АКТУАЛЬНОСТЬ
Пациенты с жалобой на кашель – частые посетители врачей первичного звена, пульмонологов и других специалистов, особенно в осенне-зимний период. В реальной клинической практике за медицинской помощью чаще обращаются пациенты с острым кашлем, обусловленным острыми респираторными вирусными инфекциями (ОРВИ) [1, 2]. В связи с высокой распространенностью острый кашель, сопровождающий ОРВИ, является глобальной проблемой и требует существенных расходов на рецептурные и безрецептурные препараты [3, 4].
КАШЕЛЬ ПРИ ОСТРЫХ РЕСПИРАТОРНЫХ ИНФЕКЦИЯХ
В большинстве случаев кашель – защитный сложнорефлекторный акт, направленный на удаление из дыхательных путей мокроты, частиц пыли и дыма. В случае, когда «эскалаторный» механизм мукоцилиарного аппарата перестает справляться со своей функцией, собственно, и возникает кашель [5, 6].
Первостепенная задача врача при жалобе пациента на кашель – установление его причины. Кашель как симптом может быть обусловлен многими причинами, такими как гастроэзофагеальная рефлюксная болезнь, применение ряда лекарственных средств (например, ингибиторов АПФ), бронхиальная астма, хроническая обструктивная болезнь легких (ХОБЛ), патология ЛОРорганов, хроническая сердечная недостаточность и др. [1, 5, 6].
По длительности кашель подразделяют на острый (продолжительность до 3 нед), подострый (3–8 нед) и хронический (продолжительность свыше 8 нед). В зависимости от наличия выделения мокроты выделяют продуктивный (влажный) и непродуктивный (сухой) кашель [1, 3, 4].
В 2022 г. в России в структуре всех нозологий численно преобладал диагноз «Острые инфекции верхних дыхательных путей множественной или неуточненной локализации» (к этой категории относится и ОРВИ, являющаяся наиболее частой причиной кашля) – 42 176 449 случаев, что оказалось на 10% больше, чем в 2020 г. [7]. У пациентов с ОРВИ продуктивный или непродуктивный кашель нередко выступает ведущим симптомом заболевания. Помимо кашля, пациентов беспокоят общее недомогание, повышение температуры тела, утомляемость, слабость, снижение аппетита, головные боли, боли в горле, затруднение носового дыхания, ринорея, осиплость голоса, увеличение лимфоузлов, боли в животе (при аденовирусной инфекции) [2]. У больных острым вирусным риносинуситом, вирусным тонзиллофарингитом частота встречаемости симптома кашля в первые 48 ч заболевания достигает 83%, к 14-му дню снижается до 26% [8, 9]. Особенности кашля при различных нозологических формах представлены в таблице.
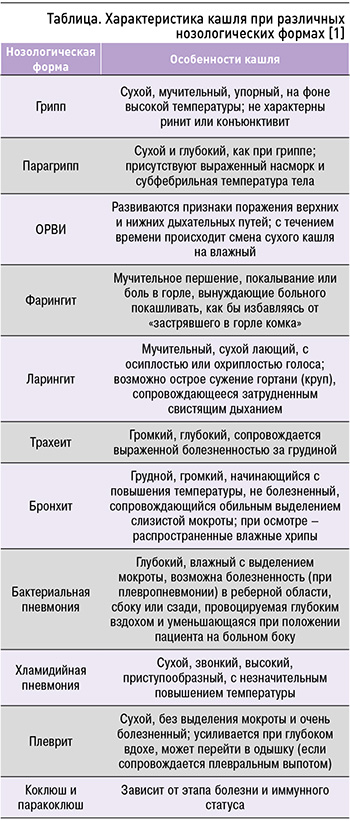
В клинической практике распространенной причиной острого кашля является острый бронхит. Под ним понимают остро возникшее воспаление нижних отделов дыхательных путей преимущественно вирусной этиологии. Кашель при остром бронхите чаще продуктивный, продолжается не более 2 нед и сочетается с характерными признаками инфекции нижних отделов дыхательной системы (хрипами, дискомфортом в грудной клетке, одышкой). При этом указанные симптомы невозможно альтернативно объяснить пневмонией, хроническим бронхитом, ХОБЛ или бронхиальной астмой [4–6].
Патогенез кашля как при ОРВИ, так и остром бронхите схож. При внедрении инфекционного агента (чаще вирусного) запускается сразу несколько путей формирования патологического кашля: продукты воспаления оказывают триггерное действие на хеморецепторы, одновременно повышается чувствительность всех кашлевых рецепторов. Нарушение мукоцилиарного клиренса, наряду с изменениями объема и вязкости мокроты, также участвует в патогенезе кашля. Непродуктивный кашель, характерный для первых дней заболевания, повышает реактивность бронхов и способствует уменьшению просвета бронхиального дерева за счет усиления воспалительных реакций [3–5, 8]. Кашель негативно влияет на качество жизни пациентов, может усугублять гипоксию и снижать сатурацию крови [1, 5].
