СПИСОК СОКРАЩЕНИЙ:
- AUC – area under the curve (площадь под кривой)
- CYР – изоферменты системы цитохрома Р450
- АВК – антагонисты витамина К
- АСК – ацетилсалициловая кислота
- БАД – биологически активная добавка
- ДАТТ – двойная антитромбоцитарная терапия
- ДИ – доверительный интервал
- ЖКК – желудочно-кишечное кровотечение
- ЖКТ – желудочно-кишечный тракт
- ИБС – ишемическая болезнь сердца
- ИПП – ингибиторы протонной помпы
- ЛС – лекарственные средства
- МНО – международное нормализованное отношение
- НПВП – нестероидные противовоспалительные препараты
- НЯ – нежелательные явления
- ОАК – оральные антикоагулянты
- ОР – отношение рисков
- ОтнР – относительный риск
- ОШ – отношение шансов
- ПОАК – прямые оральные антикоагулянты
- РКИ – рандомизированные контролируемые исследования
- СИОЗНА – селективные ингибиторы обратного захвата серотонина и норадреналина
- СИОЗС – селективные ингибиторы обратного захвата серотонина
- СО – слизистая оболочка
- ФП – фибрилляция предсердий
- ЦОГ-2 – циклооксигеназа 2 типа
ВВЕДЕНИЕ
Масштабы применения антикоагулянтной терапии как инструмента профилактики тромботических осложнений неуклонно растут во всем мире, и в развитых странах доля лиц, использующих антикоагулянтные лекарственные средства, составляет 2% от общей численности населения [1, 2].
Первыми оральными антикоагулянтами (ОАК), внедренными в клиническую практику во второй половине прошлого столетия для применения у пациентов с фибрилляцией предсердий (ФП), протезированными клапанами сердца, тромбозом вен конечностей, тромбоэмболией легочной артерии (ТЭЛА), стали антагонисты витамина К (АВК) [2]. В дальнейшем появились прямые оральные антикоагулянты (ПОАК) – дабигатрана этексилат (2009), ривароксабан и апиксабан (2011), эдоксабан (2013). Эти препараты имеют ряд преимуществ по сравнению с АВК, включая отсутствие необходимости мониторирования международного нормализованного отношения (МНО), а также меньший риск межлекарственных взаимодействий [2].
Вместе с тем терапия с применением как АВК, так и ПОАК способна приводить к развитию кровотечений в целом и кровотечений из желудочно-кишечного тракта (ЖКТ) в частности, которые могут угрожать жизни пациента [2]. Данные о величине повышения риска развития желудочно-кишечных кровотечений (ЖКК) на фоне применения антикоагулянтов разнятся в зависимости от дизайна исследований, но в среднем такой риск увеличивается в 1,45–2,8 раза [3, 4]. Так, в метаанализе [4], объединившем 43 клинических исследования и более 154 тыс. пациентов, было установлено, что в течение периода наблюдения (от 3 нед до 31 мес) назначение ПОАК сопровождалось ЖКК в 1,5% случаев, причем 89% из них являлись большими ЖКК, т.е. вели к снижению уровня гемоглобина на 20 г/л и более в течение 24 ч, требовали гемотрансфузии ≥2 единиц эритроцитарной массы, хирургического вмешательства или приводили к смертельному исходу.
ЖКК представляют собой один из наиболее часто встречающийся типов кровотечений у пациентов, получающих ОАК по поводу ФП, и, главное, несут за собой существенное бремя заболеваемости и смертности (5–15%) [5]. Несмотря на то что в последние годы наблюдается некоторое снижение частоты ЖКК (из верхних отделов – до 100 случаев на 100 000 населения, из нижних отделов – до 20 случаев на 100 000 человек), смертность от них остается высокой, находясь на уровне 10–20% [5, 6].
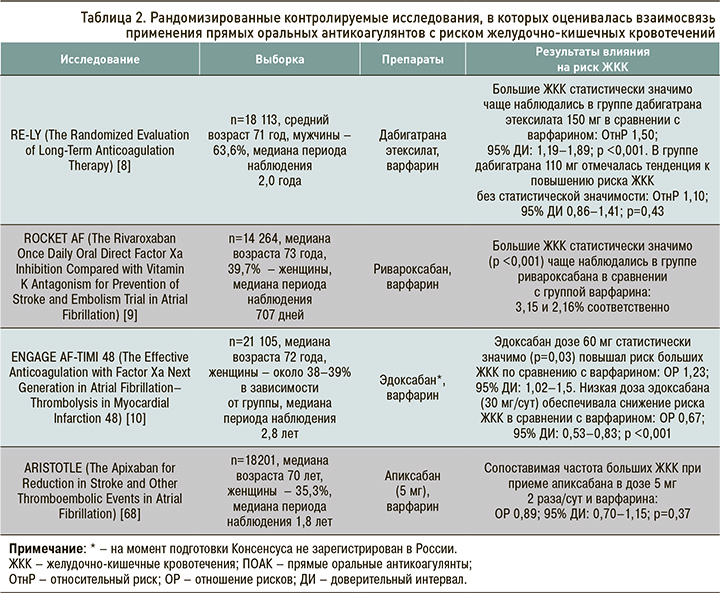
По данным опорных рандомизированных контролируемых исследований (РКИ), ПОАК в сравнении с варфарином демонстрируют различные риски развития ЖКК. В исследовании ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) [7] апиксабан показал сходный с варфарином риск ЖКК. В исследовании RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) [8] прием дабигатрана этексилата в дозе 150 мг 2 раза/сут сопровождался большим риском ЖКК по сравнению с варфарином (относительный риск (ОтнР) 1,50; 95% доверительный интервал (ДИ): 1,19–1,89); в то же время использование дабигатрана этексилата в дозе 110 мг 2 раза/сут ассоциировалось лишь с тенденцией к повышению риска ЖКК, не достигая при этом статистической значимости (ОтнР 1,10; 95% ДИ: 0,86–1,41). В исследовании ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) [9] большие ЖКК статистически значимо (p <0,001) чаще встречались в группе ривароксабана (3,2%), чем в группе варфарина (2,2%). Наконец, в исследовании ENGAGE AF-TIMI (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction Study) [10] было выявлено, что в сравнении с варфарином использование эдоксабана в низкой дозе (30 мг) ассоциируется со снижением на 33% риска больших ЖКК (p <0,001), тогда как применение эдоксабана в дозе 60 мг вело к повышению риска ЖКК на 23% (p=0,03).
Отделы ЖКТ, в которых те или иные ОАК преимущественным образом способны вызывать кровотечения, также разнятся [11]. Так, кровотечения из верхних отделов ЖКТ чаще индуцирует варфарин, тогда как среди пациентов с большими кровотечениями на фоне дабигатрана этексилата у 53% зафиксированы кровотечения из нижних отделов пищеварительной трубки [12]. Среди больных, получающих апиксабан и ривароксабан, чаще встречаются кровотечения из верхнего этажа ЖКТ (для апиксабана в 63% случаев, для ривароксабана – в 76%) [7, 13]. На фоне применения эдоксабана частота ЖКК из верхних и нижних отделов ЖКТ сопоставима [10].
