 По последним данным Всемирной организации здравоохранения (ВОЗ), ежегодно в мире более 17 млн человек умирают от сердечно-сосудистых заболеваний (ССЗ) [1]. В России в 2019 г. смертность от болезней системы кровообращения составила 633 человек на 100 тыс. населения [2]. Среди них особого внимания заслуживает ишемическая болезнь сердца (ИБС): несмотря на значительный прогресс в контроле факторов риска и лечения, включая широкое распространение современных инвазивных методов коррекции заболевания, она остается одной из ведущих кардиоваскулярных причин смертности в экономически развитых странах.
По последним данным Всемирной организации здравоохранения (ВОЗ), ежегодно в мире более 17 млн человек умирают от сердечно-сосудистых заболеваний (ССЗ) [1]. В России в 2019 г. смертность от болезней системы кровообращения составила 633 человек на 100 тыс. населения [2]. Среди них особого внимания заслуживает ишемическая болезнь сердца (ИБС): несмотря на значительный прогресс в контроле факторов риска и лечения, включая широкое распространение современных инвазивных методов коррекции заболевания, она остается одной из ведущих кардиоваскулярных причин смертности в экономически развитых странах.
Ведущим фактором риска развития ИБС выступает атеросклероз коронарных артерий (рис. 1), борьба с которым ведется на протяжении многих лет. После открытия ингибиторов синтеза холестерина в 1978 г. смертность от ИБС и иных ССЗ снизилась более чем в 5 раз [3].
ИБС И ГИПОХОЛЕСТЕРИНЕМИЧЕСКАЯ ФАРМАКОТЕРАПИЯ: ПРОБЛЕМЫ И ИННОВАЦИИ
Несмотря на значительный рост использования инвазивных методов лечения (чрескожные вмешательства, аортокоронарное шунтирование), фармакотерапия сохраняет ведущую позицию во вторичной профилактике пациентов с хронической ИБС [4]. Последние данные проведенных клинических исследований говорят о том, что применение медикаментозной терапии у пациентов, перенесших инвазивное лечение ИБС, сопровождается выраженным снижением смертности и частоты развития отсроченных осложнений [5, 6]. Например, в 2018 г. были получены результаты клинического исследования ORBITA, согласно которым у больных со значимым атеросклерозом коронарной артерии выполнение чрескожного коронарного вмешательства (ЧКВ) без последующей медикаментозной поддержки не приводит к улучшению качества жизни по сравнению с пациентами, которым подобрана корректная лекарственная терапия без эндоваскулярного вмешательства [7].
Основой медикаментозного лечения ИБС служит гиполипидемическая терапия. Общий холестерин (ОХС) и его атерогенная фракция – холестерин липопротеидов низкой плотности (ХС ЛПНП) – самые значимые показатели липидного обмена, являющиеся ключевыми факторами риска развития ССЗ [8]. Долгое время основным методом вторичной профилактики гиперхолестеринемии было применение ингибиторов 3-гидрокси-3-метилглютарил-кофермент А редуктазы (статинов) и ингибитора абсорбции холестерина в кишечнике эзетемиба. Однако, как было показано в клинических исследованиях, на фоне приема высоких доз этих препаратов только 70% пациентов достигали целевого уровня ХС ЛПНП [9], а в реальной клинической практике этот показатель был еще ниже: не более чем 21% больных [10].
В 2013–2014 гг. было проведено масштабное исследование АРГО с целью изучить уровни ОХС у пациентов высокого и очень высокого сердечно-сосудистого риска в реальной клинической практике и оценить соответствие ведения таких больных действующим клиническим рекомендациям по лечению дислипидемий [11]. В исследование были включены пациенты в возрасте 30 лет и старше, обратившиеся на прием к терапевтам и кардиологам городских поликлиник в период с октября 2013 г. по июль 2014 г. Каждый пациент заполнял анкету. Определение уровня ОХС выполнялось без специальной подготовки при помощи портативного анализатора крови, позволяющего в течение нескольких минут определить уровень ОХС [12]. После обработки 38 400 анкет в окончательный анализ были включены 18 273 пациента (58,9% женщин и 41,1% мужчин). Гиперхолестеринемия была установлена у 78,9% мужчин и 81,3% женщин. Во всех субъектах России уровень ОХС был существенно выше целевого и колебался от 5,82 до 6,10 ммоль/л, при этом гиполипидемическая терапия была назначена всего лишь половине пациентов. Среди пациентов, принимавших симвастатин, уровень общего холестерина >5 ммоль/л был выявлен в 84,7% случаев, аторвастатин – в 75,2%, розувастатин – в 59%. Пациенты очень высокого сердечно-сосудистого риска достигали целевого уровня ОХС лишь в 2,04–7,38% случаев. Из всего вышесказанного можно сделать вывод, что, несмотря на доступность информации для врачей и пациентов, ситуация с диагностикой и лечением дислипидемий в реальной клинической практике далека от идеальной.