Респираторные вирусные инфекции, в том числе вызванная SARS-CoV-2, могут приводить к нарушению мукоцилиарного клиренса в бронхиальном дереве из-за повышения вязкости слизи и ее гиперсекреции. На первом этапе инфекция распространяется по большей части слизеобразующих дыхательных путей (около 90% длины) без существенных изменений скорости выделения секрета. На втором этапе вязкость и толщина слоя слизи постепенно увеличиваются. Через некоторое время слой слизи в мелких воздухоносных путях может становиться сравнимым с их диаметром. Скопление в просвете мелких и средних бронхов секрета с неблагоприятными реологическими свойствами (повышенная вязкость, адгезивность) влечет за собой нарушение бронхиальной проходимости (воздушного потока) и клинически сопровождается приступообразным кашлем (продуктивным или непродуктивным), экспираторной одышкой и приступами удушья [9–12].
Подострый кашель тоже чаще всего вызывается острыми респираторными инфекциями и нередко представляет собой исход острого кашля. Его этиологическими возбудителями могут быть вирусы или такие атипичные внутриклеточные микроорганизмы, как Mycoplasma pneumoniae, Chlamydia pneumoniae, возбудители коклюша и паракоклюша Bordetella pertussis и Bordetella parapertussis. Подострый кашель может сопровождаться преходящей бронхиальной гиперреактивностью даже у лиц без бронхиальной астмы и без эозинофилии крови и мокроты [6, 8, 13]. В нередких случаях даже на фоне разрешения прочих местных и общих проявлений острых респираторных инфекций кашель сохраняется продолжительное время, переставая выполнять свою защитную функцию. Такой кашель принято называть постинфекционным [14, 15].
МЕДИКАМЕНТОЗНЫЕ ПОДХОДЫ К ТЕРАПИИ КАШЛЯ
Существует два основных медикаментозных подхода к купированию кашля – подавление кашлевого рефлекса (с использованием антитуссивных средств) и уменьшение проявлений кашля за счет изменения свойств и объема бронхиального секрета (с применением мукоактивных препаратов) [16, 17].
Подавление центральных и периферических звеньев кашлевого рефлекса оправдано при выраженном кашле, нарушающем активный образ жизни человека, ночной сон, при возникновении на фоне кашля боли, риске возможных осложнений. К препаратам, реализующим такой механизм действия, относятся бутамират, кодеин, глауцин, преноксдиазин, леводропропизин и др. Основная область их применения в терапии кашля – при онкологических процессах, состояниях после торакальных операций, дискинезии мембранозной части трахеи и других ситуациях, когда кашель не выполняет своей защитной функции и может нанести вред больному [18, 19]. Кроме того, непродуктивный характер имеет кашель при новой коронавирусной инфекции COVID-19. Во временных методических рекомендациях по профилактике и лечению новой коронавирусной инфекции в качестве средств симптоматической терапии рекомендовано применение таких противокашлевых препаратов, как бутамират и леводропропизин [20].
В то же время антитуссивные (противокашлевые) препараты не рекомендуется применять при наиболее распространенных болезнях органов дыхания (ХОБЛ, бронхиальная астма, пневмонии, бронхиты, бронхоэктазы и др.), требующих улучшения мукоцилиарного клиренса [17, 19, 21].
К средствам, позволяющим управлять кашлем через изменение количества и свойств бронхиального секрета, относятся мукоактивные препараты [18, 22, 23]. Они являются самыми назначаемыми препаратами в большинстве случаев кашля, включая острый бронхит, обострение хронического бронхита и внебольничную пневмонию (от 41 до 66% назначений) [21].
Среди препаратов, влияющих на отделяемое бронхов, выделяют несколько подгрупп лекарственных средств [17, 23].
- Экспекторанты (отхаркивающие, секретомоторные средства) – препараты резорбтивного или рефлекторного действия. Их эффект может развиваться после всасывания и выделения через слизистую бронхов, либо реализовываться через вагусный гастропульмонарный рефлекс. Эта группа подгруппа средств включает калия йодид, терпингидрат, гвайфенезин, а также препараты растительного происхождения, содержащие измельченные части или экстракты таких растений, как алтей, солодка, тимьян, девясил, мать-и-мачеха, душица, подорожник, термопсис, анис, первоцвет, плющ и др.
