На сегодняшний день имеются многочисленные литературные данные о том, что вакцинация служит наиболее эффективным методом профилактики распространения COVID-19-инфекции и связанных с ней осложнений [1–4]. Ниже рассмотрен клинический случай развития синдрома Гийена–Барре у пациента молодого возраста после введения вакцины «Спутник V». Это одно из первых наблюдений больного с развитием серьезных неврологических осложнений после вакцинации от COVID-19.
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ
Пациент А., 47 лет, офисный работник, поступил в стационар клиники АО «Медицина» 18.01.2021.
Жалобы при поступлении: слабость в ногах, меньше в руках, запоры, требующие применения клизмы, натуживание при мочеиспускании.
История заболевания: пациент считает себя больным с 14.01.2021, когда после местного переохлаждения дома (спал с открытым окном) его начали беспокоить головные боли в затылочной области и выраженная слабость. 16.01.2021 появилась асимметрия лица слева, слезотечение из глаз, больше слева. Обратился на прием к неврологу поликлиники и был госпитализирован в стационар с диагнозом острая периферическая невропатия лицевого нерва с двух сторон. Пациент сообщил, что 28.12.2020 с профилактической целью ему была сделана вакцинация от COVID-19-инфекции: выполнено первое введение вакцины «Спутник V».
Анамнез жизни: длительное время пациент отмечает повышение артериального давления (АД), максимально до 160–170/90 мм рт.ст. Адаптирован к показателям 130/80 мм рт.ст., постоянную антигипертензивную терапию не получает.
Наблюдается амбулаторно с диагнозом «многоузловой зоб, эутиреоз. Нарушение гликемии натощак. Аденома паращитовидной железы. Киста правой почки».
Лекарственный и аллергический анамнез: в анамнезе – отек Квинке на пенициллин.
Выкуривает 2 сигареты в сутки с 2008 г.
Состояние при поступлении: относительно удовлетворительное. Телосложение нормостеническое, питание умеренное. Кожные покровы: лицо и шея гиперемированы, обычной влажности, эластичные. Видимые слизистые: розовые, чистые, влажные, блестящие. Костно-мышечная система без видимой патологии. Лимфатические узлы без особенностей. Отеков нет. Температура тела 36,4 °C. Дыхание свободное через нос. Частота дыхания 18/мин. Голос звонкий, нёбная занавеска не висит Грудная клетка цилиндрической формы, равномерно участвует в акте дыхания. При аускультации дыхание проводится через все отделы, везикулярное, хрипов нет. Патологической пульсации в области сердца, сосудов шеи и эпигастрии не отмечается. Перкуторно границы сердца не изменены: правая – по краю грудины, левая – на 0,5 см кнутри от среднеключичной линии, верхняя – в третьем межреберье. При аускультации тоны сердца приглушены, ритмичные, шумов нет.
АД 160\100 мм рт.ст. Пульс 82 уд./мин, ритмичный, удовлетворительного наполнения и напряжения. Аппетит сохранен, но пациент ест мало из-за сложностей с жеванием. Язык влажный, чистый. Живот правильной формы, не вздут, участвует в акте дыхания равномерно, при пальпации мягкий, безболезненный. Аускультативно перистальтика выслушивается. Край печени при пальпации острый, эластической консистенции, безболезненный. Дизурических явлений нет. Область почек визуально не изменена. Симптом поколачивания отрицательный с обеих сторон.
Нервная система: пациент в сознании, ориентирован, адекватен. Менингеальной симптоматики нет. Речевых нарушений нет. Движения глазных яблок в полном объеме. Нистагма нет. Глазные щели, зрачки равны. Легкий лагофтальм с двух сторон, больше слева. Парез круговой мышцы глаз с двух сторон. Конвергенция сохранена. Носогубные складки отсутствуют с двух сторон. Оскал невозможен с двух сторон.
Язык по средней линии. Глотание, фонация не нарушены. Движения в конечностях в полном объеме. Сила и тонус существенно не изменены. Рефлексы без четкой разницы сторон. Патологических знаков, расстройств чувствительности нет. Координаторные пробы пациент выполняет удовлетворительно с двух сторон. В позе Ромберга устойчив.