Отдельно следует отметить высокую распространенность гиперхолестринемии не только среди пациентов с заболеваниями сердечно-сосудистой системы, но и в общей популяции. По данным проведенного в США исследования Multi-Ethnic Study of Atherosclerosis (n=6814; средний возраст от 45 до 84 лет, без хронических ССЗ), повышение уровня ОХС было обнаружено у 29,3% лиц, из которых 54% сообщили о постоянном приеме статинов [13]. В Китае при обследовании 11 953 пациентов в рамках национального исследования National Cholesterol Education Program Adult Treatment Panel III нарушение липидного обмена было выявлено в 16,4% случаев, а один из типов дислипидемий имели 36,9% лиц обследованной популяции [14]. В 2006 г. в Испании в рамках исследования HISPALIPID Study (n=8256; 52,4% женщин; средний возраст 62,4±12,4 лет) повышение уровня атерогенных липидов было обнаружено в 24,3% случаях [15]. Там же через 7 лет среди пациентов высокого и очень высокого сердечно-сосудистого риска (n=1137; средний возраст 63,9±9,7; 64,6% мужчин) дислипидемия была диагностирована в 27,1% случаях [16]. Учитывая все вышесказанное, многие годы продолжался поиск новых видов препаратов, снижающих уровень липидов крови.
В 2003 г. был открыт ген, отвечающий за количество белка пропротеиновой конвертазы субтилизин-кексинового типа 9 (PCSK9), играющего основную роль в уровне гомеостаза холестерина, а в 2015 г. впервые в практической медицине были применены моноклональные антитела, являющиеся ингибиторами этого белка. Они позволяют достичь целевого уровня липидных показателей более чем у 90% пациентов [17]. Значительное количество проведенных клинических исследований доказало высокую эффективность ингибиторов PCSK9 (ODYSSEY LONG TERM, ODYSSEY COMBO II, ODYSSEY FH I, ODYSSEY FH II, DESCARTES, RUTHERFORD-2, TESLA, OSLER, MENDEL-2, LA PLACE-2, FOURIER) [18].
В связи с необходимостью решения вопроса о целесообразности применения ингибиторов PCSK9 больных ИБС принято разграничивать по группам сердечно-сосудистого риска. В настоящий момент препараты этого класса рекомендуется назначать пациентам высокого, очень высокого и экстремально высокого сердечно-сосудистого риска, не достигшим целевых значений ХС ЛПНП (<1,8/ <1,4/ <1,0 ммоль/л соответственно) [19].
Группа экстремально высокого сердечно-сосудистого риска впервые была выделена на Европейском конгрессе кардиологов в 2019 г.: в нее включают лиц, перенесших 2 острых сосудистых события (острый коронарный синдром, острое нарушение мозгового кровообращения, транзиторную ишемическую атаку) в течение 2 лет [19]. Достижение целевых показателей уровня холестерина у таких пациентов – приоритетная задача в выборе тактики лекарственной терапии.
МЕХАНИЗМ ДЕЙСТВИЯ ИНГИБИТОРОВ PCSK9
Известно, что циркулирующий в крови ХС ЛПНП захватывается гепатоцитами с помощью рецепторов к ЛПНП (Р-ЛПНП). Образовавшийся комплекс доставляется в гепатоцит в составе клатриновых пузырьков, которые сливаются с эндосомами, где происходит разрушение образовавшегося комплекса. После этого Р-ЛПНП вновь возвращаются на поверхность гепатоцита, где повторно связывают и доставляют в гепатоцит новые ХС ЛПНП. Доказано, что белок PCSK9 регулирует этот процесс, связываясь с Р-ЛПНП, который в дальнейшем разрушается в лизосомах клетки, что ведет к увеличению концентрации холестерина ЛПНП в плазме крови [20]. Известно, что PCSK9 типа относится к семейству сериновых протеиназ, которое также включает такие ферменты, как PC1/3, PC2, фурин, PC4, PC5/6, PACE4, PC7, SKI-1/ S1P. Гены, кодирующие синтез этих ферментов, получили схожие названия: Pcsk1, Pcsk2, Furin, Pcsk4, Pcsk5, Pcsk6, Pcsk7, Mbtps1 и Pcsk9. Все перечисленные ферменты расщепляются самостоятельно на один или два основных остатка, кроме PCSK9, которой необходим субстрат для ее активации [21].