- Мукокинетики – препараты, влияющие на объем, вязкость, подвижность преимущественно золевого компонента бронхиального секрета. Они повышают транспортабельность секрета за счет улучшения мукоцилиарного транспорта. К ним чаще всего относят бромгексин и амброксол. Кроме них, отхождение мокроты облегчают β2-адреномиметики и метилксантины.
- Муколитики – препараты, влияющие на вязкость, эластичность и адгезивность преимущественно гелевого компонента бронхиального секрета и оказывающие свое мукоактивное действие в просвете бронхов. Количество бронхиального секрета при этом существенно не изменяется. Традиционно к муколитикам относят препараты N-ацетилцистеин, эрдостеин, дорназу-альфа.
- Мукорегуляторы – лекарственные средства, изменяющие продукцию бронхиального секрета и соотношение основных типов клеток в слизистой бронхов. При этом уменьшается число бокаловидных клеток и слизистых желез, что вызывает уменьшение образования слизи. Часть препаратов этой подгруппы обладает противовоспалительным действием, которое развивается при длительном применении. Классическим мукорегулятором является карбоцистеин. Также мукорегуляторные свойства присущи М-холиноблокаторам, ингаляционным глюкокортикоидам, макролидам.
При небольшом количестве мокроты терапия кашля может быть направлена на увеличение образования легко отделяемого бронхиального секрета с помощью экспекторантов (рефлекторного или резорбтивного действия), а также стимуляцию мукоцилиарного клиренса посредством мукокинетиков. Эти препараты уменьшают вязкость и адгезивность бронхиального секрета и обеспечивают достаточную эффективность мукоцилиарного и кашлевого клиренса.
В случае умеренного количества мокроты рекомендовано использовать мукокинетики, муколитики и мукорегуляторы. У больных со сниженным кашлевым рефлексом (пациентов пожилого и старческого возраста, детей раннего детского возраста, ослабленных больных, перенесших травмы грудной клетки и др.) оптимальным выбором будут мукокинетики. Эти лекарственные средства способны улучшать реологические свойства бронхиального секрета и стимулировать мукоцилиарный клиренс, что создает условия для облегчения экспекторации мокроты [21, 24]. Необходимо помнить о том, что одновременный прием противокашлевых и мукоактивных препаратов недопустим.
КОМБИНИРОВАННЫЕ ПРЕПАРАТЫ
В нередких случаях сочетания продуктивного кашля с бронхообструктивным синдромом применяются комбинированные препараты, обладающие как мукоактивным, так и бронхолитическим эффектами. При выборе оптимальной комбинации для терапии кашля с явлениями бронхообструкции необходимо учитывать ряд моментов [6, 17].
- Для профилактики реакций нежелательного лекарственного взаимодействия важно, чтобы препарат содержал не более трех активных компонентов из различных фармакологических групп, а также и не более одной активной субстанции из отдельной фармгруппы.
- Дозировка каждого действующего вещества должна быть эффективной, безопасной и позволяющей получать эффект синергизма компонентов.
- Состав препарата должен соответствовать типу и тяжести симптомов, которые необходимо купировать.
В медицине накоплен достаточный опыт применения фикисрованной комбинации агониста β2-адренорецепторов (бронхолитика) сальбутамола с мукоактивными средствами гвайфенезином и бромгексином. Эти активные компоненты действуют синергично, способствуя отхождению мокроты и разрешению кашля [25].
Фармакологические эффекты β2-агонистов адренорецепторов включают бронходилатацию и снижение сопротивления дыхательных путей, активизацию секреции слизи, стимуляцию восстановления реснитчатого эпителия дыхательных путей. В то же время использование адреномиметиков уменьшает одышку, связанную с бронхообструкцией, обеспечивает подавление высвобождения гистамина и лейкотриенов в бронхиальном дереве [16, 26].
Следует помнить, что при пероральном приеме сальбутамол обладает несколько иными фармакокинетическими свойствами, чем при использовании в форме аэрозоля. Для стимулирующего воздействия на мукоцилиарный клиренс при приеме внутрь необходимы бóльшие дозы β2-агонистов, чем при ингаляционном введении. Достижение таких доз возможно только при применении пероральной формы сальбутамола, чья биодоступность составляет 50%. Прием пищи снижает скорость его абсорбции, но не влияет на биодоступность. При приеме внутрь абсорбция сальбутамола высокая; 10% лекарственного вещества связывается с белками плазмы [18]. Безопасность приема перорального сальбутамола, входящего в состав фиксированной комбинации с гвайфенезином и бромгексином, отмечают 85% терапевтов [22].