Движения в позвоночнике ограничены в шейном отделе с двух сторон из-за болевого синдрома. Пальпация паравертебральных точек и точек остистых отростков резко болезненна в шейном отделе позвоночника с двух сторон. Симптомы натяжения: Нери – положительный, Лассега – отрицательный с двух сторон. Дефанс паравертебральных мышц в шейном отделе позвоночника с двух сторон. Сила экстензеров первых пальцев обеих стоп в норме. Глубокие виды чувствительности не нарушены.
Клинический анализ крови и мочи в норме.
Исследование мазка из зева и носа методом полимеразной цепной реакции (ПЦР) на COVID-19 – результат отрицательный. Антитела IgG к COVID- 19 – 5,34 (положительно), антитела IgA к COVID-19 – 10,31 (положительно).
Необходимо отметить, что уже через 18 дней после введения первой дозы вакцины у пациента был защитный титр антител к COVID-19-инфекции. Хотя данные о сроках появления антител у привитых вакциной «Спутник V» пока предварительные, однако известно, что обычно иммунный ответ формируется намного позже – через 40 дней. Таким образом, в нашем случае у пациента отмечался ранний и бурный иммунный ответ на вакцинацию.
В биохимическом анализе крови обращало на себя внимание повышение аланинаминотрансферазы (АЛТ) до 61,4 ЕД. (норма до 50,0 ЕД.), в остальном отклонений не наблюдалось.
При магнитно-резонансной томографии (МРТ) головного мозга с внутривенным контрастированием отмечается картина неравномерного расширения субарахноидальных конвекситальных пространств. Единичные очаговые изменения вещества головного мозга микроангиопатического генеза. Тяжистый глиоз, мелкая кавернома в области прецентральной извилины правого полушария. Структурные изменения аденогипофиза – кистозная микроаденома?
МРТ шейного отдела позвоночника с внутривенным контрастированием: картина дегенеративно-дистрофических изменений шейного отдела позвоночника. Протрузии дисков С5–С6, С6– С7, С7–Th1 до 2,2; 2,8 и 2,3 мм соответственно. Спондилез. Нарушение статики. Крупная гемангиома Th2 позвонка с распространением на корень левой дужки, до ≈1,95×1,45×1,97 см.
МРТ пояснично-крестцового отдела позвоночника: дегенеративно-дистрофические изменения пояснично-крестцового отдела позвоночника. Протрузии дисков L3/L4, L5/S1 3–3,5 мм. Спондилез. Локальная зона трабекулярного отека в области переднего верхнего угла тела L3 позвонка справа (не характерная для спондилоартрита). Нарушение статики.
Электрокардиограмма: ускоренный синусовый ритм. Нормальное положение электрической оси сердца. Ротация сердца верхушкой назад (SISIISIII). Неполная блокада правой ножки пучка Гиса.
Предварительно установлен диагноз «острая нейропатия лицевого нерва».
В стационаре пациенту было начато введение дексаметазона внутривенно капельно 24 мг/сут, назначены Мильгамма (2,0 мл) и Нейромидин (1,0 мл) внутримышечно, антигипертензивная терапия. На фоне проводимого лечения у пациента на вторые сутки слабость мышц лица прогрессировала, присоединилась слабость правой половины лица, однако в дальнейшем слабость мимической мускулатуры немного уменьшилась с двух сторон, улучшилась чувствительность губ. На фоне введения дексаметазона у пациента зафиксировано стойкое повышение АД до 180/110 мм рт.ст., появилась транзиторная гипергликемия, в связи с чем этот препарат был отменен.
26.01.2021 пациент в удовлетворительном состоянии был выписан домой под наблюдение невролога поликлиники.
В течение следующих 4 дней пациент отметил нарастающую общую слабость, слабость в ногах, запоры, требующие применения клизмы, и затруднения при мочеиспускании, в связи с чем 30.01.2021 был повторно госпитализирован в стационар.
Неврологический статус пациента при повторной госпитализации: в сознании, ориентирован, адекватен. Менингеальной симптоматики нет. Речевых нарушений нет. Движения глазных яблок в полном объеме. Нистагма нет. Глазные щели, зрачки равны. Веки смыкаются полностью. Парез круговой мышцы глаз с двух сторон.
Конвергенция сохранена. Носогубные складки очень слабые с двух сторон. Увеличилось движение мышц при оскале с двух сторон, больше справа. Язык по средней линии. Глотание, фонация не нарушены. Движения в конечностях в полном объеме. Нижний вялый парапарез в сгибателях бедра и голени с двух сторон 4,0 балла. Рефлексы с рук низкие без четкой разницы сторон, коленные и ахилловы рефлексы отсутствуют с двух сторон, брюшные рефлексы отсутствуют с двух сторон.
