Диагностика
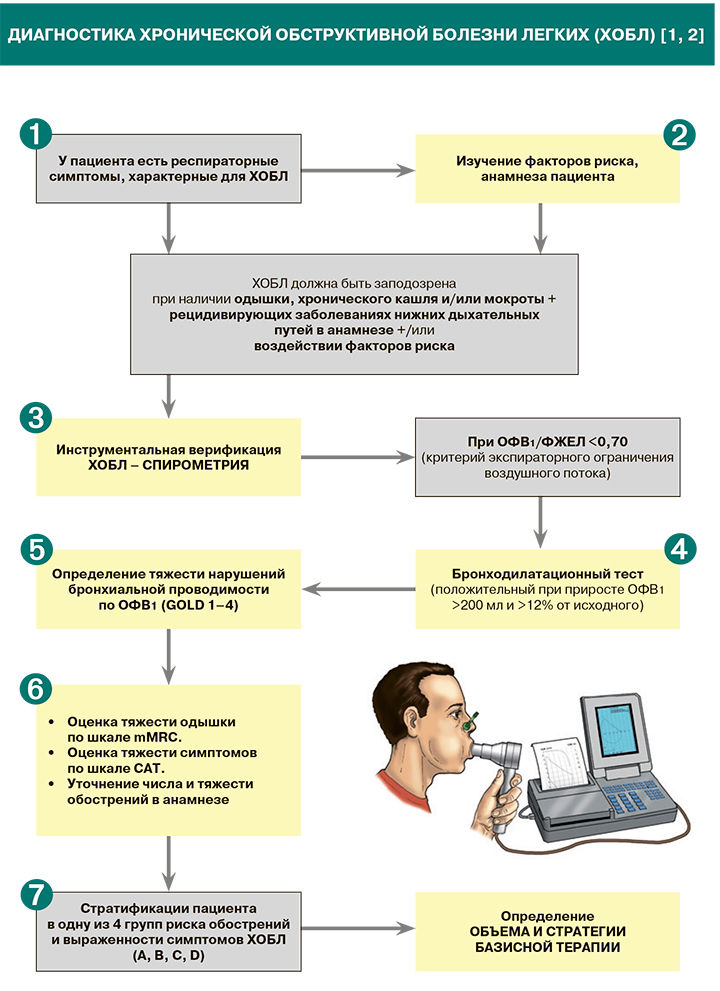
1.
• Ведущий симптом хронической обструктивной болезни легких (ХОБЛ) – одышка – обычно описывается пациентами как «необходимость дополнительных усилий для вдоха», «чувство тяжести в груди», «нехватка воздуха», «затрудненное дыхание».
• Хронический кашель при ХОБЛ может быть как продуктивным, так и непродуктивным.
• К другим возможным симптомам ХОБЛ относятся хрипы, чувство тесноты в груди, при тяжелой форме болезни – потеря в весе, анорексия [1].
2.
• Вероятность ХОБЛ возрастает в следующих обстоятельствах:
– курение (как сигарет, так и марихуаны), постоянное воздействие других вредных аэрополлютантов;
– наличие в анамнезе бронхиальной астмы (БА), рецидивирующих заболеваний нижних дыхательных путей, аллергии, синусита, назальных полипов, частых ОРЗ в детстве;
– близкие родственники с ХОБЛ (генетический фактор);
– появление симптомов во взрослом возрасте, усиление чувства нехватки воздуха во время зимних холодов;
– случаи госпитализации по поводу острых нарушений дыхательной системы в анамнезе;
– нереспираторные коморбидные заболевания, ограничивающие активность – сердечно-сосудистые, скелетно-мышечные, онкологические и др.;
– низкий социально-экономический статус пациента;
– наследственная недостаточность альфа1-антитрипсина (редко) [1, 2].