Принимая во внимание вышеописанные факты, экспертами Российского научного медицинского общества терапевтов (РНМОТ), Научного общества гастроэнтерологов России (НОГР) и Национального общества профилактической кардиологии был разработан Консенсус, цель которого – освещение современного состояния вопроса гастроэнтеропротекции у пациентов, получающих ОАК.
ФАКТОРЫ, ПОВЫШАЮЩИЕ РИСК ЖЕЛУДОЧНО-КИШЕЧНЫХ КРОВОТЕЧЕНИЙ, АССОЦИИРОВАННЫХ С ПРИЕМОМ ОРАЛЬНЫХ АНТИКОАГУЛЯНТОВ
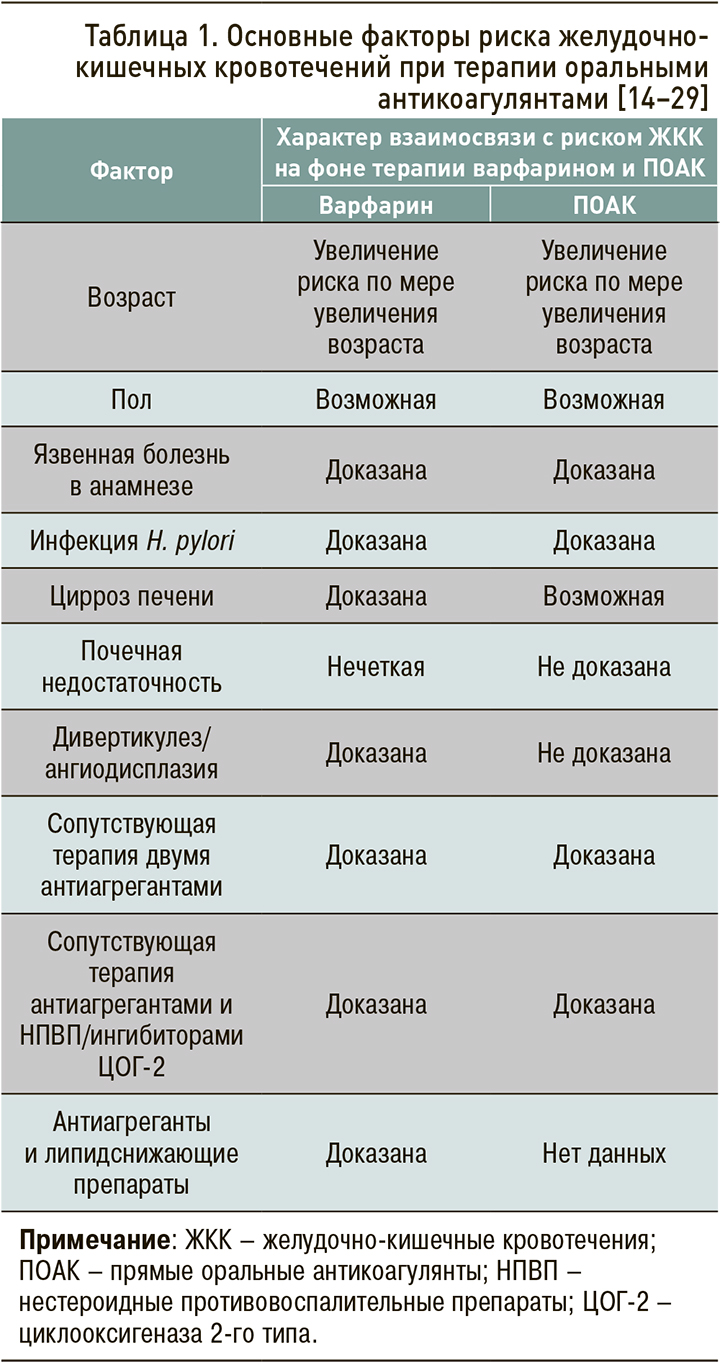
К факторам, повышающим риск ЖКК на фоне приема ОАК (табл. 1), относят возраст 65 лет и старше, нарушение функции печени и почек, низкую массу тела, язвенную болезнь желудка, дивертикулез/ангиодисплазию, сочетанный прием антитромбоцитарных средств, нестероидных противовоспалительных препаратов (НПВП), липидоснижающих препаратов, или препаратов, взаимодействующих с Р-гликопротеином и системой цитохромов Р450 [14–29].
Возраст
Возраст ≥65 лет входил в число важнейших факторов риска кровотечений, ассоциированных с ПОАК, в ряде клинических исследований [7, 29–31]. При этом остается неясным, является ли повышенный риск кровотечений в пожилом возрасте эффектом всего класса антикоагулянтов или только некоторых из них.
К настоящему времени проведен лишь один крупный метаанализ [32], в котором выполнялся сравнительный анализ возрастных подгрупп 65–74 года (пожилой возраст) и ≥75 лет (старческий возраст). В него вошли 25 РКИ и 24 нерандомизированных исследования. Было обнаружено, что у пациентов ≥75 лет дабигатрана этексилат в дозах 110 и 150 мг и ривароксабан ассоциировались с более высоким риском больших кровотечений относительно варфарина. В дополнение к этому на фоне терапии дабигатрана этексилатом в дозе 150 мг риск ЖКК у пациентов старческого возраста, по сравнению с больными пожилого возраста, оказался в 1,5 раза выше (ОтнР 1,51; 95% ДИ: 1,61–1,96). Сходным образом, в отличие от других ПОАК, назначение дабигатрана этексилата 150 мг было взаимосвязано с большим риском развития ЖКК относительно варфарина (отношение рисков, ОР 1,51) [32].
Увеличение риска ЖКК в группе пациентов 75 лет и старше показано также в метаанализе и систематическом обзоре Romanelli R.J. et al. [33]. В этой работе вероятность ЖКК у больных, получающих дабигатран в дозе 150 мг 2 раза/сут, была приблизительно на 50% выше у пациентов в возрасте ≥75 лет, чем у лиц моложе 75 лет, по сравнению с варфарином (β=1,53; 95% ДИ :1,10–2,14; р=0,020). В субанализе [12] упоминавшегося выше исследования RE-LY выявлена взаимосвязь возраста на момент кровотечения и повышенной дозы дабигарана этексилата с риском кровотечений, но эти данные не достигали статистической значимости (p=0,06). Два вышеупомянутых метаанализа [32, 33] доказали значимость возраста как фактора риска ЖКК, в особенности на фоне терапии дабигатрана этексилатом в дозе 150 мг 2 раза/сут.