Гиперестезия по полиневритическому типу с двух сторон на ногах с уровня голеностопного сустава и на руках – на подушечках пальцев. Патологических знаков нет. Координаторные пробы выполняет удовлетворительно с двух сторон. В позе Ромберга выраженное круговое пошатывание. Глубокие виды чувствительности немного снижены: путает 2–3 палец на правой ноге. Походка на носочках невозможна, на пяточках затруднена на обеих ногах.
Движения в позвоночнике не ограничены в полном объеме. Пальпация паравертебральных точек и точек остистых отростков безболезненна. Симптомы натяжения: Нери – отрицательный, Лассега – отрицательный с двух сторон. Дефанса паравертебральных мышц нет.
В клиническом анализе крови от 30.01.2021 отмечены признаки раздражения всех кровяных ростков: гемоглобин – 192,6 г/л (норма 140–180 г/л), эритроциты – 6,5×1012/л (норма 4,7–6,1×1012/л), тромбоциты – 452,4×109/л (норма 130–400×109/л), лейкоциты – 16,68×109/л (норма 3,9–10,6×109/л). Обращали на себя внимание лимфоцитопения (16,0% при норме 19–37%) и повышение уровня ретикулоцитов (до 22% при норме 2–12%).
В клиническом анализе мочи имела место небольшая эритроцитурия – до 6,0 в поле зрения (норма 0–1 в поле зрения)
В биохимическом анализе крови наблюдались сдвиги в содержании электролитов: натрий – 123 ммоль/л (норма 135–145 ммоль/л), калий – 5,7 ммоль/л (норма 3,5–5,0 ммоль/л). В дальнейшем эти показатели нормализовались на фоне терапии.
Также у пациента в период лечения сохранялась постоянная гипергликемия с повышением глюкозы максимально до 10,3 ммоль/л при небольшом повышении уровня гликозилированного гемоглобина до 6,2 % (норма 4,2–6,1%).
Для уточнения поражения периферических нервов пациенту была выполнена электронейромиография, по результатам которой обнаружены:
1. выраженное аксонально-демиелинизирующее поражение моторных волокон левого большеберцового нерва;
2. умеренное демиелинизирующее поражение моторных волокон правого большеберцового нерва;
3. снижение амплитуды ответов по сенсорным волокнам обоих икроножных нервов по полиневральному типу;
4. умеренное демиелинизирующее поражение моторных волокон правого срединного нерва. Демиелинизация сенсорных волокон со снижением амплитуды (не исключается аксональное повреждение);
5. умеренное демиелинизирующее поражение моторных волокон правого локтевого нерва со снижением амплитуды сенсорных волокон;
6. умеренное аксонально-демиелинизирующее поражение моторных волокон правого лучевого нерва со снижением амплитуды сенсорных волокон;
7. умеренное демиелинизирующее поражение моторных волокон левого срединного нерва с демиелинизацией сенсорной порции;
8. умеренное демиелинизирующее поражение сенсорных волокон левого локтевого нерва;
9. умеренное аксонально-демиелинизирующее поражение моторных волокон левого лучевого нерва с демиелинизацией и снижением амплитуды сенсорных волокон;
10. выраженное аксонально-демиелинизирующее поражение моторных волокон правого малоберцового нерва;
11. выраженное аксонально-демиелинизирующее поражение моторных волокон левого малоберцового нерва;
12. косвенные признаки вовлечения в процесс мотонейронов спинного мозга на уровне С5–Th1, L4–S1 сегментов.
Пациенту была начата терапия человеческим иммуноглобулином внутривенно капельно в дозе 0,4 г/кг/сут в течение 3 сут с положительным эффектом. Далее препарат был отменен в связи с развившейся аллергической реакцией в виде генерализованных высыпаний. Также проводилась терапия Калимином 60 мг по 1 таб. 2 раза/сут.
На фоне проводимой терапии уменьшилась слабость в ногах и слабость мимической мускулатуры. 08.02.2021 пациент был выписан домой в удовлетворительном состоянии.
