Примерно у 75% пациентов, страдающих бронхиальной астмой (БА), наблюдается легкое течение болезни, которое часто не воспринимается серьезно врачами первичного звена здравоохранения. Вместе с тем показано, что даже легкая астма существенно снижает качество жизни больных, сопровождается развитием тяжелых, в ряде случаев смертельных обострений и требует значительных затрат на лечение [1]. Это стало причиной изменения тактики ведения БА легкого течения, которая нашла отражение в последней редакции Глобальной инициативы по БА (GINA) и действующих национальных клинических рекомендациях [2, 3].
В последние годы произошли существенные изменения в определении тяжести и контроля над БА. Ранее тяжесть болезни определялась на основании выраженности клинической картины и показателей функции легких ДО начала терапии. Недостатком этого определения было то, что оно не учитывало особенности течения БА у пациентов, получающих лечение.
В настоящее время в международных и национальных рекомендациях тяжесть течения БА оценивается с учетом объема проводимой терапии, необходимого для достижения контроля. Легкая БА – это заболевание, контроль над которым достигается при использовании низких доз ингаляционных глюкокортикоидов (ИГКС) и антилейкотриеновых препаратов (1 и 2 ступени терапии по GINA).
В предыдущих версиях (до 2019 г.) международных рекомендаций первая ступень предусматривала возможность использования коротко действующих β2-агонистов (КДБА) «по требованию» в виде монотерапии. Это было одним из парадоксов в лечении БА, поскольку эти препараты не обладают противовоспалительным эффектом. Более того, их регулярное применение ассоциировано с прогрессированием воспаления дыхательных путей, повышенным риском обострений и летального исхода [4, 5].
ИГКС служат основными препаратами в лечении легкой БА. Их эффективность была доказана в нескольких международных многоцентровых исследованиях [6, 7] с участием более 8000 пациентов. Показано, что постоянное использование низких доз ИГКС связано со снижением риска обострений, улучшением контроля над БА по сравнению с плацебо, а добавление к терапии длительно действующих β2-агонистов (ДДБА) обеспечивает дополнительный эффект, увеличивая время до первого тяжелого обострения и улучшая контроль [7]. Post-hoc-анализ исследования START показал, что низкие дозы ИГКС уменьшали риск тяжелых обострений даже у пациентов с симптомами менее одного раза в неделю [8].
Однако, несмотря на убедительность данных об эффективности постоянного применения ИГКС при легкой БА, в реальной клинической практике степень приверженности к такой терапии у больных остается низкой [9, 10]. Пациенты для контроля над симптомами преимущественно используют монотерапию КДБА [11, 12].
Основными причинами отказа пациентов от приема ИГКС становятся боязнь побочных эффектов и замедленное начало действия этих препаратов.
Возможным решением проблемы низкой приверженности пациентов к терапии ИГКС служит назначение «по требованию» комбинации средств этой группы с бронхолитиком быстрого действия. Возможность интермиттирующего приема ИГКС при легкой БА стала предметом нескольких исследований. В одном из них было впервые показано, что применение комбинации беклометазон/альбутерол в режиме «по требованию» не менее эффективно, чем регулярное использование ИГКС [13].
В настоящее время опубликованы результаты двух масштабных многоцентровых клинических исследований при легкой БА (SYGMA 1 и 2 – SYmbicort Given as need in Mild Asthma), в которых принимали участие и российские центры [14, 15]. В них было установлено, что интермиттирующее применение комбинации будесонид/формотерол (БУД/ФОРМ, Симбикорт Турбухалер) в течение 52 нед достоверно уменьшает число тяжелых обострений БА и улучшает ее контроль по сравнению с монотерапией КДБА. Частота обострений астмы при использовании БУД/ФОРМ была такой же, как при регулярном лечении будесонидом, при этом кумулятивная доза последнего была на 83% ниже.
Полученные результаты были подтверждены в отрытом многоцентровом исследовании Novel START (Novel Symbicort Turbuhaler Asthma Reliver Therapy), приближенном к условиям реальной клинической практики в связи с расширенными критериями включения пациентов с легкой БА [16].
Таким образом, результаты цитируемых выше исследований подтверждают возможность интермиттирующего использования комбинации БУД/ФОРМ (Симбикорт Турбухалер) при легкой БА (на 1 и 2 ступенях терапии) вместо КДБА. Полученные данные послужили причиной изменения международных рекомендаций по лечению (табл.). Особенно важно, что в международных и национальных рекомендациях из ступенчатой терапии полностью исключена монотерапия β2-адреномиметиками короткого действия.