Стоит отметить, что PCSK9 синтезируется в виде белка про-PCSK9, содержащего 692 аминокислоты [22]. Синтез про-PCSK9 происходит в эндоплазматическом ретикулуме. Связывание про-PCSK9 с рецепторами к ЛПНП в эндоплазматическом ретикулуме способствует транспорту Р-ЛПНП из эндоплазматичского ретикулума в сторону комплекса Гольджи, где к Р-ЛНП присоединяются зрелые остатки углевода. Транспорт про-PCSK9 к комплексу Гольджи зависит от белка Sec24A. Связывание про-PCSK9 с Р-ЛНП способствует каталитическому расщеплению PCSK9 [23]. Учитывая тот факт, что зрелые Р-ЛНП и PCSK9 находятся в комплексе Гольджи, вероятно, что процесс деградации Р-ЛНП с помощью PCSK9 протекает или начинается в комплексе Гольджи или транс-Гольджи. Ингибирование PCSK9 не позволяет этому белку связываться с Р-ЛПНП и инициировать его дальнейшее разрушение, результатом чего становится увеличение числа активных Р-ЛПНП на поверхности гепатоцита [24].
Добавим, что количество белка PCSK9 в плазме крови зависит от ряда факторов. Доказано, что при голодании содержание холестерина в гепатоците увеличивается, что приводит к снижению стерол-регулирующего элемента-2 (SREBP-2) и экспрессии PCSK9 [25]. Кроме того, SREBP-2 влияет на концентрацию в плазме PCSK9 в течение суток (максимальное содержание PCSK9 приходится на ранние утренние часы и снижается к полудню) [26]. Таким образом, для корректного сравнения уровня PCSK9 в динамике необходимо проводить измерение в одно и то же время суток, желательно утром.
Наряду с этим установлено, что концентрация PCSK9 может зависеть от пола человека (у женщин выше, чем у мужчин); также она с возрастом увеличивается у женщин и уменьшается у мужчин [27].
ИНГИБИТОРЫ PCSK9: КРАТКИЙ ОБЗОР ОСНОВНЫХ КЛИНИЧЕСКИХ ИССЛЕДОВАНИЙ
В России доступно два лекарственных средства из класса ингибиторов PCSK9 – эволокумаб и алирокумаб.
Эволокумаб был изучен в обширной программе клинических исследований PROFICIO, которая включала более 35 тыс. пациентов, и в 2015 г. стал первым ингибитором PCSK9, одобренным для клинического применения под торговым названием Репата. Препарат выпускается в виде раствора для подкожного введения в дозе 140 мг/мл.
Алирокумаб был зарегистрирован двумя годами позже (2017) под торговым наименованием Пралуэнт. Препарат производится в виде раствора для подкожного введения в дозах 75 и 150 мг/мл.
В исследовании LAPLACE-2 изучалась эффективность эволокумаба (140 мг подкожно, каждые 2 нед) при добавлении к стандартной гиполипидемической терапии. Было показано, что на фоне интенсивной статинотерапии эволокумаб дополнительно снижает ХС ЛПНП до 75% по сравнению с изолированной терапией статинами. При этом 94% пациентов, получавших этот ингибитор PCSK9, достигали целевого уровня ХС ЛПНП [28].
В исследовании GAUSS-2 эволокумаб (140 мг подкожно, каждые 2 нед) использовался у пациентов с непереносимостью статинов. Было показано, что его введение привело к снижению уровня ХС ЛПНП на 56%, тогда как прием эзетимиба – на 19% [29].
Также применение эволокумаба оценивалось у больных с семейной гетерозиготной гиперхолестеринемией в исследовании RUTHERFORD-2, где на фоне максимально переносимой гиполипидемической терапии дополнительно назначали этот ингибитор PCSK9 (140 мг подкожно, каждые 2 нед) и плацебо. В группе эволокумаба было выявлено снижение уровня ХС ЛПНП на 60% по сравнению с плацебо. Помимо этого, 67% участников исследования, получавших эволокумаб, достигли целевого значения ХС ЛПНП, в то время как в группе плацебо этот показатель составил 2% [30].