Неврологический статус при выписке: в сознании, ориентирован, адекватен. Менингеальной симптоматики нет. Речевых нарушений нет. Движения глазных яблок в полном объеме. Нистагма нет. Глазные щели, зрачки равны. Веки смыкаются полностью. Парез круговой мышцы глаз с двух сторон. Конвергенция сохранена. Носогубные складки слабые с двух сторон. Увеличилось движение мышц при оскале с двух сторон, больше справа. Язык по средней линии. Глотание, фонация не нарушены. Движения в конечностях в полном объеме. Нижний вялый парапарез в сгибателях бедра и голени с двух сторон – 4,5 балла. Рефлексы с рук умеренно живые без четкой разницы сторон, появились слабые коленные рефлексы с двух сторон, ахиллов рефлекс отсутствует слева, но появился низкий справа, брюшные рефлексы отсутствуют с двух сторон. Расстройств чувствительности нет. Патологических знаков нет. Координаторные пробы пациент выполняет удовлетворительно с двух сторон. В позе Ромберга круговое пошатывание уменьшилось. Глубокие виды чувствительности немного снижены: путает 2–3 палец на правой ноге. Походка на носочках: очень легко прихрамывает на левую ногу, на пяточках – в норме. Движения в позвоночнике ограничены в полном объеме. Пальпация паравертебральных точек и точек остистых отростков безболезненна. Симптомы натяжения: Нери – отрицательный, Лассега – отрицательный с двух сторон. Дефанса паравертебральных мышц нет.
После выписки из стационара пациент продолжил наблюдение в поликлинике АО «Медицина». При очередном визите к неврологу спустя месяц после появления первых симптомов его беспокоят эпизодические боли в голенях и стопах слабой интенсивности, однако слабость в обеих ногах и в мимической мускулатуре стала значительно меньше. Также у пациента эпизодически возникают запоры, которые он купирует приемом слабительного. Мочеиспускание полностью нормализовалось. Таким образом, можно говорить об устойчивой положительной динамике, однако восстановление пока не полное.
ОБСУЖДЕНИЕ
Особенностью приведенного клинического наблюдения стало развитие неврологической симптоматики по типу синдрома Гийена–Барре (СГБ) через 18 дней после введения вакцины «Спутник V» с целью профилактики COVID-19-инфекции.
СГБ – тяжелое аутоиммунное заболевание периферической нервной системы, представляющее собой наиболее частую причину развития острого вялого тетрапареза.
В настоящее время СГБ признан самостоятельной и самой распространенной в мире формой острой полинейропатии. Частота его встречаемости варьируется от 0,6 до 2,4 случая на 100 000 населения, в среднем 1,5–2 случая на 100 000 населения ежегодно. В настоящее время выделяют четыре основные клинические формы СГБ: острую воспалительную демиелинизирующую полирадикулонейропатию (ОВДП), острую моторную аксональную нейропатию (ОМАН), острую моторно-сенсорную аксональную нейропатию (ОМСАН) и синдром Фишера [5].
Литературные данные об особенностях течения COVID-19-инфекции показывают широкую распространенность неврологической симптоматики: более 30% пациентов жалуются на головокружения, головные боли, нарушения чувствительности запахов и миалгии [6].
В июне 2020 г. было опубликовано первое клиническое наблюдение СГБ у 65-летнего пациента с подтвержденной COVID-19-инфекцией. Развитие неврологического дефицита у этого больного началось примерно через 2 нед после появления симптомов заболевания [7]. Патогенетические механизмы развития такого повреждения неизвестны, однако, вероятнее всего, речь идет о способности COVID- 19-инфекции запускать разнообразные нарушения в иммунной системе, так как ведущая роль в патогенезе СГБ отводится аутоиммунным механизмам. В литературе также описано развитие СГБ после других вирусных инфекций, в частности после гриппа [8].
После начала масштабной вакцинации от COVID-19-инфекции вопросы безопасности применяемых вакцин привлекают пристальное внимание. Результаты исследований на больших популяциях показывают высокую эффективность и безопасность применяемых вакцин. Частота побочных эффектов при их использовании не превышает 5%, причем основную часть из них составляют местные реакции. Сообщалось о единичных случаях развития локальных гипестезий, проходящих самостоятельно [9]. Также было зарегистрировано несколько случаев развития паралича Белла после применения вакцины производства Pfizer. Специалисты Центра по контролю и профилактике заболеваний (CDC, США) поставили под сомнение патогенетическую связь между введением вакцины и развитием неврологической симптоматики и рекомендовали проводить дальнейшие наблюдения.