• Основные отличия ХОБЛ от БА:
– ХОБЛ дебютирует в среднем возрасте, БА – в раннем (часто – в детском);
– для ХОБЛ характерно медленное прогрессирование симптомов, для БА – широкая вариабельность клиники изо дня в день с ухудшением симптомов ночью или ранним утром;
– большинство больных ХОБЛ курят или курили в прошлом.
Следует помнить, что у пациентов нередко имеет место сочетание двух этих заболеваний [1, 2].
3.
При спирометрии рекомендуется проводить не менее трех технически правильных дыхательных маневра форсированной жизненной емкости легких (ФЖЕЛ) до получения воспроизводимых результатов: максимальные и следующие за ними по величине показатели ФЖЕЛ и объема форсированного выдоха за 1-ю секунду (ОФВ1) должны различаться не более чем на 150 мл. Если ФЖЕЛ ≤1000 мл, то максимально допустимая разница как по ФЖЕЛ, так и по ОФВ1 не должна быть >100 мл [2].
4.
• Бронходилатационный тест выполняют с сальбутамолом в разовой дозе 400 мкг через дозированный аэрозольный ингалятор со спейсером. Повторную спирометрию следует проводить через 15–30 мин после ингаляции короткодействующего β2-агониста [2].
• GOLD-2020 допускает использование во время бронходилатационного теста не только сальбутамола, но и коротко действующего антихолинергического средства (160 мкг) или даже их комбинации [1].
• Не следует прибегать к бронходилатационному тесту на протяжении длительного периода ведения пациента, так как степень обратимости дыхательной обструкции не является критерием, определяющим необходимость расширения диагностических исследований или дифференциальной диагностики ХОБЛ и БА. Кроме того, этот показатель не позволяет прогнозировать ответ на длительную поддерживающую терапию ингаляционными бронходилататорами или глюкокортикостероидами [1].
5.
Классификация GOLD тяжести нарушений бронхиальной проводимости по ОФВ1:
– ОФВ1 ≥80% – легкая степень (GOLD 1);
– 50%≤ ОФВ1 <80% – средняя степень (GOLD 2);
– 30%≤ ОФВ1 <50% – тяжелая степень (GOLD 3);
– ОФВ1 <30% – крайне тяжелая степень (GOLD 4) [1].
6.
Принципы оценки тяжести одышки по шкале mMRC и симптомов ХОБЛ по шкале CAT отражены в таблице 1 и на рисунке.