Безусловно, требуются специально спланированные сравнительные исследования о применении различных ПОАК у лиц пожилого и старческого возраста для анализа эффективности и безопасности такой терапии. Однако на сегодняшний день можно говорить, что среди ОАК наименьшим риском ЖКК характеризуется апиксабан. Так, в ретроспективном исследовании реальной клинической практики сведения базы данных пациентов с неклапанной ФП (средний возраст 74,9–75,8 лет), которым впервые назначался варфарин (n=183 318), дабигатрана этексилат (по 150 мг 2 раза/сут; n=86 198), ривароксабан (по 20 мг 1 раз/сут; n=106 389), апиксабан (по 5 мг 2 раза/сут; n=73 039) [34]. В сравнении с варфарином только апиксабан обеспечивал снижение риска развития больших кровотечений из ЖКТ – на 48% (ОР 0,45–0,60). В то же время, согласно опорным исследованиям, при приеме других ПОАК у пациентов старше 75 лет частота ЖКК в сравнении с АВК увеличивается: при использовании дабигатрана этексилата 110 мг – на 38%, дабигатрана этексилата 150 мг – на 75%, ривароксабана – на 69%, эдоксабана – на 29% [12, 35, 36].
Низкая масса тела
После того как в клиническую практику вошел дабигатрана этексилат, более чем в половине случаев кровотечений они регистрировались у пациентов с массой тела менее 60 кг [37]. Эта особенность не наблюдалась при приеме ривароксабана. Вместе с тем среди больных с низкой массой тела фиксировались более высокие концентрации ривароксабана в плазме крови [38]. Сходным образом среди больных с низкой массой тела (менее 50 кг), принимающих апиксабан, обнаружен повышенный риск кровотечений на фоне более высоких (до 30% выше) концентраций препарата крови по сравнению с пациентами с весом 65–85 кг [39].
Сочетанное применение антиагрегантов, в том числе ацетилсалициловой кислоты и/или нестероидных противовоспалительных препаратов
Коморбидные пациенты с ФП и ишемической болезнью сердца (ИБС), наряду с ОАК, как правило, принимают антиагреганты. Известно, что приблизительно 35% больным с ФП, получающим ОАК, назначается также АСК, причем в 40% (!) случаев нецелесообразно [40]. Одновременное использование АСК и ОАК ведет к повышению риска больших кровотечений [41–43]. Аналогичным образом риск кровотечений возрастает при сочетанном использовании ОАК с НПВП [44]. Следует отметить, что, по данным исследований, риски ЖКК при сочетанном применении с варфарином в целом не различаются среди пациентов, получающих селективные и неселективные НПВП [22–25].
ОАК могут назначаться пациентам с ИБС в дополнение к двойной антитромбоцитарной терапии (ДАТТ) при наличии у них ФП или трепетания предсердий и/или ТЭЛА; в такой ситуации идет речь уже о тройной антитромботической терапии [45, 46], и вероятность кровотечений может увеличиваться более чем в 2 раза [39]. Как следует из данных регистровых [47] и когортных [48–50] исследований пожилых пациентов, риск ЖКК возрастает пропорционально числу используемых ОАК и антиагрегантов; он увеличивается еще больше, когда пожилым пациентам к терапии добавляются дабигатрана этексилат, ривароксабан и эдоксабан [51] или их комбинации с ДАТТ [52].
Повышение риска кровотечений при сочетанном применении ОАК и ДАТТ обнаружено также в исследовании APPRAISE-2 (APixaban for PRevention of Acute ISchemic Events 2) [53]. В этой работе добавление апиксабана в дозе 5 мг 2 раза/ сут к антиагрегантной терапии после острого коронарного синдрома (ОКС) у пациентов высокого риска вело к статистически значимому увеличению вероятности больших кровотечений (ОР 2,59; 95% ДИ: 1,50–4,46; p=0,001), статистически значимо не снижая риск ишемических событий.
Как предполагается, в патогенезе развития ЖКК на фоне приема ПОАК играют роль следующие факторы:
- системное антикоагулятное действие;
- наличие винной кислоты в составе капсулы отдельных прямых ингибиторов тромбина;
- нарушение репарации слизистой оболочки кишечника [54].
ШКАЛЫ ОЦЕНКИ РИСКОВ КРОВОТЕЧЕНИй У ПАЦИЕНТОВ, ПОЛУЧАЮЩИХ ОРАЛЬНЫЕ АНТИКОАГУЛЯНТЫ
Для оценки риска кровотечений у пациентов с ФП, получающих ОАК, используются различные шкалы – HAS-BLED, HEMORR2HAGES, ATRIA, или Qbleed [55, 56]. Наиболее часто применяется шкала HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) [57]. В ней учитывается наличие артериальной гипертонии, нарушения функции печении и/или почек, инсульта, кровотечения в анамнезе или предрасположенности к нему, лабильного МНО, пожилого возраста (≥65 лет), сопутствующее применение препаратов и/или употребление алкоголя. За каждый фактор присваивается 1 балл. Суммарный балл на HAS-BLED ≥3 соответствует высокому риску кровотечений, 1–2 – умеренному, 0 – низкому [53, 54]. Данная шкала прогнозирует риск развития больших кровотечений в течение одного года [53]. Под большим кровотечением в данном случае подразумевается кровотечение любой локализации, потребовавшее госпитализации и/ или гемотрансфузии и/или ассоциирующееся со снижением содержания гемоглобина более чем на 2 г/л. По шкале HAS-BLED оценивается вероятность кровотечения у пациентов, принимающих варфарин (но не ПОАК), при этом она не позволяет прогнозировать риски развития ЖКК [57, 58].
Европейское общество кардиологов (European Society of Cardiology – ESC) одобрило использование шкал HAS-BLED и HEMORR2HAGES в силу их простоты и удобства [59]. Шкалы ATRIA и HEMORR2HAGES имеют сходные параметры с HAS-BLED, однако не учитывают сопутствующий прием антиагрегантов. Также при применении этих шкал необходимо определение некоторых лабораторных и генетических маркеров. Ни одна из упомянутых шкал не была разработана для оценки риска кровотечений иных локализаций, помимо внутричерепных (в том числе для оценки риска кровотечений из ЖКТ); это связано с низкой распространенностью случаев таких кровотечений в популяции [60].
Шкала Qbleed [61] оценивает риск внутримозгового кровоизлияния или ЖКК из верхних отделов, в обоих случаях – до и после начала терапии варфарином. Однако существует более 14 вариантов расчета этого риска, кроме того, данная шкала не позволяет судить о вероятности кровотечений из нижних отделов ЖКТ (наиболее частые ЖКК), что делает ее использование непрактичным. В целом шкалы риска кровотечений имеют скромное ограниченное прогностическое значение в отношении вероятности ЖКК [62], несмотря на широкое их использование в реальной клинической практике.
Высокий риск желудочно-кишечных кровотечений
На сегодняшний день нет единой общепризнанной трактовки понятия «высокий риск ЖКК». Анализируя рекомендации Минздрава России [63, 64], можно сделать вывод, что под таковым понимается наличие у пациента следующих факторов:
- язвенной болезни или ЖКК в анамнезе;
- хронического использования НПВП или кортикостероидов;
- минимум двух из следующих признаков:
- возраст ≥65 лет;
- диспепсия;
- желудочно-пищеводный рефлюкс;
- инфицирование H. pylori;
- хроническое употребление алкоголя.
