СОСТАВ РАБОЧЕЙ ГРУППЫ:
Председатель: Л.Б. Лазебник, вице-президент РНМОТ, Президент НОГР, член президиума Общества врачей России, член президиума Национальной медицинской палаты, д.м.н., профессор, профессор кафедры поликлинической терапии ФГБОУ ВО «Московский государственный медицинский стоматологический университет им. А.И. Евдокимова» Минздрава России
Секретарь: Е.В. Голованова, д.м.н., профессор кафедры поликлинической терапии лечебного факультета ФГБОУ ВО «Московский государственный медицинский стоматологический университет им. А.И. Евдокимова» Минздрава России
ЧЛЕНЫ РАБОЧЕЙ ГРУППЫ:
С.А. Алексеенко, д.м.н., профессор, зав. кафедрой госпитальной терапии ФГБОУ ВО «Дальневосточный государственный медицинский университет» Минздрава России
О.Л. Арямкина, д.м.н., профессор, зав. кафедрой внутренних болезней БУВО Ханты-Мансийского автономного округа – Югры «Сургутский государственный университет»
И.Г. Бакулин, д.м.н., профессор, зав. кафедрой пропедевтики внутренних болезней, гастроэнтерологии и диетологии им. С.М. Рысса ФГБОУ ВО «Северо-Западный государственный медицинский университет им. И.И. Сеченова» Минздрава России
Н.В. Бакулина, д.м.н., профессор, зав. кафедрой внутренних болезней, клинической фармакологии и нефрологии ФГБОУ ВО «Северо-Западный государственный медицинский университет им. И.И. Мечникова» Минздрава России
А.Ю. Барановский, д.м.н., профессор, главный гастроэнтеролог СЗФО России, руководитель научно-клинического и образовательного центра гастроэнтерологии и гепатологии Санкт-Петербургского государственного университета, председатель общества гастроэнтерологов, гепатологов и диетологов Санкт-Петербурга, медицинский директор ММЦ «Юнион Клиник»
О.А. Бондаренко, врач – клинический фармаколог БУ «Сургутская окружная клиническая больница»
А.Н. Варганова, врач – клинический фармаколог БУ «Сургутская окружная клиническая больница»
Т.В. Волкова, врач – клинический фармаколог БУ «Сургутская окружная клиническая больница»
Л.Г. Вологжанина, к.м.н., доцент кафедры факультетской терапии № 2 с курсом курортологии и немедикаментозной терапии ФГБОУ ВО «Пермский государственный медицинский университет им. акад. Е.А. Вагнера» Минздрава России
И.А. Волчегорский, д.м.н., профессор, зав. кафедрой фармакологии ФГБОУ ВО «Южно-Уральский государственный медицинский университет» Минздрава России
Т.П. Демичева, к.м.н., доцент кафедры эндокринологии и клинической фармакологии ФГБОУ ВО «Пермский государственный медицинский университет им. акад. Е.А. Вагнера» Минздрава России
А.И. Долгушина, д.м.н., зав. кафедрой госпитальной терапии и диетологии ФГБОУ ВО «Южно-Уральский государственный медицинский университет» Минздрава России
И.В. Маев, д.м.н., профессор, академик РАН, зав. кафедрой пропедевтики внутренних болезней и гастроэнтерологии ФГБОУ ВО «Московский государственный медицинский стоматологический университет им. А.И. Евдокимова» Минздрава России
О.Н. Минушкин, д.м.н., профессор, зав. кафедрой терапии и гастроэнтерологии Центральной государственной медицинской академии медицинского центра Управления делами Президента РФ
К.Л. Райхельсон, д.м.н., профессор научно-клинического и образовательного центра гастроэнтерологии и гепатологии Санкт-Петербургского государственного университета
Е.Н. Смирнова, д.м.н., профессор, зав. кафедрой эндокринологии и клинической фармакологии ФГБОУ ВО «Пермский государственный медицинский университет им. акад. Е.А. Вагнера» Минздрава России
Л.В. Тарасова, д.м.н., профессор кафедры внутренних болезней БУВО Ханты-Мансийского автономного округа – Югры «Сургутский государственный университет»
О.В. Хлынова, д.м.н., профессор, член-корр. РАН, зав. кафедрой госпитальной терапии ФГБОУ ВО «Пермский государственный медицинский университет им. акад. Е.А. Вагнера» Минздрава России
Ю.В. Цыганова, к.м.н., ассистент кафедры факультетской и госпитальной терапии ФГБОУ ВО «Чувашский государственный университет им. И.Н. Ульянова» Минздрава России
СПИСОК СОКРАЩЕНИЙ
- АИГ – аутоиммунный гепатит
- АЛТ – аланинаминотрансфераза
- АСТ – аспарагинаминотрансфераза
- БАД – биологически активные добавки
- ВГН – верхняя граница нормы
- ГКС – глюкокортикостероиды
- ЛПП – лекарственное поражение печени
- НПВП – нестероидные противовоспалительные препараты
- NAC – N-ацетилцистеин
- ПХТ – полихимиотерапия
- ФДД – фитопрепараты и/или диетические добавки
- FDA – агентство США по санитарному надзору за качеством медикаментов
- ХЗП – хронические заболевания печени
- ЩФ – щелочная фосфатаза
ТЕРМИНЫ И ОПРЕДЕЛЕНИЯ
Острое лекарственное поражение печени (ЛПП) – изменение уровня аланинаминотрансферазы (АЛТ) и щелочной фосфатазы (ЩФ), развившееся в течение менее 3 мес от начала приема лекарственного препарата.
Хроническое ЛПП – стойкое повреждение печени, сохраняющееся более чем через 1 год после начала приема лекарственного препарата.
Персистирующее ЛПП – сохранение изменений в показателях состояния печени более 3 мес при гепатоцеллюлярном и более 6 мес при холестатическом ЛПП.
Идиосинкразическое ЛПП – гепатотоксичность проявляется лишь у отдельных восприимчивых лиц, реакция имеет менее выраженную зависимость от дозировки и в большей степени различается по длительности латентного периода, манифестации и течению.
Латентный период – время от начала приема лекарственного средства либо фитопрепарата и/или диетической добавки (ФДД) до развития ЛПП.
Период вымывания, разрешение либо проба с отменой препарата – время от развития ЛПП до возврата уровня ферментов и/или билирубина к исходным цифрам.
Проба с повторным назначением препарата – повторное назначение лекарственного средства либо ФДД пациенту, у которого уже развивалось ЛПП в ответ на данное средство.
Закон Хая: суть – эмпирическая закономерность, согласно которой у пациента есть высокий риск летального ЛПП, если лекарственный препарат вызывает печеночно-клеточную, а не холестатическую желтуху. Закон Хая состоит из трех частей:
a) трех- или более кратное превышение верхней границы нормы (ВГН) АЛТ или аспарагинаминотрансферазы (АСТ);
b) более чем в 2 раза превышен верхний предел нормы общего билирубина сыворотки, без застоя желчи (определяется как менее чем двукратное превышение ВГН ЩФ);
c) отсутствие других причин подобной комбинации повышенных аминотрансфераз и общего билирубина сыворотки: вирусного гепатита, алкоголизма, ишемии, ранее выявленного заболевания печени или иного лекарства, способного вызвать наблюдаемое поражение.
Значение R: АЛТ/ВГН или ЩФ/ВГН. Применяется для определения типа гепатотоксического повреждения: гепатоцеллюлярный (R >5), смешанный (R=2–5) и холестатический (R <2).
RUCAM: метод оценки достоверности причинно-следственной связи при ЛПП компании Roussel Uclaf, в котором используется система оценки, учитывающая клинические данные, имеющиеся литературные данные по гепатотоксичности подозреваемого лекарственного средства и пробу с повторным назначением препарата.
1. КРАТКАЯ ИНФОРМАЦИЯ
1.1. Определение
ЛПП относится к повреждению печени, вызванному всеми типами отпускаемых по рецепту или без рецепта лекарств (включая небольшие химические молекулы, биологические агенты, фитопрепараты, диетические и биологические добавки к пище), развившееся в период в среднем от 5 до 90 дней от начала приема [1, 2]. В англоязычной литературе для обозначения таких заболеваний используется термин drug-induced liver injury, в русскоязычной литературе – «лекарственные поражения печени».
1.2. Этиология и патогенез
Наиболее хорошо изученные вещества, вызывающие ЛПП, представлены в таблице 1.