В литературе обсуждается роль в патогенезе СГБ некоторых видов вакцин (противополиомиелитной, антирабической, противодифтерийной, противогриппозной и др.). Так, риск развития болезни после противогриппозной вакцинации (H1N1) составляет около 1–2 случая на 1 млн привитых [10]. Считается, что развитие заболевания обусловлено аномальным Т-клеточным ответом, индуцированным инфекционным процессом. Возникает воспалительная невропатия с перекрестной реактивностью между антителами к инфекционным агентам и антителами к нейроантигенам. Иммунопатологические реакции приводят к аутоиммунному повреждению тканей, ассоциированному с механизмами молекулярной мимикрии, участием суперантигенов и стимуляцией цитокинов.
В описанном нами клиническом случае неврологическая симптоматика также началась с поражения лицевого нерва, однако затем, несмотря на проводимое лечение, неврологический дефицит усугублялся, и у пациента появилось демиелинизирующее поражение периферических нервных волокон, подтвержденное инструментальными данными.
Диагноз СГБ устанавливается на основании международных критериев, принятых ВОЗ в 1993 г. (табл.).
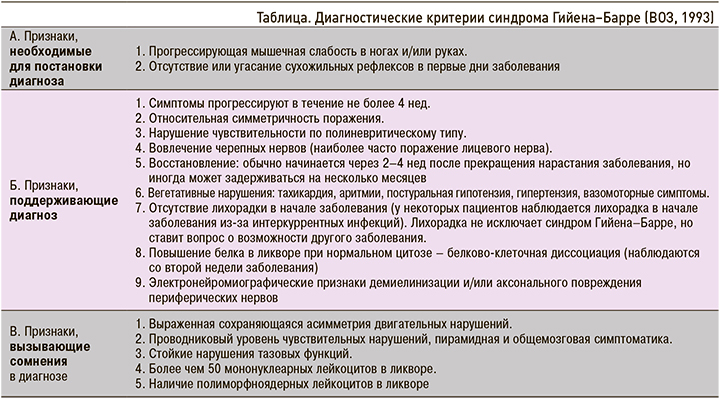
В описанном нами клиническом случае у больного присутствовала прогрессирующая мышечная слабость в ногах и угасание вплоть до отсутствия сухожильных рефлексов в первые дни заболевания. Симптомы прогрессировали около 14 дней, поражение было симметричным с развитием нарушением чувствительности по полиневритическому типу. В патологический процесс были вовлечены лицевые нервы с обеих сторон. Восстановление началось спустя 2 нед после начала симптомов. На фоне заболевания у пациента наблюдалось частое повышение АД. При электронейромиографии выявлялись признаки демиелинизации. По данным МРТ головного и спинного мозга были исключены другие причины развития патологического процесса. Все эти данные позволили поставить пациенту диагноз Синдром Гийена–Барре: острая воспалительная демиелинизирующая полиневропатия, легкая форма. Осложнения: нижний вялый парапарез 4 балла с восстановлением до 4,5 баллов. Двусторонний периферический парез лицевого нерва. Легкие тазовые нарушения.
Точные патогенетические механизмы развития СГБ и их связь с вакцинацией до настоящего времени не установлены. Однако других факторов риска, кроме проведенной незадолго до госпитализации вакцинации против COVID-19-инфекции, у нашего пациента не было. Вместе с тем известно, что иногда СГБ развивается на фоне полного здоровья, поэтому с уверенностью говорить о причинно-следственной связи вакцинации от COVID-19-инфекции и развитии СГБ нельзя, однако нельзя и оставить этот факт без внимания.
ЗАКЛЮЧЕНИЕ
Приведенное клиническое наблюдение впервые описывает развитие СГБ у пациента после введения вакцины «Спутник V» против COVID-19-инфекции. Хотя причинно-следственная связь между возникновением неврологического дефицита и введением этой вакцины не установлена, наличие хронологической связи заставляет с вниманием относиться к развитию признаков неврологического дефицита у пациентов после проведенной вакцинации. Своевременное проведение диагностических исследований, назначение патогенетического лечения и определение показаний для госпитализации в стационар позволяют эффективно вмешиваться в патологический процесс и добиваться хорошего восстановления поврежденных функций. Требуются дальнейшие клинические наблюдения за пациентами, получившими вакцинацию против COVID-19-инфекции, с целью получения всесторонней информации о возможных ранних и отдаленных ее осложнениях.