ОРАЛЬНЫЕ АНТИКОАГУЛЯНТЫ И РИСК ЖЕЛУДОЧНО-КИШЕЧНЫХ КРОВОТЕЧЕНИЙ
Данные клинических исследований
В двух метаанализах регистрационных исследований третьей фазы ПОАК повышали риск больших ЖКК на 23–25% (р=0,04–0,01) относительно варфарина у пациентов с ФП [65, 66]. Следует отметить, что такие результаты были ассоциированы с лечением ривароксбаном, дабигатрана этексилатом и эдоксабаном, в то время как апиксабан статистически значимо риск больших ЖКК по сравнению с варфарином не повышал [66].
В исследовании RE-LY [8] использование дабигатрана этексилата по 150 мг 2 раза/сут у пациентов с ФП (средний возраст 71 год) ассоциировалось с более высоким риском ЖКК по сравнению с применением варфарина (ОтнР 1,50; 95% ДИ: 1,19–1,89). В то же время при терапии дабигатрана этексилатом в дозе 110 мг 2 раза/сут статистически значимых различий в сравнении с варфарином уже не обнаруживалось (ОтнР 1,10; 95% ДИ: 0,86–1,41) [8]. В исследовании RE-LY общее повышение риска ЖКК, наблюдаемое при применении дабигатрана этексилата в дозе 150 мг, происходило за счет когорты «хрупких» пациентов (имеющих синдром старческой астении) [8]. В связи с этим в инструкции по медицинскому применению дабигатрана этексилата рекомендуется назначать препарат в дозе 150 мг 2 раза/сут пациентам с ФП в возрасте <80 лет без повышенного риска кровотечений (HAS-BLED <3 баллов), а также не принимающим одновременно верапамил; если эти условия не выполняются, доза должна быть снижена до 110 мг 2 раза/сут [67].
В дальнейшем был выполнен post hoc-анализ [67] исследования RE-LY, где производилось моделирование вышеописанного дифференциального применения дозы дабигатрана этексилата в соответствии с инструкцией по применению, и такая терапия сравнивалась с хорошо контролируемым лечением варфарином (МНО 2–3, среднее время его нахождения в терапевтическом диапазоне 67,3%). В результате такого моделирования наблюдалась лишь тенденция к повышению риска больших ЖКК в группе дабигатрана этексилата, которая не достигала статистической значимости (ОР 1,23; 95% ДИ: 0,96–1,59) [67].
В исследовании ROCKET-AF пациенты, рандомизированные в группу ривароксабана, имели статистически значимо более высокую частоту ЖКК по сравнению с лицами, получавшими варфарин (3,15 против 2,16%; p <0,001) [9].
Что касается эдоксабана, то в исследовании ENGAGE AF-TIMI 48 [10] при использовании препарата в дозе 60 мг отмечалось статистически значимое (р=0,03) повышение риска больших ЖКК по сравнению с варфарином (ОР 1,23; 95% ДИ: 1,02–1,5). В то же время у пациентов, получавших низкую дозу эдоксабана (30 мг/сут), частота ЖКК была ниже по сравнению с варфарином (ОР 0,67; 95% ДИ: 0,53–0,83; р <0,001).
В метаанализе [4], изучавшем риск ЖКК и клинически значимых кровотечений у пациентов, принимающих ПОАК по различным показаниям (ФП, профилактика тромботических осложнений после ортопедических операций, венозный тромбоз и даже ОКС), ЖКК встречались в 1,5%, из них 89% были «большими» [4]. Риск ЖКК среди пациентов, принимавших ПОАК, по сравнению с прочими стратегиями антитромботической терапии, был выше на 45% (отношение шансов (ОШ) 1,45; 95% ДИ: 1,07–1,97). Среди ПОАК статистически значимо повышали такой риск дабигатрана этексилат (ОШ 1,58; 95% ДИ: 1,29–1,93) и ривароксабан (ОШ 1,48; 95% ДИ: 1,21–1,82) [4].
Единственным представителем класса ПОАК, который, по-видимому, не приводит к увеличению риска ЖКК в большей степени, чем варфарин, является апиксабан [15]. Так, в опорном исследовании ARISTOTLE наблюдалась сопоставимая частота больших ЖКК при приеме апиксабана в дозе 5 мг 2 раза/сут и варфарина (ОР 0,89; 95% ДИ: 0,7–1,15; рис. 1) [68]. Преимущества профиля безопасности апиксабана в отношении ЖКК отмечены в публикации рабочей группы ESC в 2017 г. [69], которая предлагает использовать этот ПОАК у пациентов с ФП, имеющих высокий риск кровотечения из ЖКТ. Следует отметить, что в данной публикации [69] не приводятся критерии высокого риска ЖКК, а эксперты обращают внимание на то, что трактовка этого понятия в известной степени довольно условна. С другой стороны, до настоящего времени не проводились РКИ, в которых различные ПОАК сравнивались бы напрямую.
Еще одним клинически значимым аспектом являются различия в преимущественной локализации ЖКК, индуцируемых тем или иным ПОАК [54, 70]. ЖКК на фоне терапии дабигатрана этексилатом чаще (в 53% случаев) возникают в нижних отделах ЖКТ [12]. Это можно объяснить неполным всасыванием препарата в верхних отделах ЖКТ, ввиду чего повышается его доступность в толстой кишке, где он может индуцировать кровотечение из исходно существующих поражений, в частности эрозий слизистой оболочки и областей ангиодисплазии [54, 70]. Напротив, при приеме ривароксабана кровотечение чаще наблюдается в верхних отделах ЖКТ (в 76% случаев), а вот при использовании апиксабана 10 мг/сут различий в частоте кровотечений из верхних и нижних отделов ЖКТ не отмечается [12]. Отчасти такую закономерность локализации ЖКК можно объяснить тем, что приеме ривароксабана (который применяется 1 раз/ сут) создаются условия для более высокой пиковой концентрации препарата, чем при использовании апиксабана (который применяется 2 раза/сут) [54, 68]. Этим же механизмом, вероятно, объясняется более высокая относительная частота больших ЖКК, наблюдающаяся при приеме ривароксабана по сравнению с дабигатраном, принимаемым, в свою очередь, 2 раза/сут [2, 71].
Данные РКИ, в которых среди прочего анализировался риск развития ЖКК на фоне терапии ПОАК, представлены в таблице 2.
Данные исследований реальной клинической практики
Результаты исследований реальной клинической практики свидетельствуют о повышении частоты ЖКК при приеме отдельных ПОАК в сравнении с варфарином [72]. В ретроспективном исследовании риск ЖКК оценивался в когорте из 92 816 пациентов, принимавших ОАК: 9,2% пациентов получали дабигатран, 17,5% – ривароксабан и 73,2% – варфарин [51]. Данная работа позволила провести прямое сравнение между варфарином и каждым из двух упомянутых ПОАК как среди пациентов с наличием/отсутствием ФП, так и проанализировать локализацию кровотечений. С помощью метода псевдорандомизации было установлено, что риск ЖКК на фоне приема ПОАК был сопоставим с таковым при применении варфарина у пациентов с ФП (дабигатрана этексилат против варфарина – ОР 0,79; 95% ДИ: 0,61–1,03; ривароксабан против варфарина – ОР 0,93; 95% ДИ: 0,69–1,25) и у пациентов без ФП (дабигатрана этексилат против варфарина – ОР 1,14; 95% ДИ: 0,54–2,39; ривароксабан против варфарина – ОР 0,89; 95% ДИ: 0,60–1,32) [51]. Вместе с тем риск кровотечений увеличивался с возрастом. Он превышал таковой риск в группе варфарина у пациентов с ФП ≥76 лет, принимавших дабигатрана этексилат (ОР 2,49; 95% ДИ: 1,61–3,83), а также среди больных этой возрастной категории, получавших ривароксабан (с наличием ФП – ОР 2,91; 95% ДИ: 1,65–4,81; без ФП – ОР 4,58; 95% ДИ: 2,40–8,72) [51].