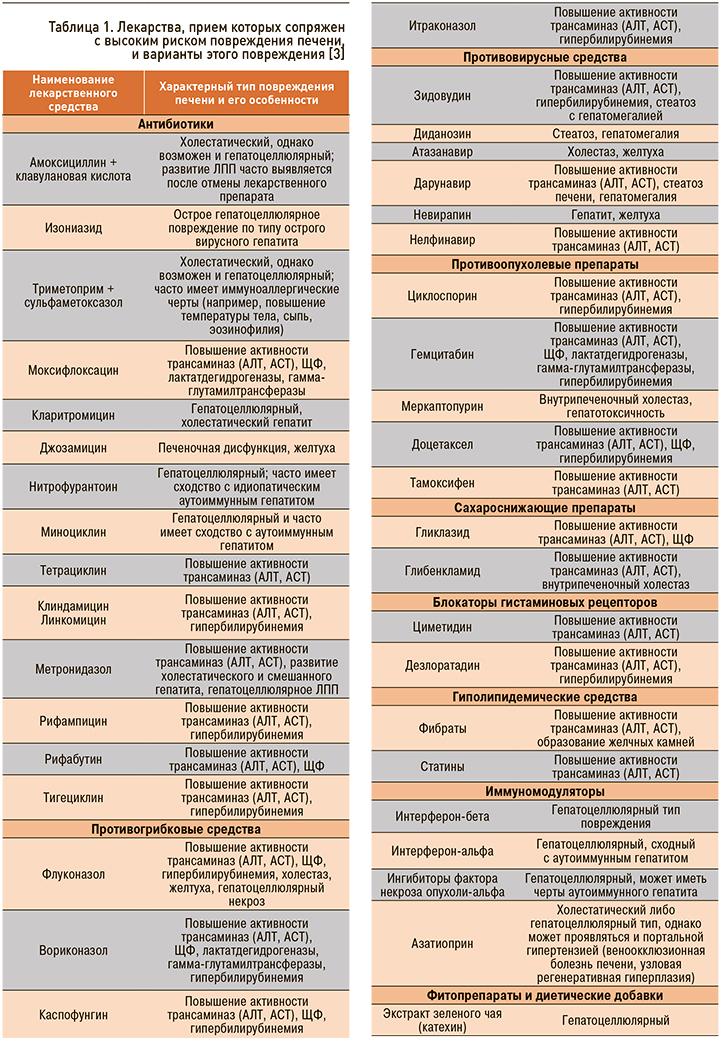
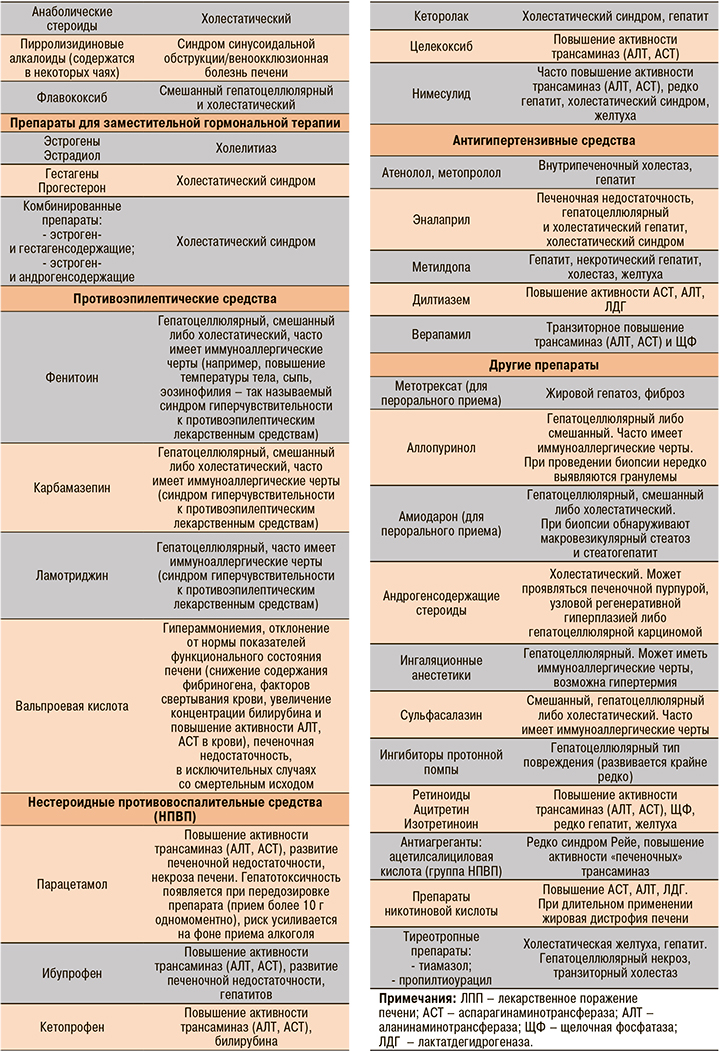
На долю антибиотиков и противосудорожных препаратов приходится более 60% всех случаев ЛПП [3]. Среди нестероидных противовоспалительных препаратов (НПВП) в этом плане лидируют диклофенак и нимесулид, в группе антибиотиков – амоксициллин + клавулановая кислота [4].
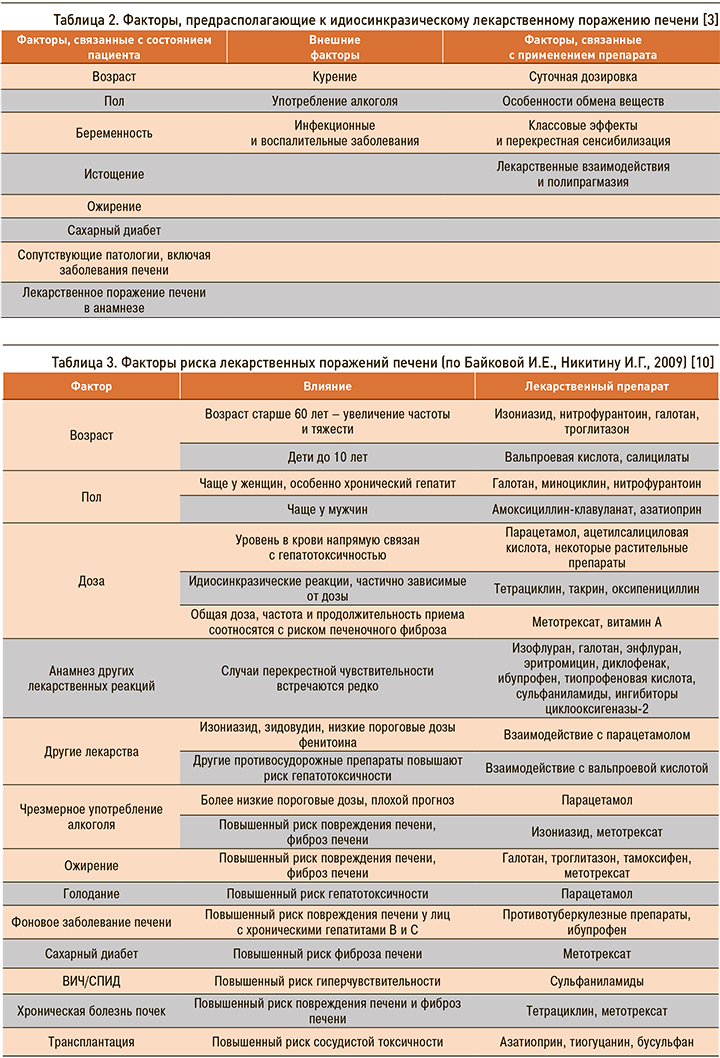
Отдельно необходимо учитывать и факторы, которые могут вызывать предрасположенность к развитию идиосинкразического ЛПП (табл. 2).
1.2.1. Патогенез лекарственного поражения печени
В связи с большим разнообразием механизмов биотрансформации лекарственных средств патогенез развития ЛПП различен:
a) прямые гепатотоксические эффекты, обусловленные нарушением реакций окисления и гидроксилирования с образованием активных промежуточных метаболитов. Этот процесс происходит в результате самых различных изменений ферментов семейства цитохромов Р450 (CYP), в том числе генетически детерминированных. В настоящее время индексировано более 1000 изоформ Р450; их номенклатура представлена на сайте htpp://www. cepalleles.ki.se [5];
b) нарушение конъюгации метаболитов с глутатионом, сульфатом и глюкуронидом, ассоциированное с блокировкой образования нетоксичных гидрофильных соединений и выведения их в кровь и желчь. На каждом из указанных этапов биотрансформации лекарственных средств возможно подключение субклеточных механизмов воспаления с активацией каспаз, фрагментацией ДНК, повреждением внутренних структур митохондрий и лизосом. В последнем случае развиваются лекарственно-индуцированные липидозы и стеатозы. Этапы образования токсических метаболитов и биотрансформации лекарственных средств в гепатоците включают прямое токсическое действие, повреждение мембраны клетки за счет нарушения сборки актинофибрилл с последующим ее лизисом, нарушение функции транспортеров солей желчных кислот (в частности, белка мультилекарственной резистентности) с последующим нарушением экскреции метаболитов ЛС с желчью, активацию иммунной системы с инициированием иммуновоспалительных реакций. Итогом этих нарушений биотрансформации выступает апоптоз клетки [6–9];
c) блокада ферментов дыхательной цепи, приводящая к снижению продукции АТФ, изменению метаболизма жирных кислот и инициированию различных вариантов стеатоза.
1.2.2. Факторы риска развития лекарственных поражений печени
В отношении некоторых лекарственных препаратов имеются исследования, показавшие наличие факторов риска, которые многократно усиливают риск повреждения печени. Наиболее значимые факторы риска ЛПП, согласно исследованиям Байковой И.Е. и Никитина И.Г. (2009), приведены в таблице 3.
1.3. Эпидемиология
Истинная распространенность лекарственных поражений печени остается и, по-видимому, останется неизвестной, однако можно констатировать, что в клинической практике данный диагноз формулируется неоправданно редко. Это обусловлено несколькими факторами, среди которых наиболее важное значение имеют:
- нежелание пациента сообщать о приеме некоторых препаратов (антидепрессантов, нейролептиков, средств для повышения потенции и др.);
- нежелание врачей документировать ятрогенные заболевания;
- неверная трактовка весьма разнообразной симптоматики [11].
Поражения печени, возникающие вследствие применения лекарственных средств, остаются актуальной проблемой медицины и выступают основной причиной острой печеночной недостаточности в США и Западной Европе [12]. Во всем мире отмечается рост ЛПП вследствие приема растительных средств и пищевых биологически активных добавок (БАД). Ежегодно в США выполняется около 2 тыс. трансплантаций печени из-за развития ЛПП. Частота ЛПП составляет 1–19 случаев на 100 000 населения в год, по другим данным, 3–6% от всех случаев применения лекарственных средств [3, 13]. Во всем мире в структуре больных, госпитализируемых с патологией печени, 2–5% составляют пациенты с лекарственной желтухой (холестазом), 10% – с лекарственным гепатитом [2].
Анализ базы данных Всемирной организации здравоохранения (ВОЗ), регистрирующей побочные реакции лекарственных средств с 1968 г. (http://www.who-umc.org), позволил выявить существенный рост количества ЛПП, начиная с 1990- х гг. [1, 14]. Среди них наиболее частыми причинами летальных исходов у пациентов с ЛПП были прием парацетамола, средств, применяемых в лечении ВИЧ-инфекции, троглитазона, антиконвульсантов (вальпроата), анальгетиков, антибиотиков и противоопухолевых средств.
Эти данные согласуются с результатами исследований, проведенных в странах Европы. Так, в Испании за последнее десятилетие наиболее частые причины зарегистрированных случаев ЛПП (n=461) были связаны с приемом амоксициллина + клавулановой кислоты, бентазепама, аторвастатина и каптоприла. Согласно другим исследованиям, ЛПП возникали в результате применения парацетамола, антиретровирусной терапии, антибиотиков, гиполипидемических средств и антиконвульсантов.