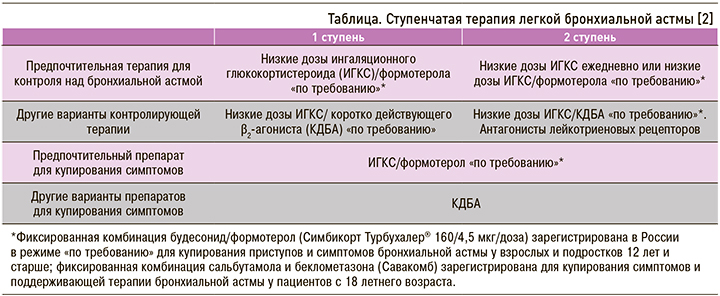
Принципиально важным являются рекомендации по раннему назначению низких доз ИГКС, а также применение для поддерживающего лечения в режиме «по требованию» и купирования симптомов комбинации ИГКС/формотерол (БУД/ФОРМ, Симбикорт Турбухалер®), которую называют «противовоспалительный бронхолитик».
В случае, когда пациент применяет этот препарат для купирования симптомов, он получает и ИГКС, обладающий противовоспалительным эффектом. Это снижает вероятность развития обострений при более низкой кумулятивной дозе ИГКС.
Антагонисты лейкотриеновых рецепторов (монтелукаст и не зарегистрированный в настоящее время в России зафирлукаст) относят к дополнительным средствам для лечения БА легкого течения (см. табл.). Они показаны больным с сопутствующим аллергическим ринитом и пациентам, которые в силу разных причин не желают или не могут использовать ИГКС. Антагонисты лейкотриеновых рецепторов обладают слабым и вариабельным бронхорасширяющим эффектом, уменьшают выраженность симптомов и частоту обострений астмы, уменьшают бронхиальную гиперреактивность и хроническое воспаление дыхательных путей, но их противовоспалительный эффект слабее, чем у ИГКС. Они применяются в терапии аспириновой астмы, БА физического усилия и аллергического ринита [3].
Аллерген-специфическая иммунотерапия (АСИТ) используется для лечения легкой атопической БА. Это метод заключается в повторных введениях больным лечебных аллергенов (аллерговакцин) с целью снижения чувствительности пациентов к их воздействию. Классический способ иммунотерапии представляет собой подкожные инъекции стандартизованных водно-солевых растворов аллергенов (клещевых, пыльцевых, за рубежом эпидермальных) в постепенно возрастающих концентрациях по специальным схемам и далее поддерживающее лечение в течение 3–5 лет. В последние годы в России, как и в других странах мира, активно используется сублингвальная АСИТ. Показано, что она улучшает контроль, снижает потребность больных в ИГКС и частоту обострений астмы [17, 18], в том числе БА легкого течения [19]. Метод имеет ряд ограничений, противопоказан при обострениях астмы, отсутствии контроля, множественной сенсибилизации (более 3 аллергенов), наличии ряда тяжелых фоновых заболеваний и др. АСИТ используется врачами – аллергологами-иммунологами у ряда пациентов с легкой атопической астмой.
Кроме применения лекарственных препаратов, важными аспектами ведения БА являются обучение больного, которое служит условием для установления партнерских отношений между врачом и пациентом и повышает приверженность к лечению, а также устранение факторов риска развития обострений (аллергенов, поллютантов, лекарственных средств и др.) и терапия сопутствующих заболеваний. Если у пациента в течение 2–3 мес применения рекомендованной терапии не удается установить контроль над заболеванием, то в первую очередь необходимо исключить факторы, которые могут к этому приводить: оценить технику ингаляции, выполнение назначений, вероятность неполной элиминации значимого аллергена, вероятный вклад сопутствующей патологии (курения, ожирения, гастроэзофагеальной рефлюксной болезни, артериальной гипертензии) [2]. Если этих факторов нет, следует пересмотреть терапию в сторону повышения ступени лечения.
Таким образом, легкая БА представляет в настоящее время значимую проблему как для пациентов, так и для системы здравоохранения в целом. При отсутствии адекватного лечения снижается качество жизни больных, возрастает угроза тяжелых (в том числе фатальных) обострений, прогрессирования болезни и формирования ремоделирования дыхательных путей. В связи с этим такие пациенты не должны получать монотерапию КДБА, которые не обладают противовоспалительным эффектом. Результаты выполненных исследований позволяют рекомендовать применение комбинации ИГКС/формотерол «по потребности» как для поддерживающего лечения, так и для купирования симптомов легкой БА в качестве основного режима фармакотерапии.