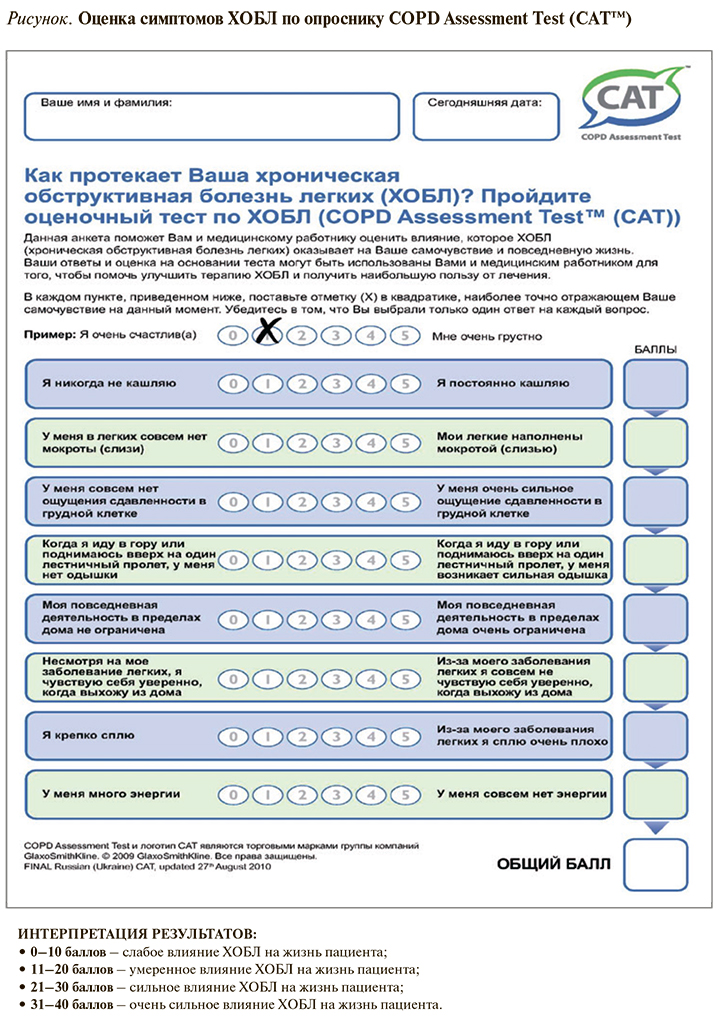
Качество жизни пациента в значительной мере определяется формированием «воздушных ловушек» в легких (гиперинфляция) из-за неполного опорожнения альвеол во время выдоха. Это приводит к увеличению остаточного объема и снижению емкости вдоха, прогрессирующей при физической нагрузке (динамическая гиперинфляция).
7.
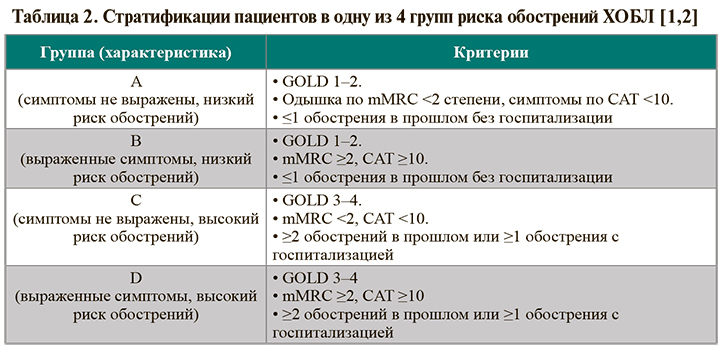
• Принципы стратификации пациентов в одну из 4 групп риска обострений ХОБЛ приведены в таблице 2. Принадлежность больного к той или иной страте во многом определяет начальную медикаментозную терапию ХОБЛ.
• Еще один вариант стратификации пациентов с ХОБЛ – разделение по фенотипу заболевания:
1. кахектический фенотип (эмфизематозный, «синий отечник») – преобладание эмфиземы, снижение мышечной массы, отеки ног, раннее развитие сердечной недостаточности, низкий ответ на бронхолитическую и стероидную терапию;
2. метаболический фенотип (бронхитический, «розовый пыхтельщик») – преобладание обструктивного бронхита, избыточная масса тела, нарушения липидного и углеводного обмена, артериальная гипертензия, удовлетворительный ответ на бронхолитическую и стероидную терапию.
Такая стратификация приобретает важное значение при выборе дополнительной (фенотип-специфической) терапии в тех случаях, когда обострения ХОБЛ повторяются, несмотря на использование комбинаций длительно действующих антихолинергических средств (ДДАХ) + длительно действующих β2-агонистов (ДДБА) ± ингаляционных глюкокортикостероидов (ИГКС) (см. комментарии к схемам лечения) [1, 2].
Лечение


1.
• Нефармакологические методы лечения ХОБЛ у пациентов всех групп (A, B, C, D) включают [1, 2]:
– отказ/ограничение курения;
– физическую активность;
– вакцинацию от гриппа и пневмококковой инфекции (PCV13, PPSV23) в тех регионах, где эти инфекции актуальны;
– обучение технике ингаляций и основам самостоятельного контроля заболевания.
• Больным групп B, C, D при постоянной одышке, непереносимости физических нагрузок дополнительно рекомендована легочная реабилитация [1].