Особого внимания заслуживает существенное возрастание за последнее десятилетие (преимущественно в странах Азии) количества случаев ЛПП в результате приема средств для похудения и препаратов, применяющихся в нетрадиционной медицине.
Помимо базы данных ВОЗ, регистрация случаев ЛПП проводится во многих странах мира. Так, в США с 2003 г. учет токсичности лекарственных средств осуществляет Food and Drug Administration (FDA), случаи ЛПП регистрируются в специально созданной базе данных (Drug Induced Liver Injury Network).
Аналогичная база данных существует в Испании и других странах Европы. В России за последние годы также проводится регистрация случаев ЛПП, главным образом врачами, клиническими фармакологами на сайте www.regmed.ru [2].
В 2012 г. в США впервые был создан веб-сайт LiverTox (http://www.livertox.nih.gov) [15], а в 2014 г. Американский колледж гастроэнтерологии (ACG) опубликовал первое клиническое руководство, нацеленное на диагностику и ведение больных с ЛПП [3]. Сегодня на сайте LiverTox существует информация о более 700 препаратов с гепатотоксическими реакциями, причем наиболее частой причиной развития ЛПП в Америке является применение таких препаратов, как антибактериальные лекарственные средства, фитопрепараты и диетические добавки.
В 2014 г. Китай также создал веб-сайт HepaTox (http://www.hepatox.org), где представлено уже более 400 видов обычных лекарств, способных приводить к ЛПП [16]. Важно заметить, что это относится и к традиционным китайским лекарствам: китайским лекарственным травам и нелетучим веществам, их готовым срезам или подготовленным соединениям, состоящим из нескольких трав и/или не травяных компонентов, произведенных по рецептам традиционной китайской медицины [1, 16].
В России острые медикаментозные поражения печени регистрируются у 2,7% госпитализированных больных. Как правило, они связаны с применением противотуберкулезных, антибактериальных препаратов, анальгетиков, гормональных, цитостатических, гипотензивных и антиаритмических средств [17]. Опубликованные основательные обзоры российских авторов также доказывают необходимость изучения практическим врачом этой сложной патологии.
Особого внимания заслуживают случаи ЛПП, ассоциированные с приемом различных БАДов и средств народной медицины. Их гепатотоксичность обычно недооценивается как пациентами, так и врачами. Между тем частота употребления этих средств населением достаточно высока. Только в США до 50–70% жителей страны принимают добавки для поддержания здоровья, моделирования фигуры или лечения различных заболеваний. Известна гепатотоксичность растений, использующихся в китайской и аюрведической медицине, таких как чистотел, цимицифуга, дубровник, блоховник, составляющие Гербалайфа и многие другие. Важное значение имеет взаимодействие между различными фитокомпонентами [18].
Истинная распространенность и заболеваемость ЛПП, ассоциированных с приемом БАД, неизвестны. Испанские ученые считают, что до 2% токсических поражений печени у взрослых и до 5% у детей обусловлены применением лекарственных трав и БАДов. В американском исследовании DILIN отмечено увеличение доли случаев ЛПП, связанных с БАДами, в период с 2004 по 2012 г. Они были причиной 16% всех случаев ЛПП, 1/3 из них были вызваны средствами для бодибилдинга, 1/4 – добавками для снижения веса [18].
Во многих странах Азии и Африки население для лечения в основном использует методы нетрадиционной медицины. Поэтому неудивительно, что, например, в Сингапуре лекарственные средства являются причиной до 71% случаев ЛПП.
Важно помнить о потенциальной гепатотоксичности этих средств, так как даже в развитых странах разработка БАДов не контролируется так же строго, как фармацевтических препаратов. В частности, для их регистрации не нужны доклинические и клинические токсикологические исследования, клинические испытания безопасности и эффективности [19, 20].
1.4. Кодирование по МКБ-10
К71 Токсическое поражение печени.
Включены:
- лекарственная идиосинкразическая (непредсказуемая) болезнь печени;
- токсическая (предсказуемая) болезнь печени.
При необходимости идентифицировать токсическое вещество используют дополнительный код внешних причин (класс ХХ).
Исключены:
- алкогольная болезнь печени (К.70);
- синдром Бадда–Киари (I82.0).
К71.0 Токсическое поражение печени с холестазом. Холестаз с поражением гепатоцитов. «Чистый» холестаз.
К71.1 Токсическое поражение печени с печеночным некрозом. Печеночная недостаточность (острая) (хроническая), обусловленная лекарственными средствами.
К71.2 Токсическое поражение печени, протекающее по типу острого гепатита.
К71.3 Токсическое поражение печени, протекающее по типу хронического персистирующего гепатита.
К71.4 Токсическое поражение печени, протекающее по типу хронического лобулярного гепатита.
К71.5 Токсическое поражение печени, протекающее по типу хронического активного гепатита. Токсическое поражение печени, протекающее по типу люпоидного гепатита.
К71.6 Токсическое поражение печени с картиной гепатита, не классифицированное в других рубриках.
К71.7 Токсическое поражение печени с фиброзом и циррозом печени.
К71.8 Токсические поражение печени с картиной других нарушений печени. Токсические поражение печени с очаговой узелковой гиперплазией, печеночными гранулемами, пелиозом печени, веноокклюзионной болезнью печени.
К71.9. Токсическое поражение печени неуточненное.
1.5. Классификация
Патогенетическая классификация ЛПП предусматривает 2 варианта повреждения органа [2]:
a) прямое повреждающее действие (тип А);
b) непрямое повреждающее (идиосинкразическое) действие (тип В).
I. Основные характеристики прямого повреждающего действия лекарственных средств (тип А):
- дозозависимый эффект – при достижении определенной дозы препараты оказывают непосредственное повреждающее действие на печень;
- эффект воспроизводим и предсказуем;
- латентный период короткий;
- механизм – непосредственное повреждение клеточных структур;
- гистологические изменения – некроз гепатоцитов и/или жировая дистрофия печени.
II. Основные характеристики непрямого повреждающего действия лекарственных средств (тип В):
- токсический эффект не зависит от дозы;
- токсический эффект идиосинкразический – индивидуален, невоспроизводим и непредсказуем, зависит от генетических особенностей пациента;
- латентный период – от нескольких дней до нескольких месяцев;
- механизм – образование гепатотоксических метаболитов в реакциях I или II фазы или индивидуальная гиперчувствительность (идиосинкразия).
Типы поражения печени (клинико-лабораторные варианты ЛПП) [2]:
a) гепатоцеллюлярный. Характеризуется повышением активности АЛТ более чем в 2 раза в сравнении с верхней границей нормы (N) или соотношением АЛТ/ЩФ ≥5. Эту форму ЛПП по сравнению с холестатической и смешанной отличает более тяжелая степень поражения печени. Типично острое течение, а сочетание гепатоцеллюлярного типа ЛПП с гипербилирубинемией обусловливает тяжелое повреждение печени и высокую смертность (0,7–1,3 случая на 100 тыс. пациентов);
b) холестатический. Типично повышение активности ЩФ >2N или соотношение АЛТ/ЩФ ≤2 при хроническом течении;
c) смешанный. Характерно повышение активности АЛТ >2N и соотношение 2< АЛТ/ЩФ и <5 при хроническом течении.
Морфологические виды ЛПП: стеатоз, гепатит, фиброз, цирроз, сосудистые, опухолевые и комбинированные поражения.
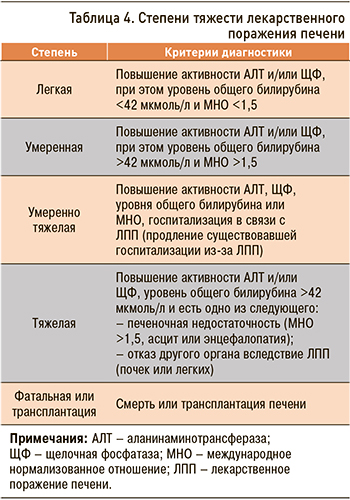 Степени тяжести ЛПП представлены в таблице 4 (исследование DILIN – Drug-Induced Liver Injury Network) [21, 22].
Степени тяжести ЛПП представлены в таблице 4 (исследование DILIN – Drug-Induced Liver Injury Network) [21, 22].
1.6. Клиническая картина
Диапазон клинических проявлений ЛПП разнообразен: от небольшого повышения уровня аминотрансфераз, не сопровождающегося клинической симптоматикой, до молниеносных гепатитов и развития циррозов. Изменения показателей ферментов могут свидетельствовать о гепатоцеллюлярном повреждении печени при повышении уровня аминотрансфераз в качестве преобладающего симптома или холестатическом повреждении при повышении уровня ЩФ с гипербилирубинемией или без нее.
ЛПП могут напоминать почти все существующие заболевания печени: острый гепатит, стеатогепатит, острую печеночную недостаточность, сосудистые реакции, холестатическое поражение и др. Лекарственные гепатиты, сопровождающиеся желтухой, могут протекать по цитолитическому, холестатическому или смешанному варианту. В ряде случаев развивается псевдохирургическая симптоматика (боли в животе, лихорадка, желтуха, увеличенный желчный пузырь). К лекарственным средствам, способным вызвать острую псевдохирургическую симптоматику, относятся цитостатики, антидепрессанты, антиаритмические препараты. Фактором, способствующим переходу гепатита в хроническое состояние, является длительный прием лекарственных средств [19].
2. ДИАГНОСТИКА
2.1. Критерии установления диагноза
• В настоящее время клинический диагноз ЛПП остается диагнозом исключения и может быть установлен после рекомендации всестороннего анализа «лекарственного» анамнеза у пациента, клинических признаков поражения печени, динамической оценки изменений в биохимических тестах печеночных синдромов, после оценки истинной или идиосинкразической гепатотоксичности конкретного препарата, если это возможно, а также при исключении других причин поражения печени. При необходимости может быть проведено гистологическое исследование печеночной ткани [1, 3].
Уровень убедительности рекомендаций B (уровень достоверности доказательств 1).