Недавно был выполнен ретроспективный анализ пациентов с неклапанной ФП, получавших дабигатрана этексилат, ривароксабан или апиксабан [71]. В нем сопоставлялись данные трех групп пациентов в зависимости от получаемой ими терапии: терапия ривароксабаном по сравнению с дабигатраном (n=31 574); лечение апиксабаном по сравнению с дабигатраном (n=13 084); прием апиксабана по сравнению с применением ривароксабана (n=13 130). ЖКК чаще встречались на фоне использования ривароксабана относительно дабигатрана этексилата (ОР 1,20; 95% ДИ: 1,00–1,45). Апиксабан обеспечивал статистически значимо более низкий риск ЖКК, чем дабигатрана этексилат (ОР 0,39; 95% ДИ: 0,27–0,58; p <0,001) и ривароксабан (ОР 0,33; 95% ДИ: 0,22–0,49; p <0,001). Во всех исследуемых группах частота кровотечений увеличивалась среди пациентов старше 75 лет [56]. Апиксабан имел преимущества [71] в снижении риска ЖКК и у пожилых пациентов как по сравнению с дабигатрана этексилатом (ОР 0,45; 95% ДИ: 0,29–0,71), так и ривароксабаном (ОР 0,39; 95% ДИ: 0,25–0,61).
В другом ретроспективном когортном исследовании [73] обнаружено, что частота госпитализаций по поводу кровотечения из верхних отделов ЖКТ среди больных, принимавших ПОАК или варфарин, была ниже, если пациенты получали сопутствующую терапию ингибиторами протонной помпы (ИПП).
На сегодняшний день имеются исследования реальной клинической практики, базирующиеся на сведениях фармаконадзорных баз данных. Так, в исследовании, выполненном в Италии [74], анализировались данные по всем ПОАК, поступившие из системы отчетов о нежелательных лекарственных реакциях Национальной сети фармаконадзора с января по декабрь 2017 г. В результате было установлено, что ЖКК на фоне терапии ПОАК представляют собой наиболее частый тип кровотечений (12–15%) и могут рассматриваться как класс-специфический эффект рассматриваемых препаратов. В то же время принципиально подчеркнуть, что такие кровотечения редко сопровождались развитием анемии, а это указывает на их относительно легкое течение и низкую вероятность развития неблагоприятных исходов.
Данные исследований реальной клинической практики, в которых оценивался в том числе риск развития ЖКК на фоне терапии ПОАК, представлены в таблице 3.

ВОЗМОЖНЫЕ ВЗАИМОДЕЙСТВИЯ С ДРУГИМИ ЛЕКАРСТВЕННЫМИ СРЕДСТВАМИ, БИОЛОГИЧЕСКИМИ АКТИВНЫМИ ДОБАВКАМИ, ПИЩЕВЫМИ ПРОДУКТАМИ
Механизмы развития кровотечений вследствие межлекарственных взаимодействий для того или иного представителя класса ПОАК имеют некоторые отличия (табл. 4). Так, для дабигатрана они преимущественно обусловлены ингибированием P-гликопротеина, так как препарат не метаболизируется изоферментами цитохрома P450 [75].

Ривароксабан также представляет собой субстрат P-гликопротеина, но в отличие от дабигатрана активно (примерно на 2/3) метаболизируется цитохромами печени CYP3A4, CYP3A5, CYP2J2, а также CYP-независимыми путями. Следовательно, увеличение риска кровотечений на фоне приема ривароксабана будет происходить при ингибировании изоферментов цитохрома Р450 печени и P-гликопротеина, например, при сочетанном использовании ритонавира, кетоконазола и прочих лекарственных средств. Исходя из этого не рекомендуется назначать ривароксабан одновременно с азоловыми противогрибковыми препаратами или ингибиторами протеазы вируса иммунодефицита человека. Аналогичное ограничение применимо и к другим ингибиторам Xа-фактора. Следует отметить, что риски кровотечения прямо пропорциональны величине ингибирования цитохрома и/или P-гликопротеина: чем более мощный ингибитор применяется, тем больше концентрация ПОАК в крови и тем выше риск кровотечения.
Необходимо подчеркнуть, что вопрос взаимодействия ПОАК и продуктов питания изучен не до конца, а имеющиеся литературные сведения довольно противоречивы. В большей степени это обусловлено различиями в количественном и качественном составе одного и того же продукта питания, выращенного на различных территориях, а также количеством, в котором его потребляет пациент.
Механизмы взаимодействия ПОАК с пищей аналогичны тем, которые наблюдаются при межлекарственном взаимодействии и связаны с ингибированием Р-гликопротеина, цитохрома Р450 печени, а также с возможными фармакодинамическими взаимодействиями. В частности, в отдельных официальных инструкциях по медицинскому применению ПОАК указано, что «взаимодействия с продуктами питания и молочными продуктами отсутствуют» [75], однако потенциально эти лекарственные средства способны взаимодействовать с продуктами, являющимися ингибиторами ферментов цитохрома P-450 (грейпфрутовый сок, куркумин и др.), ингибиторами P-гликопротеина (апельсиновый сок, зеленый чай, розмарин и др.), или продуктами, обладающими антикоагулянтной и/или антиагрегантной активностью (имбирь, хрен обыкновенный, гинкго и др.; см. табл. 4).
В целом на сегодняшний день еще недостаточно данных для того, чтобы однозначно делать выводы о рисках сочетанного применения ПОАК, биологически активных добавок и употребления отдельных продуктов питания. Тем не менее следует учитывать потенциальные фармакодинамические взаимодействия, способные менять уровень ПОАК в крови и их биодоступность.
СТРАТЕГИИ МИНИМИЗАЦИИ РИСКА ЖЕЛУДОЧНО-КИШЕЧНЫХ КРОВОТЕЧЕНИЙ ИЗ ВЕРХНИХ ОТДЕЛОВ ЖЕЛУДОЧНО-КИШЕЧНОГО ТРАКТА
Первоочередной этап в стратегии минимизации риска ЖКК – определение модифицируемых и немодифицируемых факторов риска кровотечения, которое необходимо проводить до назначения ПОАК. Для снижения риска кровотечений рекомендуется [14]:
- избегать назначения ПОАК без соответствующих показаний;
- регулярно анализировать лист врачебных назначений пациента и оценивать самостоятельно принимаемые больным биологически активные вещества и безрецептурные препараты;
- обращать особое внимание на назначение ПОАК пациентам, которым также требуется антиагрегантная терапия или применение ингибиторов CYP3A4 и/или Р-гликопротеина (в этой ситуации обязательно следует обсудить с пациентов риск ЖКК);
- не использовать для купирования болевого синдрома НПВП.