В плацебо-контролируемом двойном слепом международном исследовании в параллельных группах FOURIER (n=27 564; 49 стран; 1272 исследовательских центра) изучалась клиническая эффективность и безопасность эволокумаба у пациентов 40–85 лет (средний возраст 62,3 года, 75% мужчин) с клинически выраженным атеросклерозом: целью было показать, что добавление эволокумаба к стандартной гиполипидемической терапии снижает частоту основных нежелательных сердечно-сосудистых явлений. Для включения в исследование пациент должен был иметь дополнительно один и более факторов риска ССЗ, а также уровень ХС ЛПНП ≥1,8 ммоль/л (или ХС неЛПВП ≥2,6 ммоль/л) на фоне терапии аторвастатином (в дозе ≥20 мг или при приеме эквивалентной дозы другого статина) ± эзетимибом. Комбинированной первичной конечной точкой служили сердечно-сосудистая смерть, инфаркт миокарда, инсульт, госпитализация по поводу нестабильной стенокардии или ЧКВ, ключевой вторичной конечной точкой – сердечно-сосудистая смерть, инфаркт миокарда или инсульт. Основными сопутствующими заболеваниями и факторами риска у участников были артериальная гипертензия (80% участников), сахарный диабет (36%) и курение (28%). У 81% исследуемых в анамнезе имел место перенесенный острый инфаркт миокарда, у 19% – ишемический инсульт, у 13% – атеросклероз периферических артерий. Пациенты находились на адекватной гиполипидемической терапии: 70% получали терапию статинами высокой интенсивности (аторвастатин более 40мг), около 30% – умеренной интенсивности (аторвастатин 20–40 мг); также 5% пациентов применяли эзетимиб. У участников, радомизированных в первую группу, использовался эволокумаб подкожно в дозе 140 мг 1 раз в 2 нед или 420 мг/мес (на выбор пациента), в группу контроля – плацебо 1 раз в 2 нед или 1 раз в месяц (на выбор пациента). Медиана наблюдения в исследовании составила 2 года 2 мес. В результате была подтверждена высокая гиполипидемическая эффективность эволокумаба: уровень ХС ЛПНП снизился на 59% от исходных значений – с 2,4 до 0,78 ммоль/л. Также было продемонстрировано положительное влияние этого ингибитора PCSK9 на сердечно-сосудистые исходы. Так, риск развития первичной конечной точки в группе эволокумаба оказался на 15%, а вторичной на 20% ниже, чем в группе плацебо. Через год наблюдения в группе пациентов, получавших эволокумаб, риск развития острого инфаркта миокарда и инсульта снизился на 19%, а к концу наблюдения этот показатель достиг 33% [31].
Самое длительное исследование эволокумаба (более 4 лет) называлось OSLER-1, результаты которого были опубликованы в 2017 г. Было доказано, что после отмены препарата отсутствует «эффект рикошета», т.е. через 12 мес уровень ХС ЛПНП достигал первоначальных значений и не превышал их [32]. Также было показано, что на фоне длительного применения эволокумаба уровень ХС ЛПНП поддерживался на одном уровне: через 1 год он снизился на 61%, через 4 года – на 57% от начальных показателей [33]. Таким образом, клиническая эффективность эволокумаба сохраняется на протяжении всего периода лечения и не зависит от индивидуальных особенностей пациентов.