Комментарий: диагностика ЛПП традиционно включает оценку интервала между началом приема лекарственных средств и развитием поражения печени, клинические признаки, длительность и течение периода восстановления, оценку конкретных факторов риска ЛПП, исключение других причин поражения печени, учет предшествующих данных о гепатотоксичности лекарственных средств. Неинвазивные методы оценки фиброза (лабораторные расчетные методики, эластография) могут быть использованы при хронических ЛПП. Однако их данные следует трактовать как ориентировочные, поскольку эти методы не адаптированы для применения при ЛПП [3, 9].
Согласно рекомендациям Международной рабочей группы экспертов, наличие ЛПП можно обсуждать, если на фоне приема препарата (БАД и пр.) наблюдается:
a) повышение активности АЛТ >2 ВГН;
b) или повышение уровня связанного билирубина >2ВГН;
c) или сочетание повышения активности АСТ, ЩФ и уровня общего билирубина (один из показателей >2 ВГН).
При этом необходимо учитывать наличие существующих заболеваний печени [21].
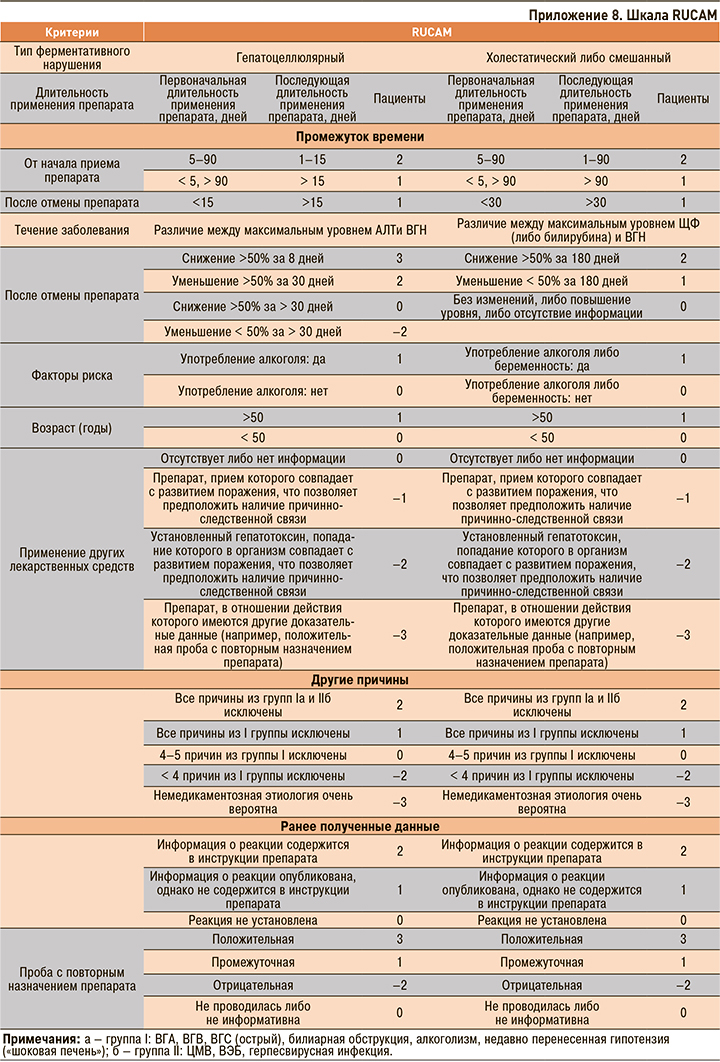
• Шкала RUCAM рекомендуется для использования в качестве полуколичественной системы оценки для определения возможного ЛПП конкретным препаратом [1, 3, 9].
Уровень убедительности рекомендаций B (уровень достоверности доказательств 1).
Комментарий: при оценке возможной ЛПП по шкале RUCAM (Приложение 8) величина баллов ≥9 указывает на то, что корреляция между подозреваемым лекарством (препаратами) и ЛПП «весьма вероятна», 6–8 баллов – «вероятна», 3–5 баллов – «возможна», 1–2 балла – «маловероятна». Количество баллов ≤0 означает «исключение» возможности ЛПП при приеме данного лекарственного средства.
• С целью дифференциальной диагностики АИГ-подобного ЛПП с аутоиммунным гепатитом (АИГ) рекомендуется тщательно собирать «лекарственный» анамнез у пациента, анализировать аутоиммунные индексы, наблюдать в динамике клинико-лабораторные сдвиги при отмене препарата и реакции на введение стероидов (если показано) и при необходимости выполнять гистологическое исследование печени для дальнейшей дифференциальной диагностики [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• В окончательный диагноз ЛПП рекомендуется включать название участвующего препарата, клинический тип ЛПП, острый или хронический вид ЛПП, показатель RUCAM и степень тяжести ЛПП [1, 3, 9].
Уровень убедительности рекомендаций B (уровень достоверности доказательств 1).
• Диагностический алгоритм при ЛПП рекомендуется использовать и при подозрении на гепатококсичность, связанную с ФДД. Это означает, что другие причины повреждения печени должны быть исключены посредством тщательного сбора анамнеза, соответствующего лабораторного обследования и визуализирующих исследований гепатобилиарной системы. При исключении других причин на фоне недавнего приема ФДД может быть достоверно установлен диагноз связанной с ФДД гепатотоксичности [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
2.2. Физикальное обследование
Физикальное обследование при подозрении на ЛПП включает осмотр кожных покровов, перкуторное и пальпаторное исследование органов дыхания, пищеварения, сердечно-сосудистой и выделительной систем. Иными словами, проводится полноценный физикальный осмотр больного для констатации патологии печени и выявления или исключения возможной патологии со стороны других органов и систем.
2.3. Лабораторная диагностика
• У лиц с подозрением на гепатоцеллюлярное либо смешанное ЛПП рекомендуется исключить острые вирусные гепатиты (A, B и C) и АИГ при помощи стандартных серологических исследований и исследования на РНК и ДНК вирусных гепатитов [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Исследование крови на IgM к вирусу гепатита Е не может быть рекомендовано в связи с неоднозначностью и отсутствием унификации технических параметров существующих в настоящее время коммерческих тестов. Тем не менее следует рассмотреть возможность обследования в случае повышенной клинической настороженности (например, при недавних поездках в эндемичные регионы) [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 2).
• Рекомендуется обследование на острую инфекцию, вызванную цитомегаловирусом, вирусом Эпштейна–Барр либо вирусом герпеса простого при исключении распространенных вирусных гепатитов (А, В, С) либо при наличии клинических признаков, характерных для указанных заболеваний, например атипичного лимфоцитоза, лимфаденопатии [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• В соответствующих клинических случаях рекомендуется исключить наличие болезни Вильсона–Коновалова и синдрома Бадда–Киари [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Выполнение серологических исследований на первичный билиарный цирроз рекомендуется пациентам с отсутствием явных признаков патологии билиарного тракта по результатам визуализирующих исследований органов брюшной полости [3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
2.4. Инструментальная диагностика
• У лиц с подозрением на лекарственный холестаз во всех случаях рекомендуется проводить визуализирующие исследования (ультразвуковое исследование, либо компьютерную томографию, либо магнитно-резонансную томографию) органов брюшной полости с целью исключения патологии билиарного тракта и инфильтративных процессов [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Проведение эндоскопической ретроградной холангиографии рекомендуется ограничить случаями, когда рутинные визуализирующие исследования не позволяют исключить наличие конкрементов в общем желчном протоке, первичный склерозирующий холангит либо панкреатобилиарные злокачественные процессы [1, 3, 9].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Выполнение биопсии печени рекомендуется рассмотреть в тех случаях, когда одной из возможных причин повреждения печени служит АИГ и предполагается проведение иммуносупрессивной терапии [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Биопсия печени рекомендуется при продолжающемся повышении уровня ферментов печени либо наличии признаков ухудшения функции печени, несмотря на отмену подозреваемого лекарственного средства [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Биопсия печени рекомендуется, если максимальный уровень АЛТ не снизился на более чем 50% через 30–60 дней после развития патологии при гепатоцеллюлярном ЛПП либо если максимальный уровень ЩФ не уменьшился более чем на 50% через 180 дней после развития патологии при холестатическом ЛПП, несмотря на отмену подозреваемого лекарственного препарата [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 2).
• Биопсия печени рекомендуется в тех случаях ЛПП, когда предполагается продолжение приема подозреваемого лекарственного средства либо его повторное назначение [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Биопсия печени рекомендуется при сохранении патологического уровня ферментов печени спустя 180 дней после отмены лекарственных средств для исключения хронического заболевания печени (ХЗП), не связанного с приемом лекарств, и/или диагностики хронического течения ЛПП [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 2).
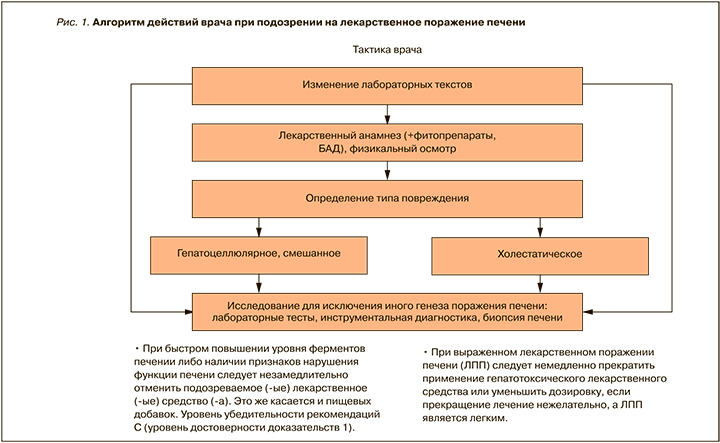
Алгоритм действий врача при подозрении на ЛПП представлен на рисунке 1. Варианты действий врача при выявлении ЛПП перечислены в Приложении 6.