У больных с сердечно-сосудистыми заболеваниями и язвенной болезнью в анамнезе особое внимание в контексте минимизации риска развития ЖКК необходимо уделять выявлению и эрадикации Helicobacter pylori (H. pylori), причем по возможности это следует делать до инициации антитромботической терапии [14, 78, 79]. Доказано, что у пациентов с ФП, принимающих дабигатрана этексилат, предшествующая H. pylori-инфекция ассоциируется с 4-кратным повышением риска ЖКК (ОР 4,75; 95% ДИ: 1,93–11,68) [27].
У пациентов с ФП, получающих ПОАК и нуждающихся в длительном приеме АСК или ДАТТ и у которых в анамнезе имеются верифицированные ЖКК, следует добавлять к терапии ИПП для снижения риска повторного ЖКК из верхних отделов ЖКТ [80–83]. Есть данные, что ИПП способны эффективно предотвращать повторные ЖКК из верхних отделов ЖКТ на фоне терапии АСК, даже при неполной эрадикации H. pylori и одновременном приеме прочих НПВП (не АСК) [78]. В исследовании Chan E.W. et al. [84] у пациентов с язвенной болезнью в анамнезе, получающих дабигатрана этексилат, на фоне назначения ИПП было обнаружено уменьшение риска кровотечений из верхних отделов ЖКТ приблизительно на 50% (коэффициент заболеваемости 0,53; 95% ДИ 0,35 – 0,77).
В 2018 г. вышло в свет центральное исследование по рискам ЖКК на фоне применения ОАК [73]. До этой публикации оставалось неясным, обеспечивает ли прием ИПП снижение риска кровотечений из верхних отделов ЖКТ при их комбинации со всеми ОАК или только с отдельными препаратами (варфарином, дабигатрана этексилатом). В работу вошли пациенты в возрасте ≥30 лет, получавшие апиксабан, дабигатрана этексилат, ривароксабан, варфарин (из исследования исключали пациентов, которые получали различные ОАК в течение периода наблюдения) в период с 01.01.2011 по 30.09.2015. Данные по эдоксабану не анализировались, поскольку терапия этим препаратом была инициирована у очень небольшого числа пациентов. Всего в работу вошли 1 643 123 пациента. Изучаемая когорта включала 754 389 человеко-лет лечения ОАК без сопутствующей терапии ИПП (апиксабан – 43 970, дабигатрана этексилат – 79 739, ривароксабан – 114 168, варфарин – 516 512 человеко-лет) и 264 447 человеко-лет – с сопутствующим приемом ИПП (апиксабан – 14 989, дабигатрана этексилат – 26 572, ривароксабан – 38 958, варфарин – 183 929 человеко-лет). Больные всех подгрупп в подавляющем большинстве случаев были в возрасте ≥65 лет (от 87,3 до 98,1%), количество пациентов в возрасте ≥85 лет варьировало от 18,2 до 23,0%. У больных, получавших ИПП, чаще встречались факторы риска ЖКК. В то же время независимо от наличия/отсутствия сопутствующего назначения ИПП у лиц, получавших апиксабан, исходно был самый высокий риск развития ЖКК, а у пациентов, принимавших дабигатрана этексилат, – самый низкий. У пациентов, получающих лечение ОАК без сопутствующего назначения ИПП, частота госпитализаций в связи с кровотечениями из верхних отделов ЖКК составила 115 на 10 000 человеко-лет (95% ДИ: 112–118). Частота госпитализаций в связи с ЖКК на фоне терапии ривароксабаном (144 на 10 000 человеко-лет; 95% ДИ: 136–152) была статистически значимо выше, чем на фоне терапии апиксабаном (73 на 10 000 человеко-лет; коэффициент заболеваемости, 1,97; 95% ДИ: 1,73–2,25), дабигатрана этексилатом (120 на 10 000 человеко-лет; коэффициент заболеваемости 1,19; 95% ДИ: 1,08–1,32) и варфарином (113 на 10 000 человеко-лет; коэффициент заболеваемости 1,27; 95% ДИ: 1,19–1,35). Частота госпитализаций вследствие кровотечения из верхних отделов ЖКК на фоне лечения апиксабаном была статистически значимо ниже, чем при терапии дабигатрана этексилатом (коэффициент заболеваемости 0,61; 95% ДИ: 0,52–0,70) и варфарином (коэффициент заболеваемости 0,64; 95% ДИ: 0,57–0,73). У пациентов, получающих сопутствующую терапию ИПП, частота госпитализаций в связи с кровотечением из верхних отделов ЖКТ (76 случаев на 10 000 человеко-лет) была статистически значимо ниже, чем у больных, не получавших лечение ИПП (коэффициент заболеваемости 0,66; 95% ДИ: 0,62–0,69). Вероятность госпитализации по поводу ЖКК верхних отделов ЖКТ была выше в подгруппах пациентов с высоким риском его развития. В соответствии с данными рассматриваемого исследования [73] это были пациенты пожилого возраста, чаще проживавшие в домах долговременного ухода; они чаще страдали синдромом старческой астении, начали антикоагулянтную терапию в недавнем времени (в течение предшествующих 90 дней), имели в анамнезе ранее перенесенное ЖКК из верхних отделов ЖКТ, чаще принимали другие лекарственные средства, способные повышать риск кровотечений (НПВП, антитромбоцитарные препараты, иные антикоагулянты, системные кортикостероиды, селективные ингибиторы обратного захвата серотонина, антибиотики), имели коморбидные сердечно-сосудистые заболевания и показания для назначения АСК, в течение предшествовавшего года чаще обращались за медицинской помощью в связи с наличием симптомов со стороны ЖКТ. Исходя из результатов этого масштабного исследования, авторы заключили, что у пациентов, начинающих лечение ОАК, частота госпитализаций по поводу кровотечений из верхних отделов ЖКТ была самой высокой в случае назначения ривароксабана, а самой низкой – апиксабана. Для любого из изучаемых антикоагулянтов частота госпитализаций по поводу кровотечений из верхних отделов ЖКТ была ниже в той подгруппе пациентов, которым назначалась сопутствующая терапия ИПП. Эти выводы очень важны и для выбора конкретного антикоагулянта, и для решения вопроса о назначении ИПП.
Несмотря на то что к настоящему времени отсутствуют однозначные данные о нежелательных межлекарственных взаимодействиях ИПП с ПОАК, потенциальные риски таковых на фармакокинетическом уровне все-таки существуют [77]. Наиболее высока вероятность взаимодействия между ИПП и дабигатрана этексилатом. При этом ингибиторы Xа фактора характеризуются меньшим риском возможных межлекарственных взаимодействий (табл. 5) [77].