Влияние алирокумаба на частоту возникновения больших сердечно-сосудистых событий изучали в исследовании ODYSSEY OUTCOMES у пациентов после перенесенного острого коронарного синдрома (ОКС). Помимо этого, в задачи исследования входило изучение безопасности и переносимости алирокумаба, а также его влияние на уровни различных фракций липопротеидов и выработку антител. В исследовании приняли участие 18 924 пациента старше 40 лет, имеющих в анамнезе ОКС в течение последнего года, но не менее одного месяца до отбора. К моменту назначения препарата пациенты длительное время получали интенсивную статинотерапию и не достигли целевых значений ХС ЛПНП. Обязательным условием для включения в исследование было соответствие хотя бы одному из следующих критериев: ХС ЛПНП ≥70 мг/дл (≥1,81 ммоль/л), или аполипопротеин B ≥ 80 мг/дл (≥0,8 г/л), или ХС ЛПнеВП ≥100 мг/ дл (≥2,59 ммоль/л). Критериями исключения служили гепатит В/С, повышение уровня триглицеридов (ТГ) более 4,52 ммоль/л, аланинаминотрансферазы (АЛТ)/аспартатаминотрансферазы (АСТ)/креатинкиназы (КФК) более чем в 3 раза, скорость клубочковой фильтрации (СКФ) менее 30 мл/мин/1,73 м2, применение фибратов (кроме фенофибрата или фенофиброевой кислоты) и повторный ОКС в течение 2 нед перед рандомизацией. Рандомизацию проводили не ранее чем через 4 нед после развития ОКС, но не включали пациентов, у которых планировалось проведение ЧКВ, или оно была выполнено менее чем за 2 нед до рандомизации. После отбора пациенты были рандомизированы для назначения алирокумаба или плацебо. Исходные группы были сопоставимы между собой, возрастная медиана составляла 58 лет, в обеих группах преобладали мужчины (74,7% в группе алирокумаба и 74,9% плацебо), в анамнезе у большинства пациентов имелась артериальная гипертония (65,6% в группе алирокумаба и 63,9% в группе плацебо), сахарный диабет (28,5 и 29,1% соответственно), перенесенный инфаркт миокарда (18,9 и 19,5% соответственно).
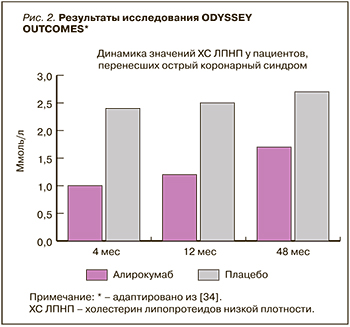 На протяжении всего исследования пациенты получали алирокумаб или плацебо каждые 2 нед. Начальная доза ингибитора PCSK9 составила 75 мг. В дальнейшем в зависимости от значений ХС ЛПНП его дозу увеличивали до 150 мг или переводили пациента на плацебо. Длительность исследования составила 2 года 8 мес. За 44% пациентами продолжали наблюдение в дальнейшем более трех лет. Преждевременно лечение прекратили 14,2% пациентов в группе алирокумаба и 15,8% в группе плацебо. 7,7% пациентов были переведены с алирокумаба на плацебо, основанием чему было снижение ХС ЛПНП менее 0,3 ммоль/л. На фоне лечения алирокумабом по сравнению с плацебо уровень ХС ЛПНП уменьшился на 62,7% через 4 мес исследования, на 61,0% – через 12 мес и на 54,7% – через 2 года. В абсолютных значениях через 4 мес исследования уровень ХС ЛПНП в группе алирокумаба составил 1 ммоль/л, в группе плацебо – 2,4 ммоль/л, через 12 мес – 1,2 и 2,5 ммоль/л соответственно, через 48 мес – 1,7 и 2,7 ммоль/л соответственно (рис. 2). После исключения из результатов пациентов, преждевременно прекративших лечение или переведенных на плацебо, динамика значений ХС ЛПНП была еще более впечатляющей: в группе алирокумаба по сравнению с плацебо отмечалось снижение ХС ЛПНП на 62,7% через 4 мес, на 61,0% – через 12 мес, на 54,7% – через 48 мес терапии. Помимо этого, наблюдалось выраженное снижение ХС липопротеидов невысокой плотности, аполипопротеина B, ТГ и липопротеина (а).