3. ЛЕЧЕНИЕ
3.1. Общие рекомендации по терапии лекарственных поражений печени
Ведение пациентов с легкой и умеренной степенью тяжести ЛПП возможно в амбулаторных условиях. Госпитализация показана пациентам с тяжелым ЛПП, признаками печеночно-клеточной недостаточности и выраженной клинической симптоматикой (рвота, обезвоживание, кровотечение, признаки печеночной энцефалопатии) и признаками плохого прогноза (см. Закон Хая). Обо всех случаях тяжелого ЛПП необходимо сообщать в центр трансплантации печени, поскольку своевременное оперативное лечение спасает около 40% пациентов с ЛПП [9]. В настоящее время изучается применение стволовых клеток у этой категории пациентов [9].
• При подозрении на ЛПП, особенно при быстром повышении уровня ферментов печени либо наличии печеночно-клеточной недостаточности (особенно быстро прогрессирующей), рекомендуется незамедлительно отменить подозреваемое(-ые) лекарственное(-ые) средство(-а) [1,3].
Уровень убедительности рекомендаций А (уровень достоверности доказательств 1).
Комментарий: поводом для отмены «причинного» препарата служит любое повышение активности АЛТ в сочетании с изменением одного из функциональных печеночных тестов: билирубина, альбумина или протромбинового времени – международного нормализованного отношения (ПВ–МНО).
• Крайне не рекомендуется повторно назначать препарат, вероятно вызвавший гепатотоксическое повреждение, особенно в тех случаях, когда первичное повреждение печени было связано со значительным повышением уровня аминотрансфераз (например, >5 ВГН, закон Хая либо развитие желтухи). Исключением из данной рекомендации могут быть угрожающие жизни состояния при невозможности проведения альтернативного лечения [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Пациентам с подозрением на развитие связанной с ФДД гепатотоксичности рекомендуется отменить все ФДД, обладающие подобным действием, и в дальнейшем наблюдать за восстановлением у них функции печени [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Рекомендуется отмена или неиспользование потенциально гепатотоксичного препарата во избежание риска обострения или рецидива основных заболеваний на фоне его приема.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
Комментарий: если «виновный» препарат жизненно необходим пациенту, он должен быть отменен при развитии следующих признаков:
а) АЛТ или АСТ сыворотки >8 ВГН;
б) AЛT или AСT >5 ВГН в течение ≥2 нед;
в) AЛT или AСT >3 ВГН, билирубин >2 ВГН или МНО >1,5;
г) AЛT или AСT >3 ВГН, цитолиз сопровождается постепенно прогрессирующей слабостью, усталостью, симптомами желудочно-кишечной диспепсии и/или эозинофилией (>5%) [1, 3].
• Назначение пациентам с ХЗП лекарственных средств, обладающих гепатотоксическим действием, рекомендуется базировать на оценке риска и пользы рассматриваемого вида лечения в каждом конкретном случае [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Не рекомендуется комбинировать два или более типа противовоспалительных и гепатопротекторных средств для лечения или профилактики ЛПП [1, 3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
3.2. Медикаментозное лечение
• N-ацетилцистеин (NAC)
На сегодняшний день FDA одобрено и рекомендовано применение NAC только для лечения ЛПП, вызванных парацетамолом [1, 3].
Уровень убедительности рекомендаций А (уровень достоверности доказательств 2).
При подозрении на ЛПП, вызванный парацетамолом, рекомендуется начать лечение N- ацетилцистеином (NAC) как можно раньше.
Уровень убедительности рекомендаций А (уровень достоверности доказательств 1).
Комментарий: исходя из степени тяжести ЛПП, NAC рекомендуется назначать по 50–150 мг/ кг/ сут в течение как минимум 3 дней [1, 3].
• L-карнитин
Карнитин рекомендовано рассмотреть при ЛПП в силу описанных в исследованиях положительных эффектов этого средства.
Уровень убедительности рекомендаций С (уровень достоверности доказательств 3).
Комментарий: карнитин (природное вещество, родственное витаминам группы В) синтезируется в организме человека в достаточном объеме и присутствует в тканях поперечнополосатых мышц и печени. В исследовании с участием 92 пациентов с тяжелой формой ЛПП, обусловленного вальпроатом, показано, что почти половина больных, получавших L-карнитин, выжили в сравнении с пациентами, получавшими исключительно поддерживающую интенсивную терапию (их выживаемость составила 10%). Кроме того, было продемонстрировано преимущество парентерального введения L-карнитина перед пероральным в дозе 0,5 г внутривенно или внутримышечно 1 раз/сут в течение 10–14 дней [9].
• Глицирризиновая кислота
Глицирриновая кислота не рекомендуется при ЛПП из-за отсутствия доказательств ее эффективности.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: влияние глицирризиновой кислоты на течение ЛПП хоть и представляет собой большой практический интерес, однако изучено недостаточно. Большинство работ, посвященных этому аспекту использования экстракта солодки и глицирризиновой кислоты, характеризуются низким качественным уровнем. Например, при изучении вопроса терапии поражений печени противотуберкулезными средствами, которые хорошо известны своей гепатотоксичностью, Liu Q. et al. обнаружили всего 4 статьи, посвященные использованию глицирризиновой кислоты при этой патологии; при этом ни одна из них не отвечала современным требованиям доказательной медицины, что сделало невозможным вывод об эффективности этого средства [23, 24].
• Силимарин
Силимарин не рекомендуется при ЛПП из-за отсутствия доказательств его эффективности.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: имеется ряд исследований эффективности силимарина при ЛПП, связанных с приемом винкристина, преднизолона, метотрексата, меркаптопурина, тиогуанина, такрина (средства лечения болезни Альцгеймера), психотропных и противотуберкулезных препаратов [24, 25]. Метаанализ 14 рандомизированных плацебо-контролируемых клинических исследований, два из которых включали пациентов с ЛПП, показал, что применение силимарина при ХЗП не приводит к значимому снижению активности трансаминаз. В связи с этим данный препарат не рекомендуется в лечении лекарственной гепатотоксичности [2].
• Адеметионин
Рекомендуется рассмотреть применение адеметионина при ЛПП, поскольку в ряде клинических исследований показана его эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: препараты адеметионина зарегистрированы с показанием «внутрипеченочный холестаз» на основании нескольких двойных слепых плацебо-контролируемых рандомизированных клинических исследований, а также открытых исследований с референс-контролем и ряда рандомизированных исследований, демонстрирующих эффекты адеметионина и при цитолитическом синдроме [26]. Адеметионин может быть использован у пациентов с ЛПП химиотерапевтическими средствами при наличии цитолитического синдрома [26].
Имеются экспериментальные исследования, свидетельствующие об эффективности S-аденозилметионина у лабораторных мышей с гепатотоксическим поражением печени в результате введения парацетамола [2]. В российском многоцентровом проспективном наблюдательном исследовании 105 пациентов с псориазом, получавших иммуносупрессивную терапию (метотрексат, циклоспорин, глюкокортикостероиды), показана эффективность адеметионина при ЛПП с холестазом. В качестве инициации гепатопротективной терапии пациенты получали исследуемый препарат парентерально в дозе 400–800 мг/сут в течение 2 нед, а на поддерживающем этапе – 800–1600 мг/сут перорально 4 нед [13, 23].
Santini D. et al. исследовали эффективность адеметионина у 50 пациентов с ЛПП химиотерапевтическими средствами. Манзюк Л.В. с соавт. изучали влияние адеметионина на течение ЛПП, вызванных химиотерапевтическими средствами, у 19 пациентов с раками различной локализации [26]. В исследовании Vincenzi B. и Daniele S. 78 больных с колоректальным раком дополнительно к основному курсу химиотерапии (капецитабин + оксалиплатин) получали бевацизумаб, а часть пациентов еще и адеметионин [26].
Рекомендуемая схема назначения адеметионина:
а) 1-й этап: по 800 мг/сут внутривенно в течение 2 нед;
b) 2-й этап: по 800–1600 мг/сут перорально в 2 этапа в течение 4 нед (если используется доза 400 мг в одной таблетке) или по 1000–1500 мг/сут перорально в 2 этапа в течение 4 нед (если используется доза 500 мг в одной таблетке).
Возможен и более длительный прием препарата [9].
- L-орнитина L-аспартат
- L-орнитина L-аспартат рекомендован при ЛПП в случае наличия печеночной энцефалопатии [27].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарии: L-орнитина L-аспартат обладает детоксикационным действием, снижая повышенный уровень аммиака в организме, в частности, при заболеваниях печени. Действие препарата связано с его участием в орнитиновом цикле мочевинообразования (активирует работу цикла, восстанавливая активность ферментов клеток печени – орнитин-карбамоилтрансферазы и карбамоил-фосфатсинтетазы). L-орнитина L-аспартат способствует выработке инсулина и соматотропного гормона, улучшает белковый обмен при заболеваниях, требующих парентерального питания, способствует уменьшению астенического, диспептического и болевого синдромов, а также нормализации повышенной массы тела (при стеатозе и стеатогепатите).
Ряд работ продемонстрировал эффективность L-орнитина L-аспартата при отравлении психотропными средствами (барбитуратами, бензадиазепинами) [24].
Способ применения L-орнитина L-аспартата:
а) внутрь после еды по 1 пакетику гранулята, предварительно растворенного в 200 мл жидкости, 2–3 раза/сут;
b) внутривенно – обычно 20 г (4 ампулы); при печеночной энцефалопатии, в зависимости от степени тяжести состояния, до 40 г (8 ампул) в сутки. Максимальная скорость инфузии 5 г/ч.
• Урсодезоксихолевая кислота (УДХК)
Рекомендуется рассмотреть применение УДХК при ЛПП, поскольку в ряде клинических исследований показана ее эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: имеются сообщения об эффективности УДХК в лечении холестатического и смешанного вариантов ЛПП, развившихся вследствие приема метотрексата, флутамида (антиандрогенного противоопухолевого средства), флувастатина и других препаратов. В рекомендациях EASL приводятся данные об эффективности УДХК в лечении 2/3 случаев холестатических ЛПП [28, 29].
При цитолитическом синдроме, развившемся на фоне приема статинов, назначение УДХК приводило к нормализации биохимических проб печени и положительной динамике в структуре печени (уменьшение индекса гистологической активности, нормализация диаметра портальных трактов, уменьшение их фиброза и инфильтрации лимфоцитами, отсутствие перипортальных некрозов, гиперплазии ретикулоэндотелиальной системы, уменьшение признаков холестаза) по данным морфологического исследования [30, 31].