• Всем пациентам также рекомендовано лечение сопутствующих заболеваний. При необходимости проводится оценка потребности длительной кислородотерапии и неинвазивной вентиляции легких [1, 2].
• Если пациент с ХОБЛ не способен самостоятельно прекратить/сократить курение, рекомендовано применение специализированных лекарственных средств – варениклина, бупропиона с пролонгированным высвобождением различных форм никотина (жевательные таблетки, спрей, ингаляции) [1, 2].
• Пневмококковые вакцины в первую очередь рекомендованы пациентам с ХОБЛ от 65 лет и старше, а также более молодым больным с серьезными сопутствующими заболеваниями, включая сердечно-сосудистые и легочные [1].
2.
• Коротко действующие бронхолитики вызывают краткосрочное уменьшение выраженности симптомов, но не снижают риск обострений ХОБЛ [1, 2].
• Согласно обзору рандомизированных клинических исследований коротко действующий антихолинергический препарат (КДАХ) ипратропия бромид несколько превосходит коротко действующие β2-агонисты (КДБА – сальбутамол и фенотерол) в плане улучшения легочной функции и общего состояния здоровья у больных ХОБЛ [1].
• Коротко действующие бронходилататоры зарегистрированы в России как в форме монопрепаратов, так и в фиксированных комбинациях (сальбутамол + ипратропия бромид, фенотерол + ипратропия бромид) [3]. Комбинации КДБА + КДАХ при обострениях ХОБЛ в большей степени, чем монотерапия, улучшают объем форсированного выдоха за 1-ю секунду (ОФВ1) и уменьшают выраженность симптомов [1].
3.
• Длительно действующие бронхолитики вызывают продолжительное уменьшение выраженности симптомов ХОБЛ, а также снижают риск обострений заболеваний [1, 2].
• Из длительно действующих β2-агонистов (ДДБА) в России в виде монопрепаратов зарегистрированы индакатерол, олодатерол и формотерол, из длительно действующих антихолинергических препаратов (ДДАХ) – тиотропия бромид, аклидиния бромид, умеклидиния бромид и гликопиррония бромид. Скорость наступления и длительность сохранения бронходилатирующего эффекта КДБА, КДАХ, ДДБА и ДДАХ приведена в таблице 1.

• ДДАХ в большей степени, чем ДДБА, редуцируют частоту обострений ХОБЛ и уменьшают риск госпитализации, в связи с чем GOLD-2020 рекомендует именно ДДАХ в качестве стартовой терапии у пациентов группы C [1]. По влиянию на ОФВ1 и одышку ДДБА по крайней мере сравнимы с тиотропия бромидом, но по влиянию на риск среднетяжелых/тяжелых обострений уступают этому ДДАХ [2].
• У пациентов с ХОБЛ и сопутствующими сердечно-сосудистыми заболеваниями рекомендуется использование ДДАХ [2].
• Тиотропия бромид (самый изученный на сегодня ДДАХ) повышает эффективность легочной реабилитации у больных ХОБЛ повышая способность к выполнению физических упражнений [1].
• Если российские клинические рекомендации (Российское респираторное общество, 2018 г.) предлагают использовать у больных ХОБЛ группы A ДДАХ или ДДБА [2], то в GOLD-2020 оговаривается возможность применения у этой категории пациентов ДДАХ или ДДБА или коротко действующих бронхолитиков (КДБА, КДАХ) [1]. При этом использование коротко действующих бронходилататоров на постоянной основе не рекомендуется [1]. Их следует применять по потребности, при ухудшении симптомов, несмотря на применение длительно действующих средств. В таких ситуациях необходимо тщательно оценить технику выполнения ингаляций длительно действующих бронходилататоров [2].
4.
• В России зарегистрирован целый ряд фиксированных комбинаций ДДАХ + ДДБА: аклидиния бромид + формотерол, умеклидиния бромид + вилантерол, гликопиррония бромид + индакатерол и тиотропия бромид + олодатерол [3].