На протяжении всего исследования пациенты получали алирокумаб или плацебо каждые 2 нед. Начальная доза ингибитора PCSK9 составила 75 мг. В дальнейшем в зависимости от значений ХС ЛПНП его дозу увеличивали до 150 мг или переводили пациента на плацебо. Длительность исследования составила 2 года 8 мес. За 44% пациентами продолжали наблюдение в дальнейшем более трех лет. Преждевременно лечение прекратили 14,2% пациентов в группе алирокумаба и 15,8% в группе плацебо. 7,7% пациентов были переведены с алирокумаба на плацебо, основанием чему было снижение ХС ЛПНП менее 0,3 ммоль/л. На фоне лечения алирокумабом по сравнению с плацебо уровень ХС ЛПНП уменьшился на 62,7% через 4 мес исследования, на 61,0% – через 12 мес и на 54,7% – через 2 года. В абсолютных значениях через 4 мес исследования уровень ХС ЛПНП в группе алирокумаба составил 1 ммоль/л, в группе плацебо – 2,4 ммоль/л, через 12 мес – 1,2 и 2,5 ммоль/л соответственно, через 48 мес – 1,7 и 2,7 ммоль/л соответственно (рис. 2). После исключения из результатов пациентов, преждевременно прекративших лечение или переведенных на плацебо, динамика значений ХС ЛПНП была еще более впечатляющей: в группе алирокумаба по сравнению с плацебо отмечалось снижение ХС ЛПНП на 62,7% через 4 мес, на 61,0% – через 12 мес, на 54,7% – через 48 мес терапии. Помимо этого, наблюдалось выраженное снижение ХС липопротеидов невысокой плотности, аполипопротеина B, ТГ и липопротеина (а).
Крупные сердечно-сосудистые события были зарегистрированы у 903 пациентов (9,5%) в группе алирокумаба и 1052 в группе плацебо (11,1%). Общая смертность была ниже в группе ингибитора PCSK9: 3,5 против 4,1% у плацебо. Наиболее выраженным гиполипидемический эффект оказался у пациентов с уровнем ХС ЛПНП >2,6 ммоль/л. В этой группе кардиоваскулярный риск оказался на 24% ниже, чем в группе плацебо, а риска смерти от любых причин – на 29% [34].
ИНГИБИТОРЫ PCSK9: ОБЗОР БЕЗОПАСНОСТИ
В клинических исследованиях эволокумаба продемонстрирован хороший показатель безопасности препарата, сопоставимый с плацебо. В целом после проведения 12 основных исследований препарата частота всех нежелательных явлений составила 51,1% в группе ингибитора PCSK9 и 49,6% в группе плацебо. Значимые нежелательные явления были зарегистрированы у 2,1% пациентов, получавших плацебо, и у 2,8% – инъекции эволокумаба. Отмена препарата потребовалась 2,3% людей в группе эволокумаба и 1,9% в группе плацебо [35].
В упоминавшемся длительном исследовании FOURIER (2 года 2 мес) доказано отсутствие значимых различий в плане нежелательных явлений между эволокумабом и плацебо. С целью изучения влияния этого препарата на когнитивные функции было проведено исследование EBBINGHAUS, куда вошли 1972 пациента из исследования FOURIER. Анализ когнитивных функций был выполнен с помощью планшетного метода CANTAB. Медиана наблюдения за пациентами составила 20 мес. Было установлено, что в группе эволокумаба не происходило значимого снижения когнитивных функций на протяжении всего исследования и при достижении минимальных значений ХС ЛПНП [36].
Наиболее длительное исследование (более четырех лет) OSLER-1 говорит о том, что на всех этапах лечения эволокумабом не выявлено ни одного случая образования антител к этому препарату [33].
В исследованиях ODYSSEY OUTCOMES получены схожие результаты относительно безопасности этого ингибитора PCSK9: не было выявлено различий в частоте развития нежелательных реакций между группами алирокумаба и плацебо. Положительная реакция на наличие антител к алирокумабу была выявлена у 0,7% пациентов в группе применения алирокумаба и у 0,4% пациентов в группе плацебо, нейтрализующие антитела были выявлены у 0,5% в группе алирокумаба и у менее 0,1% пациентов в группе плацебо [34]. Частота нежелательных эффектов со стороны опорно-двигательной, нервной системы, а также когнитивные нарушения регистрировались с одинаковой частотой в группах алирокумаба, эзетимиба и плацебо [37].
Полученные данные по безопасности ингибиторов PCSK9 свидетельствуют о том, что их применение характеризуется хорошей переносимостью.
ЗАКЛЮЧЕНИЕ
Применение максимально переносимых доз ингибиторов статинов и эзетемиба не позволяет достичь целевых показателей ХС ЛПНП у всех пациентов с ИБС из группы высокого и очень высокого сердечно-сосудистого риска. Применение ингибиторов белка PCSK9, появившихся в арсенале врачей в 2015 г., позволяет достичь целевого уровня липидных показателей более чем у 90% пациентов. Вместе с тем в отношении этой группы гиполипидемических препаратов остается ряд нерешенных вопросов, ответы на которые могут быть получены из последующих исследований.