Ряд работ продемонстрировал эффективность УДХК в лечении ЛПП после трансплантации печени и при проведении иммуносупрессивной терапии, однако необходимы крупные высококачественные исследования [24].
Рекомендуемый режим применения УДХК: 13–15 мг/кг/сут в 2–3 приема; возможен длительный многомесячный прием препарата до разрешения явлений повреждения печени [9].
• Эссенциальные фосфолипиды (ЭФЛ)
Рекомендуется рассмотреть применение ЭФЛ при ЛПП, поскольку в ряде клинических исследований показана их эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: использование ЭФЛ (2С) при ЛПП противотуберкулезными средствами упоминается в систематическом обзоре Liu Q. et al. [32]. Статистически значимые отличия между группами пациентов были отмечены только для показателей холестатического, но не гепатоцеллюлярного типа поражения [31].
ЭФЛ уменьшали активность АЛТ, наблюдаемое после длительной терапии ловастатином [24, 33].
Рекомендуемый режим применения ЭФЛ: внутривенное введение от 500 до 1000 мг в течение 7–10 дней с последующим переходом на прием внутрь в дозе 1800 мг/сут, разделенной на три приема. Длительность лечения определяется выраженностью цитолитического синдрома и составляет от 4 до 12 нед. Возможно назначение препарата внутрь c первого дня лечения, если нет возможности парентерального введения [9].
• Препараты янтарной кислоты
Рекомендуется рассмотреть применение препаратов янтарной кислоты при ЛПП, поскольку в ряде клинических исследований показана их эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: имеются исследования применения комплексного препарата янтарной кислоты (янтарная кислота 5,28 г, N-метилглюкамин (меглюмин) 8,725 г, рибоксин (инозин) 2 г, метионин 0,75 г, никотинамид 0,25 г) при ЛПП на фоне доцетаксела, эрлотиниба, гемцитабина, иматиниба, иринотекана, паклитаксела, сорафениба, топотекана, винорелбина. Согласно клиническим рекомендациям Общества специалистов поддерживающей терапии в онкологии [23], эти онкологические средства требуют профилактического применения указанного препарата на основе янтарной кислоты.
Режим введения комплексного препарата янтарной кислоты при полихимиотерапии (ПХТ) в онкологии:
а) для профилактики ЛПП – 400 мл внутривенно капельно 1 раз/сут не менее 4 инфузий;
b) для лечения ЛПП – 400 мл внутривенно капельно 2 раза/сут не менее 4 дней после каждого курса ПХТ при различных формах онкопроцесса [9, 23].
Режим введения для лечения и профилактики ЛПП, индуцированных противотуберкулезными препаратами: 400 мл внутривенно 1 раз/сут в течение 10 дней [9].
• Таурин
Рекомендуется рассмотреть применение таурина при ЛПП, поскольку в ряде клинических исследований показана его эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: таурин (2-аминоэтансульфоновая кислота) – наиболее распространенная свободная аминокислота в организме человека, играющая важную роль в таких биологических процессах, как конъюгация желчных кислот, поддержание гомеостаза кальция, осморегуляция и стабилизация мембран. Мембраностабилизирующие, антиоксидантные и гепатопротекторные свойства позволяют рассматривать таурин в качестве средства для профилактики и лечения CYP2E1-ассоциированных повреждений печени, в том числе при передозировке парацетамола [34].
Показано, что в дозе 1000 мг/сут в течение месяца таурин может быть использован для профилактики и лечения ЛПП у пациентов, получающих противотуберкулезную терапию [35].
В ряде исследований продемонстрирована возможность применения таурина в качестве гепатопротектора у пациентов с онихомикозом, получающих терапию противогрибковыми препаратами [36].
• Гидролизат плаценты человека
Рекомендуется рассмотреть применение гидролизата плаценты человека при ЛПП, поскольку в ряде клинических исследований показана его эффективность у этой категории больных.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: биологически активные вещества и факторы роста, находящиеся в гидролизате плаценты человека, стимулируют регенерацию (пролиферацию) гепатоцитов, процессы детоксикации, снижают отложение липидов и холестерина в печеночных клетках, повышают активность тканевого дыхания, активизируют обмен веществ в печени, снижают интенсивность развития соединительной ткани в печени. Имеются работы по изучению применения гидролизата плаценты при лекарственных гепатитах, развившихся при лечении вирусных гепатитов В и С [37–39].
В парацетамоловой модели острого отравления применение гидролизата плаценты приводило к нормализации уровня креатинина, уменьшению жировой инфильтрации печени и повреждения паренхимы, стимуляции регенерации гепатоцитов [40].
Способ применения гидролизата плаценты человека:
a) внутримышечно по 2 мл/сут. В зависимости от тяжести заболевания частота введения может составлять 2–3 раза/сут;
b) при внутривенном капельном применении 4 мл препарата следует растворить в 500 мл 5% раствора декстрозы и вводить через локтевую вену в течение 1,5–2 ч. Инъекции проводятся ежедневно, курс лечения 2–3 нед.
• Глюкокортикостероиды (ГКС)
ГКС рекомендуются при подозрениях на аутоиммуноподобный фенотип ЛПП.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: при подозрении на аутоимунный фенотип ЛПП дифференцировать истинный лекарственно-индуцированный АИГ можно лишь оценкой варианта ответа на терапию ГКС и динамическое наблюдение [9]. При этом аутоиммуноподобное ЛПП после отмены ГКС не рецидивирует в отличие от истинного АИГ.
Преднизолон назначается в дозе 20–40 мг/сут с последующим постепенным снижением дозы после нормализации биохимических показателей в течение 6 мес [9].
3.3. Хирургическое лечение
Трансплантация печени рекомендуется для пациентов с острым и подострым ЛПП и декомпенсированным циррозом печени [1, 3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
Комментарий: в случае неэффективности всех проводимых мероприятий единственным методом лечения, способным спасти жизнь больного, может быть трансплантация печени [2, 9].
4. ЛЕКАРСТВЕННЫЕ ПОРАЖЕНИЯ ПЕЧЕНИ У БЕРЕМЕННЫХ
4.1. Эпидемиология лекарственных поражений печени у беременных
Не менее 80–90% женщин принимают различные лекарственные средства во время беременности по назначению врача и без врачебного назначения [41]. Согласно результатам российского исследования «Эпидемиология использования лекарственных средств у беременных», среднее количество одновременно назначенных препаратов этой группе женщин составляет 3,8 (от 0 до 16), а в I триместре – 3,2 (от 0 до 10) [42]. В связи с этим в клинической практике нарастает актуальность проблемы гепатотоксичности лекарственных средств у беременных женщин.
4.2. Этиология и патогенез лекарственных поражений печени у беременных
Теоретически любое лекарственное средство является потенциально опасным для печени беременных, поскольку функциональная нагрузка на нее в этот период существенно возрастает. Из-за изменения функционирования печени и других органов и систем, гипоальбуминемии, вызванной эффектом «разведения», склонности к развитию внутрипеченочного и внепеченочного холестаза, нарушающего выведение лекарственных средств и их метаболитов, появления дополнительного плацентарного «круга кровообращения» у беременных заметно изменяется фармакокинетика лекарств [41, 43]. К особой группе риска по развитию ЛПП следует отнести пациенток с экстракорпоральным оплодотворением (ЭКО), так как эта процедура подразумевает под собой большую лекарственную (в том числе гормональную) нагрузку [44].
Лекарственная терапия может спровоцировать и развитие специфической патологии печени у беременных, такой как гестоз и внутрипеченочный холестаз беременных, а также усугубить выраженность их клинических проявлений и последствий. Установлено, что потенциально фатальное осложнение, наблюдаемое у беременных, – острый жировой гепатоз беременных – в 21% случаев ассоциируется именно с приемом ряда лекарственных средств, включая поливитаминно-минеральные комплексы, препараты железа, кальция и др. [45]. Вместе с тем как минимум в 4,3% случаев острый лекарственный гепатит с гепатоцеллюлярным механизмом повреждения обусловлен приемом половых гормонов. Роль гормональных препаратов в развитии ЛПП у беременных обусловлена еще и тем, что они способствуют нарушению желчевыделения, а следовательно, задержке в печени токсичных метаболитов [46].
Развитие ЛПП определяется многими факторами: дозой препарата, продолжительностью его приема, концентрацией в сыворотке крови, возрастом больных (дети и старшие возрастные группы более чувствительны к лекарствам), генетическими факторами, одновременным приемом нескольких лекарственных средств, предшествующими заболеваниями печени, нарушением функции почек, сердечно-сосудистой системы и др. [41, 46]. По данным Ереминой Е.Ю., наиболее высокая активность трансаминаз отмечалась у женщин, принимающих одновременно более 6 наименований лекарственных средств, особенно у пациенток, в схемы лечения которых входили гормональные препараты (дюфастон, утрожестан) в сочетании с поливитаминно-минеральными комплексами. Именно у этой категории пациенток чаще всего выполнялось прерывание беременности по медицинским показаниям и преждевременное родоразрешение [46].
Выделить конкретный препарат, ответственный за ЛПП у беременной в условиях полипрагмазии, чрезвычайно сложно. Данные о безопасности лекарственных средств в период беременности основаны, как правило, на результатах экспериментальных исследований, которые не всегда подтверждаются в клинической практике [42].
4.3. Клиническая картина лекарственных поражений печени у беременных
Различные клинические варианты ЛПП, независимо от наличия беременности, начиная от субклинических форм, таких как стеатоз и малоактивный лекарственный гепатит, заканчивая фульминантной печеночной недостаточностью, описаны приблизительно для 1200 лекарственных средств. Это могут быть как прямые гепатотоксические эффекты (цитолитический, холестатический или смешанный механизм ЛПП), так и реакция идиосинкразии, характеризующаяся индивидуальной непереносимостью лекарственного средства вследствие иммуноопосредованных воспалительных реакций печени на препарат, либо образования высокотоксичных метаболитов лекарства в процессе биотрансформации [46].
