
ОБСУЖДЕНИЕ И РЕКОМЕНДАЦИИ
Для данной пациентки с уровнем HbA1c, превышающим целевой более чем на 2,5%, была целесообразна замена пероральных сахароснижающих средств (препарат сульфонилмочевины и ингибитор дипептидилпептидазы-4) на комбинированную терапию, в основе которой препараты, влияющие на разные звенья патогенеза СД 2 типа и имеющие низкие риски гипогликемических явлений:
- инсулин длительного действия гларгин, интенсивно снижающий уровень гликемии, в частности, натощак, и позволяющий поддерживать более ровный профиль гликемии в течение суток;
- аГПП-1 ликсисенатид, в большей степени контролирующий постпрандиальную гипергликемию;
- метформин, активно уменьшающий инсулинорезистентность.
После смены терапии при оценке гликимического профиля была отмечена выраженная положительная динамика, пациентку перестали беспокоить жажда и сухость во рту, отмечалось достижение целевых показателей гликемии.
При выписке пациентке были даны рекомендации по изменению питания и образа жизни: частое, дробное питание, исключение из рациона быстро усваиваемых углеводов, ограничение употребления жиров животного происхождения, аэробные физические нагрузки не менее 150 мин в неделю. Также было рекомендовано продолжить назначенную сахароснижающую терапию: комбинированный препарат, в состав которого входит инсулин гларгин и ликсисенатид (12 ЕД в сутки) + метформин (1000 мг 2 раза/сут).