СТРАТЕГИИ МИНИМИЗАЦИИ РИСКА ЖЕЛУДОЧНО-КИШЕЧНЫХ КРОВОТЕЧЕНИЙ ИЗ ВЕРХНИХ И НИЖНИХ ОТДЕЛОВ ЖЕЛУДОЧНО-КИШЕЧНОГО ТРАКТА
Как известно [86], ИПП оказывают свое действие лишь на уровне верхних отделов ЖКТ, в связи с чем представляется актуальным вопрос защиты нижних отделов ЖКТ на фоне антитромботической терапии. Следует отметить, что в настоящее время на российском рынке доступно лекарственное средство ребамипид (препарат Ребагит, «ПРО.МЕД.ЦС Прага а.о.», Чехия), обладающее комплексными протективными эффектами на всем протяжении ЖКТ. Его уникальной особенностью является способность восстанавливать целостность плотных контактов в эпителии слизистой оболочки (СО), благодаря чему он поддерживает нормальную барьерную функцию стенки кишечника [86].
Ребамипид представляет собой оптически активную α-аминокислоту, производное 2(1Н)-хинолинона. Препарат был впервые одобрен для клинического применения в 1990 г. в Японии, в связи с чем к настоящему времени имеет длительный опыт использования в реальной практике, равно как и широкую доказательную базу своей эффективности и безопасности [87]. Ребамипид обладает широким спектром плейотропных влияний на уровне ЖКТ, среди которых индукция синтеза простагландинов и собственно слизи (муцинов) в СО ЖКТ, элиминация гидроксильных радикалов, подавление активации нейтрофилов, угнетение процессов воспаления в СО, регуляция активности апоптоз-ассоциированных генов, ингибирование нитрования тирозина. Все это способствует снижению выраженности оксидативного стресса и токсического действия на СО [87]. Особого внимания заслуживает тот факт, что ребамипид реализует свои эффекты на всех трех структурных уровнях СО на всем протяжении ЖКТ: на преэпителиальном уровне, стимулируя образование слизи; на уровне эпителия, восстанавливая плотные контакты и ускоряя регенерацию клеточных элементов; на субэпителиальном уровне, улучшая микроциркуляцию с собственной пластинке СО [87]. Клинически значимой особенностью ребамипида являются механизмы его элиминации из организма: лишь около 10% лекарственного средства выводится почками, остальная часть – с кишечным содержимым. Это, безусловно, служит преимуществом при назначении препарата пациентам с поражением почек [87].
Возможности ребамипида в цитопротекции ЖКТ на фоне терапии ПОАК изучались в открытом многоцентровом проспективном рандомизированном сравнительном исследовании в параллельных группах [88] с участием 309 пациентов с неклапанной ФП, которым впервые в жизни назначался дабигатрана этексилат. Нежелательные явления (НЯ) со стороны ЖКТ анализировались с помощью опросника Global Overall Severity (GOS), в нем использовалась шкала из 7 баллов. Больных с GOS ≥3 баллов рандомизировали на три группы терапии: получавших ИПП, блокаторы Н2-антигистаминных рецепторов или ребамипид на протяжении 4 нед. Симптомы диспепсии были выявлены в 17,2% случаев, причем 77% НЯ были зарегистрированы в первые 10 дней от старта терапии. Вследствие НЯ прием дабигатрана прекратили 5 пациентов. Через 4 нед лечения средний балл по GOS составил 3,5±1,7, при этом доля пациентов с GOS≥3 баллов снизилась со 100 до 11,3%. Многофакторный регрессионный анализ не обнаружил ни одного фактора, значимо влияющего на частоту или тяжесть симптомов диспепсии. Большинство пациентов (83–100%) отметило уменьшение выраженности симптомов на фоне лечения (GOS ≤2 баллов), при этом ИПП, блокаторы Н2-рецепторов гистамина и ребамипид были одинаково эффективны для купирования симптомов диспепсии, связанной с приемом дабигатрана.
Как уже говорилось выше, сочетанное применение с ОАК антиагрегантов и НПВП (в том числе АСК) существенно повышает риск ЖКК. Это справедливо как для терапии варфарином (риск кровотечений в этой ситуации возрастает почти в 5–9 раз) [2], так и ПОАК (риски ЖКК увеличиваются почти в 1,8–2,6 раз) [31, 71, 89].
Следует отметить, что ребамипид доказал свою эффективность в отношении профилактики и регресса повреждений слизистой оболочки ЖКТ, индуцированных приемом АСК. Так, в многоцентровом рандомизированном двойном слепом плацебо-контролируемом исследовании [90] анализировались эффекты ребамипида с позиций динамики поражения СО тонкого кишечника у больных, получающих АСК в дозе 100 мг/сут с целью профилактики сердечно-сосудистых событий на протяжении не менее чем 3 мес и имеющих как минимум 3 участка поражения СО в виде эрозий или язв, подтвержденных при видеокапсульной эндоскопии. Заметим, что ребамипид в данном исследовании назначался в дозе, в три раза превышающей стандартную терапевтическую: 300 мг 3 раза/сут. Период наблюдения составлял 8 нед. В конце этого периода на фоне терапии ребамипидом наблюдалось статистически значимое снижение количества очагов повреждения СО тонкого кишечника – в среднем с 4,0 до 2,0 (р=0,046). При этом в группе плацебо статистически значимых изменений данного показателя обнаружено не было (р=0,08). Ребамипид приводил к уменьшению выраженности поражения СО, оценивавшейся по индексу Lewis, который базируется на визуализируемых эндоскопических признаках (р=0,02). Кроме того, на фоне приема ребамипида полной ремиссии поражения СО достигли 32% пациентов, тогда как в группе же плацебо только 1%. Добавим, что используемая тройная терапевтическая доза ребамипида отлично переносилась пациентами: нежелательных лекарственных реакций зарегистрировано не было.
ЗАКЛЮЧЕНИЕ
Таким образом, терапия ОАК, несмотря на исключительные преимущества в виде снижения риска тромбоэмболических осложнений, может ассоциироваться с повышением риска ЖКК, в том числе угрожающих жизни. Дополнительные факторы, повышающие риск кровотечений и часто встречающиеся в современной клинической практике, включают сочетанный прием антитромбоцитарных препаратов и/или НПВП. При инициации терапии ОАК, в том числе при назначении ПОАК, необходимо оценивать факторы риска ЖКК, и при их наличии оптимизировать профиль безопасности антитромботической терапии с точки зрения защиты ЖКТ. Согласно данным РКИ и исследований реальной клинической практики, рациональными и доказавшими свою эффективность стратегиями являются использование ИПП, ребамипида, а также приоритетное назначение апиксабана в соответствии с показаниями к его применению в качестве ОАК первой линии у пациентов, нуждающихся в антикоагулянтной терапии.