• Если Российское респираторное общество рекомендует пациентам с ХОБЛ группы B комбинацию ДДАХ + ДДБА [2], то GOLD-2020 в данном случае в качестве варианта лечения рассматривает либо комбинацию ДДАХ + ДДБА, либо монотерапию [1]. При этом в GOLD-2020 оговаривается, что при монотерапии пациентов группы B нет доказательств того, что какая-либо из групп длительно действующих бронхолитиков превосходит другую по эффективности [1].
• Больным самой тяжелой группы ХОБЛ (D) GOLD-2020 рекомендован один из трех вариантов стартовой терапии:
– ДДАХ, или
– при тяжести симптомов по CAT >20 – ДДАХ + ДДБА, или
– при эозинофилии крови ≥300 кл/мкл – ДДБА + ингаляционный глюкокортикостероид (например, формотерол + будесонид).
• Комбинации ДДАХ + ДДБА показали преимущество перед плацебо и своими монокомпонентами по влиянию на минимальный ОФВ1, одышку и качество жизни, при этом не уступая им по безопасности [2]. При сравнении с тиотропия бромидом все комбинации ДДАХ + ДДБА показали свое преимущество по действию на легочную функцию и качество жизни. По влиянию на одышку преимущество не было продемонстрировано для комбинации умеклидиния бромид + вилантерол [4], а по влиянию на легочную гиперинфляцию только комбинация тиотропия бромид + олодатерол достоверно превосходила монотерапию тиотропия бромидом [5].
• Комбинации ДДАХ + ДДБА пока не продемонстрировали преимуществ перед монотерапией тиотропия бромидом по влиянию на риск среднетяжелых/тяжелых обострений ХОБЛ [4, 6, 7].
5.
• Если эффективность ранее назначенного лечения (учитывая следующие критерии – частота обострений, степень одышки) оказалась недостаточной, перед принятием решения о расширении объема (эскалации) медикаментозной терапии предлагается убедиться в том, что пациент технически правильно делает ингаляции, точно соблюдает все предписания врача, в том числе по части самообучения и легочной реабилитации [1].
• Способами регуляции эффективности фармакотерапии ХОБЛ, помимо ее эскалации, могут служить смена типа ингаляционного устройства (табл. 2), замена какого-либо из назначенных препаратов на другое средство той же группы, в ряде случаев – деэскалация (сокращение объема) терапии, но только не за счет бронходилататоров [1].

6.
Показатель эозинофилии – содержание эозинофилов в периферической крови вне обострения ≥300 клеток [1, 2].
7.
• Наряду с бронхиальной астмой в анамнезе и эозинофилией вне обострения добавление ингаляционных глюкокортистероидов (ИГКС) к длительно действующим бронходилататорам также рекомендуется в случаях ≥2 среднетяжелых обострений ХОБЛ в течение 1 года или хотя бы одного тяжелого обострения, потребовавшего госпитализации. Больным ХОБЛ с высоким риском обострений и без эозинофилии крови с одинаковой степенью доказательности рекомендуется назначать ДДАХ или ИГКС + ДДБА [2].
• Поддерживающая монотерапия ИГКС при ХОБЛ не рекомендована [1, 2].
• Если при бронхиальной астме лечебные и нежелательные эффекты ИГКС зависят от используемой дозы, то при ХОБЛ подобная дозозависимость отсутствует [2]. Ответ пациентов с ХОБЛ на лечение ИГКС невозможно прогнозировать на основании ответа на лечение пероральными ГКС, результатов бронходилатационного теста или наличия бронхиальной гиперреактивности [8, 9].
8.
• Комбинации ДДБА + ИГКС вызывают продолжительное уменьшение выраженности симптомов ХОБЛ, а при длительном (>6 мес) применении снижают частоту обострений ХОБЛ и улучшают качество жизни пациентов [9].