Особенность ЛПП у беременных – длительная асимптомность или малосимптомность клиники, манифестация на стадии выраженных нарушений функций печени. Наиболее тяжелое течение ЛПП отмечается в III триместре беременности. Оно характеризуется прогрессивным ростом показателей печеночного цитолиза и высоким риском осложнений беременности, что в ряде случаев требует преждевременного родоразрешения [46].
4.4. Диагностика лекарственных поражений печени у беременных
ЛПП у беременных могут иметь самую разнообразную клиническую картину, и их довольно сложно дифференцировать, поскольку требуется проведение значительного объема диагностических исследований за короткий срок. К тому же часть высокоинформативных исследований, таких как компьютерная томография, магнитно-резонансная томография, эндоскопическая ретроградная панкреатохолангиография, а иногда даже эзофагогастродуоденоскопия (ЭГДС), невозможно или нежелательно проводить в период беременности. В этих случаях могут помочь общие принципы диагностики ЛПП, к которым прежде всего относятся тщательное изучение лекарственного анамнеза как минимум в течение 3 мес и исключение иного генеза поражения печени (вирусного, аутоиммунного гепатита, наследственных гепатозов) [46].
4.5. Лечение лекарственных поражений печени у беременных
• Лечение беременной с ЛПП рекомендуется осуществлять совместно гастроэнтерологу/терапевту, акушеру-гинекологу и клиническому фармакологу с детальным мониторингом (в ряде случаев ежедневным) функций печени, почек, системы гемостаза и состояния плода [46].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: для построения оптимальной тактики лечения беременной с ЛПП принципиально важно определение основного механизма токсического действия лекарственного средства на печень: прямого гепатотоксического, токсического действия метаболитов лекарства, идиосинкразии или иммуноаллергического с последующим выбором метода лекарственной коррекции ЛПП: терапия преднизолоном, адеметионином, УДХК и др.
• Предпочтительный способ родоразрешения беременных с ЛПП – кесарево сечение (с учетом высокого риска кровотечения вследствие коагулопатии) под перидуральной анестезией (с учетом гепатотоксичности анестетиков, применяемых для общей анестезии).
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
• Лечение ЛПП рекомендуется продолжить у родившей женщины и в послеродовом периоде (в родовспомогательном учреждении, а затем в амбулаторных условиях у гастроэнтеролога или терапевта).
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: следует учитывать, что в раннем послеродовом периоде в течение нескольких дней может отмечаться продолжающийся рост активности показателей печеночного цитолиза. Сведения о женщине, перенесшей ЛПП в период беременности, должны быть активно переданы в амбулаторно-поликлиническое учреждение (обычно по месту жительства).
• На период лечения ЛПП кормление ребенка грудью не рекомендуется или противопоказано в зависимости от применяемых лекарственных средств.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
• После завершения кормления ребенка грудью рекомендуется проведение комплексного обследования женщины на предмет формирования хронического лекарственного гепатита или иной хронической патологии гепатобилиарной системы, индуцированной лекарственными средствами в период беременности (аутоиммунного гепатита, первичного билиарного цирроза печени, желчнокаменной болезни и др.).
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: поскольку в настоящее время отсутствуют данные об отдаленных последствиях перенесенных ЛПП, в том числе во время беременности, но существует вероятность их хронизации, особенно при иммуноаллергическом механизме ЛПП, динамическое наблюдение за женщиной следует продолжить до полной нормализации показателей функционального состояния печени (чаще активности трансаминаз).
5. ПРОФИЛАКТИКА ЛЕКАРСТВЕННЫХ ПОРАЖЕНИЙ ПЕЧЕНИ
• Для пациентов с существующим ХЗП или множественными факторами рисками поражения печени рекомендуется тщательно подходить к выбору лекарственного препарата с потенциальной гепатотоксичностью. Важно уметь различать обострение (декомпенсацию) основного заболевания (заболеваний) печени от ЛПП, что важно для дальнейшего правильного лечения [1, 3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
• Рекомендуется предупреждать пациентов насчет необходимости сообщения лечащему врачу о приеме ФДД, также следует предоставлять пациентам информацию о том, что пищевые добавки не проходят столь тщательные проверки на безопасность и эффективность, как рецептурные и даже безрецептурные лекарственные средства [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 1).
• Рекомендуется применять различные стратегии и методы для управления рисками развития ЛПП, включая идентификацию пациентов с высоким риском, отмену препаратов, снижение дозировки лекарственных средств, мониторинг изменений биохимических показателей печени на исходном уровне и последующие наблюдения, а также постоянный контроль пользы терапии и риска ЛПП [1,3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
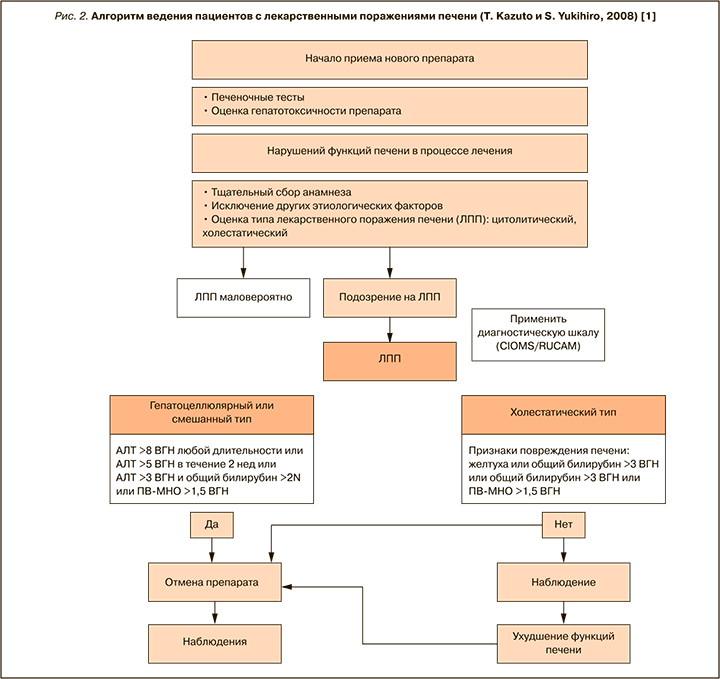
Комментарий: среди различных алгоритмов ведения пациентов с ЛПП наиболее обоснованным представляются рекомендации японских исследователей Kazuto T. и Yukihiro S., 2008 (рис. 2) [1].
• Рекомендуется назначать препараты в соответствии с показаниями при строгом соблюдении принципов совместимости и несовместимости лекарственных средств. Необходимо повышать грамотность специалистов и населения о возможности рисков ЛПП, о потенциальной гепатотоксичности лекарственных средств, в том числе растительных препаратов, а также БАДов, продуктов для здоровья и др. [1, 3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
• Рекомендуется использование врачами и пациентами ресурсов интерактивных веб-сайтов, таких как HepaTox и LiverTox. Создание, развитие и совершенствование этих платформ будет способствовать лучшему пониманию ЛПП медицинским персоналом и общественностью и должно быть активно использовано в клинической практике и научных исследованиях [1,3].
Уровень убедительности рекомендаций В (уровень достоверности доказательств 1).
Комментарий: специфические алгоритмы контроля биохимических показателей печени при назначении лицам с установленной ХЗП лекарственных средств, обладающих гепатотоксичностью, отсутствуют. Инструкции по применению лекарственных препаратов часто содержат неполную либо не оказывающую помощи в этом вопросе информацию. Следует рекомендовать пациентам незамедлительно сообщать о развитии любых новых симптомов, таких как желтое окрашивание склер, ощущение боли/дискомфорта в животе, тошнота/рвота, зуд кожных покровов либо темный цвет мочи [1, 3].
• Рекомендуется контролировать уровень биохимических показателей состояния печени в сыворотке крови с интервалом 4–6 нед, особенно в течение первых 6 мес лечения лекарственным средством, обладающим гепатотоксичностью [1, 3].
Уровень убедительности рекомендаций С (уровень достоверности доказательств 2).
• Для минимизации риска повторного ЛПП врачу, выявившему побочную реакцию на препарат, рекомендуется в обязательном порядке сделать соответствующую запись в истории болезни с указанием подозреваемого или причинного лекарственного средства [34] и выдать пациенту медицинскую документацию с описанием побочной реакции и этиологического фактора [1, 3].
Уровень убедительности рекомендаций А (Уровень достоверности доказательств 1).
Комментарий: для сообщения о нежелательной реакции используется форма «Извещение о побочном действии, нежелательной реакции или отсутствии ожидаемого терапевтического эффекта лекарственного средства», которая доступна на сайте Росздравнадзора http://www.roszdravnadzor.ru/drugs/monitpringlp/faq/25. Заполненные формы следует направлять по адресу: pharm@roszdravnadzor.ru.
5.1. Профилактика лекарственных поражений печени у беременных
• Назначение любой лекарственной терапии беременным рекомендуется только при наличии строгих индивидуальных медицинских показаний, а ее ожидаемая польза должна превалировать над возможным риском для здоровья самой женщины и плода.
Уровень убедительности рекомендаций А (уровень достоверности доказательств 2).
Комментарий: терапию следует проводить только теми препаратами, которые допустимы для соответствующего срока беременности. При клинически обоснованной необходимости применения лекарственных средств, потенциально небезопасных для беременной, течения беременности и плода женщина должна быть подробно информирована о необходимости медикаментозной терапии и ее возможных последствиях.
• При назначении медикаментозной терапии беременным рекомендуется избегать полипрагмазии, которая увеличивает риск нежелательных побочных реакций и ЛПП.
Уровень убедительности рекомендаций А (уровень достоверности доказательств 2).
Комментарий: при назначении столь распространенной в настоящее время профилактической терапии витаминами и микроэлементами в каждом конкретном случае следует придерживаться принципа «наличие строгих показаний». Также необходимо соблюдать этапность применения таких средств, предусматривающую их последовательное назначение с учетом срока гестации и индивидуальных потребностей женщины.
• Любое назначение лекарственных средств беременной рекомендуется осуществлять с учетом особенностей их фармакокинетики у этой категории пациенток. При этом следует руководствоваться принципом назначения минимально эффективной дозы на минимальный промежуток времени.