Гвайфенезин – природное соединение, известное более 5 столетий. Он стимулирует секрецию жидкой части бронхиальной слизи, вызывает деполимеризацию кислых мукополисахаридов, уменьшает поверхностное натяжение и адгезивные свойства мокроты, увеличивает активность мерцательного эпителия и перистальтические движения бронхиол, способствует продвижению мокроты по дыхательным путям и ее выведению. Гвайфенезин является ингредиентом многих безрецептурных лекарств от кашля во многих странах мира, который зарекомендовал себя как лекарственное средство с благоприятным профилем безопасности и переносимости у взрослых и детей [27]. Кроме того, гвайфенезин уменьшает тревожность и психогенную вегетативную симптоматику, обладает некоторым миорелаксирующим действием [28, 29]. С учетом влияния на центральную нервную систему можно предположить, что этот активный компонент фиксированной комбинации может уменьшать возможное негативное воздействие на психическое состояние адреномиметика сальбутамола.
Бромгексин – муколитическое средство, характеризующееся высоким уровнем безопасности и минимальным числом нежелательных эффектов [18, 30]. Механизм его действия заключается в деполимеризации и разрушении кислых мукопротеинов и мукополисахаридных полимерных молекул мокроты, что снижает вязкость бронхиального секрета. Препарат активирует реснички мерцательного эпителия, улучшает отхождение мокроты. Дополнительными свойствами бромгексина являются стимуляция синтеза эндогенного сурфактанта, обеспечивающего стабильность альвеолоцитов в процессе дыхания и поддерживающего реологические свойства бронхолегочного секрета, а также стимуляция синтеза секреторного IgA. Кроме того, бромгексин способствует проникновению антибиотиков в легочную ткань [30–32].
В недавнем исследовании Gholami A. et al. была обнаружена способность бромгексина конкурентно ингибировать фермент липазу, которую некоторые патогенные бактерии используют для разрушения внеклеточного матрикса организмовхозяев. В частности, различные внеклеточные ферменты, такие как липаза, в качестве фактора патогенности использует Pseudomonas [33].
В ряде других исследований [34–37] было показано, что применение комбинации сальбутамола с гвайфенезином и бромгексином позволяло эффективно контролировать такие симптомы, как кашель и одышка. При этом серьезных нежелательных явлений на фоне такой терапии не отмечалось. Препарат продемонстрировал высокую эффективность в лечении кашля, связанного с инфекциями нижних дыхательных путей и ХОБЛ, хорошо переносился пациентами, а его эффективность была оценена очень высоко.
Использование комбинации сальбутамола, гвайфенезина и бромгексина представляется наиболее оправданным в начальный период заболевания при относительно небольшом количестве отделяемой мокроты. В этой ситуации муколитик бромгексин позволяет увеличить количество и гидратацию мокроты, мукокинетик гвайфенезин – нормализовать вязкость, адгезивность и мукоцилиарный клиренс, а β2-адреномиметик (сальбутамол) – увеличить частоту биения ресничек мерцательного эпителия и скорость мукоцилиарного клиренса. При этом сальбутамол, гвайфенезин и бромгексин действуют синергично, что дает возможность оптимизировать дозировку этих действующих веществ, сохранив их терапевтический эффект и минимизировав негативные нежелательные явления [37, 38].
Недавно на фармрынке России появился новый препарат, содержащий данную комбинацию компонентов, – Бромгекомб производства компании «Фармстандарт». Он представлен двумя лекарственными формами, подходящими для использования всей семьей, – таблетками Бромгекомб, разрешенными к приему с 6 лет, и сиропом Бромгекомб экспекторант, который можно использовать уже с 2-летнего возраста [40, 41]. При этом, по данным компании, препараты имеют сравнительно доступную стоимость (розничный аудит аналитической компании IQVIA в 2022 г.: средние розничные цены на лекарственные препараты с МНН бромгексин + гвайфенезин + сальбутамол в лекарственной форме сироп, формы выпуска 100 мл, 200 мл).
Бромгекомб экспекторант в форме сиропа рекомендуется взрослым и детям старше 12 лет по 10 мл (2 ч.л.) 3 раза/сут, детям в возрасте от 6 лет до 12 лет – 5–10 мл (1–2 ч.л.) 3 раза/сут, от 2 до 6 лет – 5 мл (1 ч.л.) 3 раза/сут. Препарат Бромгекомб в форме таблеток у взрослых и детей старше 12 лет используется по 1 таблетке 3 раза/сут, у детей от 6 до 12 лет – по 1/2 или 1 таблетке 3 раза/сут [40, 41].
ЗАКЛЮЧЕНИЕ
Таким образом, комбинация сальбутамола, бромгексина и гвайфенезина представляет собой синергичное сочетание активных веществ, оказывающих одновременно бронхолитическое, муколитическое, мукокинетическое и легкое седативное действие. Множественность действия препарата определяет целесообразность его применения практически при всех заболеваниях органов дыхания, сопровождающихся явлениями мукостаза и кашля с компонентом бронхообструкции.