• Фиксированные комбинации ДДБА + ИГКС, зарегистрированные в России, и формы их выпуска представлены в таблице 3. При этом фиксированных комбинаций ДДАХ + ИГКС в России нет (возможно лишь сочетанное применение монопрепаратов) [3].

• Комбинации ДДБА + ИГКС более эффективны по сравнению с монотерапией в улучшении легочной функции, качества жизни и уменьшении обострений у пациентов с умеренной, тяжелой и очень тяжелой формами ХОБЛ [1]. При этом преимущество у комбинаций ДДБА + ИГКС перед бронходилататорами по влиянию на риск обострений наблюдается только при эозинофилии крови [10].
• Терапия ДДБА + ИГКС не влияет на скорость снижения ОФВ1 и летальность при ХОБЛ [2].
• Тройная терапия ХОБЛ изучалась в исследованиях, где добавление фиксированной комбинации ИГКС + ДДБА к ДДАХ тиотропия бромиду приводило к улучшению легочной функции, качества жизни и дополнительному уменьшению частоты обострений, особенно тяжелых [11]. Тем не менее тройная терапия требует дополнительного изучения в более продолжительных исследованиях [2].
• В России в настоящее время зарегистрирована лишь одна фиксированная тройная комбинация ДДБА + ДДАХ + ИГКС – вилантерол + умеклидиния бромид + флутиказона фуроат [3].
9.
• Возможные варианты фенотип-специфической терапии:
– тяжелая или очень тяжелая степень нарушений бронхиальной проводимости по ОФВ1 (GOLD 3–4) + хронический бронхит: рофлумиласт. Этот ингибитор фосфодиэстеразы-4 (ФДЭ-4) улучшает легочную функцию и редуцирует средние и тяжелые обострения ХОБЛ, сокращает частоту обострений у пациентов, получающих комбинацию ДДАХ + ИГКС [1]. При этом его не следует назначать для уменьшения выраженности симптомов ХОБЛ, хотя во время длительного лечения у пациентов, получающих салметерол или тиотропия бромид, рофлумиласт дополнительно увеличивает ОФВ1 на 50–80 мл [2];
– бронхитический фенотип в отсутствие ИГКС-терапии: муколитики (ацетилцистеин, карбоцистеин, эрдостеин). Прием этих препаратов у указанной группы пациентов с ХОБЛ может редуцировать обострения и умеренно повышать качество жизни [1];
– бронхоэктазы и частые гнойные обострения: макролиды (азитромицин, эритромицин и др.). Длительный (в течение 1 года) прием азитромицина (по 250 мг/сут или 500 мг 3 раза в неделю) или эритромицина (по 500 мг 2 раза/сут) у пациентов, склонных к обострениям ХОБЛ, сокращал риск этих обострений по сравнению со стандартным режимом применения антибиотиков. При этом использование азитромицина было сопряжено с повышением риска бактериальной резистентности, удлинением интервала QT. Дополнительный прием макролидов может быть рассмотрен у бывших курильщиков, имеющих обострения ХОБЛ, несмотря на адекватное ингаляционное лечение [1].
• При сохранении дыхательной недостаточности, несмотря на адекватную медикаментозную терапию, показана длительная кислородотерапия (1–3 л/мин), применение которой ограничено развитием гиперкапнии преимущественно у пациентов с эмфизематозным (кахектическим) фенотипом ХОБЛ. При гиперкапнической дыхательной недостаточности показано назначение двухуровневой неинвазивной вентиляции легких (BiPAP). При выраженных нарушениях откашливания рекомендуется использовать экспираторные дыхательные тренажеры с осцилляцией [12].
10.
Схема последовательной коррекции терапии ХОБЛ разработана GOLD-2020 на основе доказательных данных клинических исследований для унификации подходов к эффективному лечению у любых пациентов независимо от стажа и тяжести заболевания [1].