Уровень убедительности рекомендаций А (уровень достоверности доказательств 2).
• При проведении медикаментозной терапии у беременной женщины рекомендуется постоянный контроль состояния пациентки с особым вниманием к функционированию печени.
Уровень убедительности рекомендаций А (уровень достоверности доказательств 2).
Комментарий: мониторинг функционального состояния печени, включающий как минимум исследование активности сывороточных трансаминаз (АЛТ и АСТ), ЩФ, гамма-глютамилтранспептидазы и уровня билирубина, должен осуществляться в динамике в течение всего срока использования любого лекарственного средств, растительного препарата или БАД.
6. ОРГАНИЗАЦИЯ ОКАЗАНИЯ МЕДИЦИНСКОЙ ПОМОЩИ
Организация оказания медицинской помощи больным с ЛПП проводится на основании Приказа Минздрава России от 12.11.2012 № 906н «Об утверждении порядка оказания медицинской помощи населению по профилю „Гастроэнтерология”» (зарегистрировано в Минюсте России 21 января 2013 г. № 2664).
- Медицинская помощь оказывается поэтапно:
- амбулаторный этап: сбор жалоб и анамнеза, физикальный осмотр, лабораторные и инструментальные методы обследования (клинический и биохимический анализы крови, ультразвуковое исследование органов брюшной полости, ЭГДС и др.);
- стационарный этап: госпитализация больных ЛПП рекомендована для проведения диагностики в неясных случаях для уточнения причины поражения печени (если необходимые исследования не могут быть проведены амбулаторно), интенсивной терапии при выраженном цитолитическом синдроме (достижении уровня печеночных трансаминаз >10 норм), прогрессирующей печеночно-клеточной недостаточности, печеночной энцефалопатии, решения вопроса о трансплантации печени.
Уровень убедительности рекомендаций В (уровень достоверности доказательств 2).
Комментарий: в большинстве случаев наблюдение и лечение больных ЛПП проводится в амбулаторных условиях.
• Амбулаторный этап включает проведение лечения, наблюдение (в том числе диспансерное), профилактику обострений, реабилитацию, лечение заболевания, по поводу которого пациент принимал лекарственное средств, вызвавшее развитие ЛПП.
7. КРИТЕРИИ ОЦЕНКИ КАЧЕСТВА МЕДИЦИНСКОЙ ПОМОЩИ
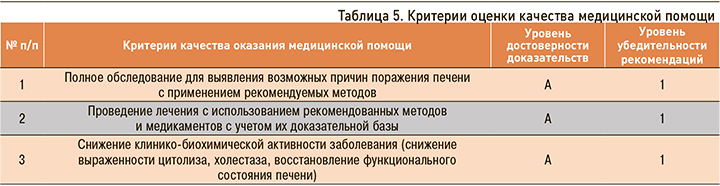
Критерии оценки качества медицинской помощи должны соответствовать Приказу Минздрава России от 10.05.2017 № 203н «Об утверждении критериев оценки качества медицинской помощи» (табл. 5).
8. ПРИЛОЖЕНИЯ
Приложение 1. Методология разработки клинических рекомендаций
Настоящие Рекомендации созданы с учетом:
- действующего Федерального закона «О внесении изменений в статью 40 Федерального закона „Об обязательном медицинском страховании в Российской Федерации” и Федерального закона „Об основах охраны здоровья граждан Российской Федерации”» по вопросам клинических рекомендаций, принятого Государственной Думой 19 декабря 2018 г. и одобренного Советом Федерации 21 декабря 2018 г.;
- Приказа Минздрава России от 28.02.2019 № 103н «Об утверждении порядка и сроков разработки клинических рекомендаций, их пересмотра, типовой формы клинических рекомендаций и требований к их структуре, составу и научной обоснованности включаемой в клинические рекомендации информации» (зарегистрировано в Минюсте России 08.05.2019 № 54588).
Методы, используемые для отбора информации, доказательств: анализ публикаций по теме, входящих в базы данных EMBASE, PubMed и MEDLINE, а также материалов, опубликованных ведущими профильными медицинскими журналами, изданных не ранее чем за последние 10 лет.
Методы, используемые для оценки достоверности (доказательности) информации:
- консенсус экспертов;
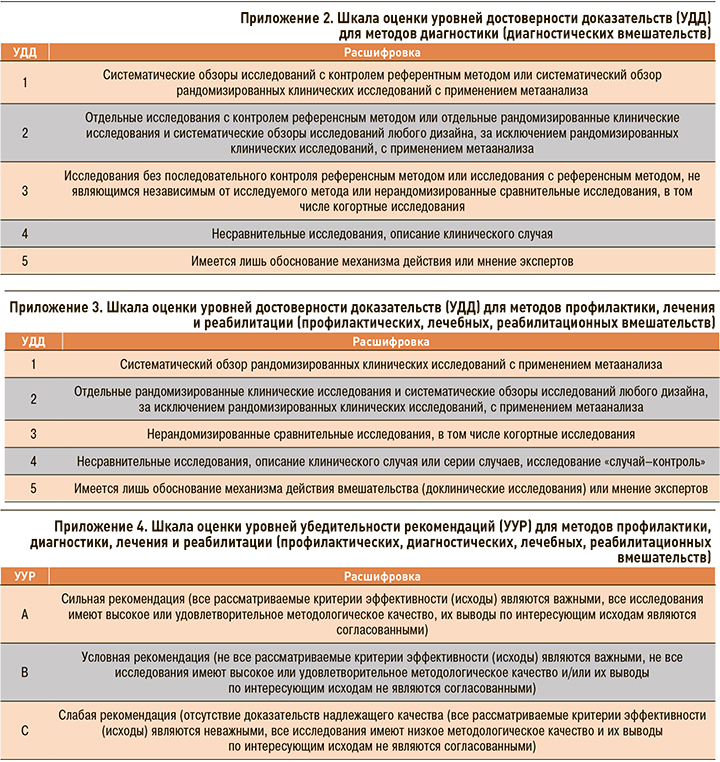
- оценка уровня доказательности в соответствии с рейтинговыми шкалами (Приложения 2, 3, 4).
Описание методов, используемых для анализа доказательств: вся информация, используемая в клинических рекомендациях, имеет доказательную базу, апробирована на практике и одобрена Российским научным медицинским обществом терапевтов (РНМОТ) и Научным обществом гастроэнтерологов России (НОГР).
Экономический анализ: анализ стоимости не проводился, и публикации по фармакоэкономике не анализировались.
Целевая аудитория: настоящие рекомендации разработаны для практических врачей первичного звена здравоохранения (врачей общей практики, терапевтов, гастроэнтерологов) с целью систематизации имеющихся данных по лекарственным поражениям печени, их диагностике, лечению и профилактике.
Этот документ является результатом коллективной работы специалистов-экспертов гастроэнтерологов, терапевтов, клинических фармакологов. По мере появления новых российских и международных данных по лекарственному поражению печени рекомендации будут обновляться в будущем в надлежащее время.
Рекомендации применимы при осуществлении медицинской деятельности в рамках Порядка оказания медицинской помощи населению при заболеваниях терапевтического и гастроэнтерологического профиля.
Порядок пересмотра рекомендаций – 1 раз в три года.
Приложение 5. Связанные документы
- Федеральный закон «О внесении изменений в статью 40 Федерального закона „Об обязательном медицинском страховании в Российской Федерации” и Федерального закона „Об основах охраны здоровья граждан Российской Федерации”» по вопросам клинических рекомендаций, принятого Государственной Думой 19 декабря 2018 г. и одобренного Советом Федерации 21 декабря 2018 г.
- Приказ Минздрава России от 28.02.2019 № 103н «Об утверждении порядка и сроков разработки клинических рекомендаций, их пересмотра, типовой формы клинических рекомендаций и требований к их структуре, составу и научной обоснованности включаемой в клинические рекомендации информации» (зарегистрировано в Минюсте России 08.05.2019 № 54588).
- Приказ Минздрава России от 12.11.2012 № 906н «Об утверждении порядка оказания медицинской помощи населению по профилю „Гастроэнтерология”» (Зарегистрировано в Минюсте России 21 января 2013 г. № 2664).
- Приказ Минздрава России от 10.05.2017 № 203н «Об утверждении критериев оценки качества медицинской помощи».
Приложение 6. Варианты действий врача при выявлении лекарственного поражения печени
- Полная отмена препарата – дэмеджера (от англ. damage – повреждение) до восстановления функций печени.
- Замена препарата на нетоксичный аналог.
- Продолжение приема препарата по жизненным показаниям под прикрытием препаратом с гепатопротективными свойствами.
- При легкой и умеренной степени ЛПП ведение пациента осуществляется амбулаторно.
- Госпитализация показана при тяжелой степени ЛПП, печеночно-клеточной недостаточности, коморбидности, неблагоприятном прогнозе.
Приложение 7. Информация для пациента
В случае необходимости или собственного желания пациента приема каких-либо лекарственных препаратов, в том числе фитопрепаратов, а также БАДов, фитодобавок и т.д., необходимо проконсультироваться с врачом. Самостоятельный прием подобного рода медикаментов и пищевых добавок может быть очень опасным. Самостоятельное изучение инструкции по применению лекарственных средств часто не оказывает помощи пациенту в этом вопросе, поскольку этот документ может содержать неполную либо непонятную для некомпетентного в медицине лица информацию.
Также необходимо консультироваться с врачом при необходимости приема одновременно нескольких лекарственных препаратов, поскольку эта ситуация усиливает риск развития лекарственного поражения печени. Необходимо помнить, что потенциально смертельным вследствие развития острой печеночной недостаточности может стать прием больших доз такого широко распространенного жаропонижающего препарата, как парацетамол.
Следует незамедлительно сообщать о развитии любых симптомов, появившихся на фоне или вскоре после приема новых лекарственных средств или биодобавок. Особенно должны насторожить следующие симптомы: желтое окрашивание склер, ощущение боли/дискомфорта в животе, тошнота/рвота, зуд кожных покровов, появление светлого кала и темный цвет мочи, лихорадка, выраженная слабость. Самолечение в случаях появления симптоматики недопустимо и может быть очень опасным.