ПРАКТИЧЕСКИЕ РЕКОМЕНДАЦИИ
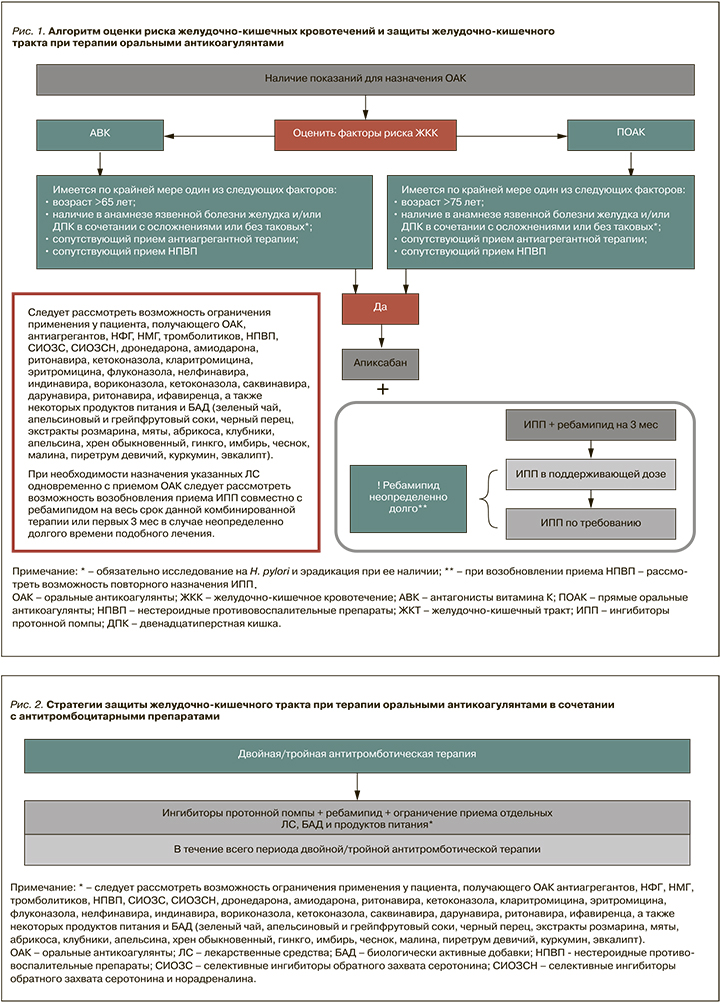
1. С точки зрения минимизации риска кровотечений приоритетным ОАК является апиксабан. При наличии у пациентов соответствующих показаний этот ПОАК должен быть препаратом первого выбора, поскольку он обладает наилучшим профилем безопасности в отношении риска ЖКК.
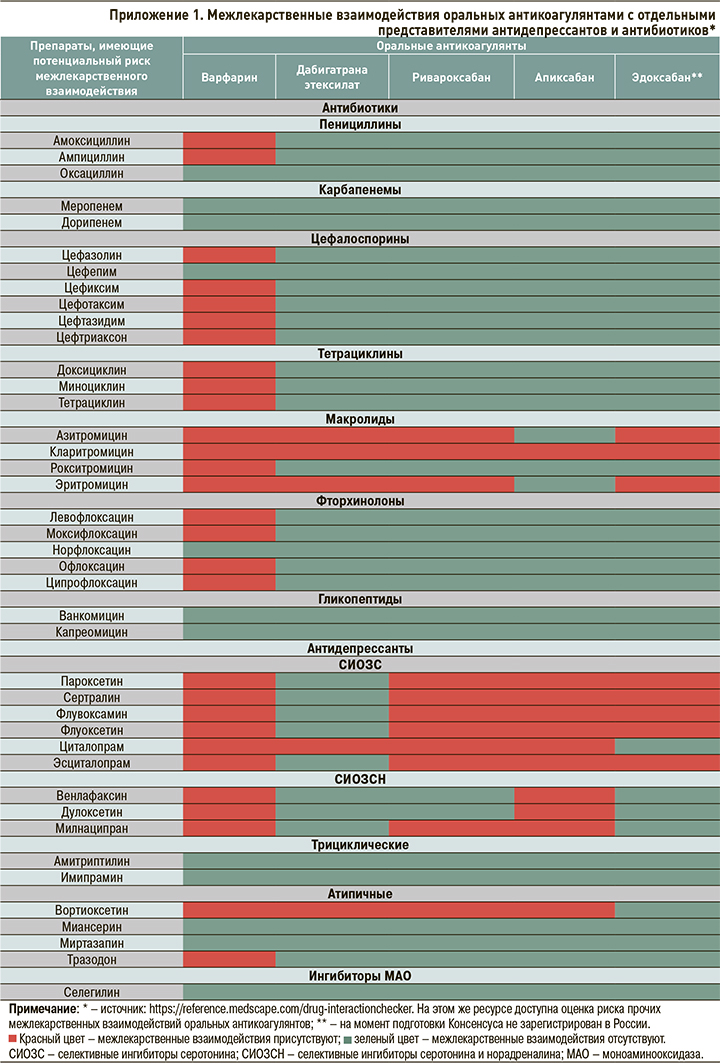
2. При наличии у пациента, получающего ОАК, факторов риска ЖКК для защиты ЖКТ рекомендуется применять ИПП в сочетании с ребамипидом как минимум в течение 3 мес (рис. 1). В последующем необходимо рассмотреть возможность перехода на прием ИПП в поддерживающей дозе и далее по требованию. При этом ребамипид должен применяться на протяжении всего периода терапии ОАК, т.е. в реальной клинической практике, как правило, неопределенно долго. В ситуации, если пациенту требуется назначение антиагреганта, НПВП, системных кортикостероидов, некоторых антидепрессантов и антибиотиков (Приложение 1), следует рассмотреть возможность повторного назначения ИПП в дополнение к ребамипиду, при этом продолжительность повторного применения ИПП должна совпадать с продолжительностью терапии вышеперечисленными лекарственными средствами.
3. Ввиду потенциального повышения риска ЖКК, в том числе в силу возможного взаимодействия с ОАК фармакокинетического и фармакодинамического типов, следует рассмотреть возможность ограничения применения у пациента, получающего ОАК, отдельных ЛС и групп ЛС (антиагрегантов, нефракционированного гепарина, низкомолекулярных гепаринов, тромболитических препаратов, НПВП, СИОЗС, СИОЗCН, хинидина, дронедарона, амиодарона (кроме апиксабана), ритонавира, кетоконазола, кларитромицина, эритромицина, флуконазола, нелфинавира, индинавира, вориконазола, кетоконазола, саквинавира, дарунавира, ритонавира, ифавиренца), а также некоторых продуктов питания и БАД, (зеленого чая; экстракта розмарина; апельсинового сока; черного перца; экстрактов мяты, абрикоса, клубники, апельсина, хрена обыкновенного, гинкго, имбиря, чеснока, малины, пиретрума девичьего, куркумина, грейпфрутового сока, эвкалипта) (рис. 2).
4. В случае необходимости назначения перечисленных в пункте 3 лекарственных средств одновременно с ОАК следует рассмотреть возможность возобновления приема ИПП совместно с ребамипидом на весь срок данной комбинированной терапии или первых 3 мес в случае неопределенно долгого времени такого лечения.
5. Наличие H. pylori-инфекции у пациентов, получающих ОАК и АСК, ассоциируется с повышением риска ЖКК, что указывает на необходимость тестирования лиц данной категории на H. pylori и эрадикации этой инфекции при ее выявлении по возможности до инициации антитромботической терапии.