ВВЕДЕНИЕ
Коронавирусная инфекция COVID-19, охватившая всю планету в конце 2019 г., к сожалению, и в настоящее время остается главной проблемой мирового здравоохранения. Это связано как с появлением новых, клинически более «тяжелых» штаммов коронавируса, так и с тем, что некоторые симптомы перенесенной инфекции могут персистировать длительное время, выходя далеко за пределы острого периода.
Несмотря на то что SARS-CoV-2 главным образом поражает дыхательную систему, проявления COVID-19 многообразны и, помимо классических респираторных симптомов, таких как одышка, боль в грудной клетке, кашель, включают желудочно-кишечные (диарея, рвота), скелетно-мышечные (миалгии, артралгии) и неврологические симптомы (головная боль, головокружение, аносмия, агевзия) [1]. Неврологическая симптоматика может быть обусловлена в том числе и побочными эффектами препаратов, часто применяемых в лечении COVID-19: например, на фоне применения глюкокортикостероидов (ГКС) возможно развитие инсомнии, аффективных и когнитивных расстройств [2]. Карантинные меры, необходимость изоляции также способствовали тому, что исходно респираторный вирус все чаще стал обсуждаться как «источник» нейропсихиатрических проявлений – депрессии, тревоги, апатии, когнитивных нарушений [3, 4]. При этом некоторые симптомы, такие как астения и ангедония (невозможность получения удовольствия), могут развиваться уже после клинического выздоровления, даже если в острый период и в анамнезе у пациентов не отмечались никакие психические расстройства [5, 6]. Так, по данным El Sayed S. et al. (2021) [7] и Goyal К. et al. (2020) [8], в течение двух недель после выздоровления у 75% пациентов постепенно нарастает выраженная утомляемость, анорексия, инсомния и сниженный фон настроения, которые остаются значимыми в течение нескольких месяцев и даже лет после перенесенной инфекции [9].
Несмотря на то что для отсроченных проявлений COVID-19 нет единого общепринятого термина, в литературе встречаются такие названия, как «лонг-COVID», «постковидный синдром» и др. [10]. Длительно сохраняющиеся после COVID-19 симптомы в совокупности напоминают хроническое мультисистемное заболевание, имеющее несколько разных названий – «синдром хронической усталости» / «миалгический энцефаломиелит» / «синдром постинфекционной астении»; по сути, в данном случае мы имеем дело с частным случаем постинфекционной астении (ПА), которой и будет посвящен данный обзор.
ЭПИДЕМИОЛОГИЯ И ДИАГНОСТИЧЕСКИЕ КРИТЕРИИ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
ПА представляет собой хроническое мультисистемное заболевание, проявляющееся различными конституциональными и нейрокогнитивными симптомами. Ее распространенность составляет 0,17–0,89% среди населения в целом и чаще встречается у женщин [11].
По данным некоторых авторов, к факторам риска ПА относят возраст, психиатрические заболевания в анамнезе, социально-экономический статус и уровень физической активности. Хотя патогенез заболевания не совсем понятен, предполагается, что многие случаи ПА обусловлены инфекцией [12]. Так, крупное ретроспективное исследование 837 пациентов выявило симптомы острой инфекции (лихорадка, инфекция верхних дыхательных путей, гриппоподобное состояние или гастроэнтерит), предшествовавшие началу ПА у 77% пациентов [13]. Часто наблюдалось наличие стрессогенных ситуаций (эмоциональных травм) за 3 мес до манифестации ПА; описаны случаи ее развития после многократных прививок, воздействия химических токсинов, при наступлении менопаузы у женщин [14]. Вместе с тем в достаточно большом проценте случаев пусковой фактор ПА установить не удалось [15].
В связи с отсутствием общепринятого определения ПА, а также ее специфических симптомов и биомаркеров существуют сложности в создании единых диагностических критериев, которые бы обладали высокой чувствительностью и специфичностью [16]. Отсутствие универсального инструмента диагностики (в настоящий момент насчитывается около 25 их различных вариантов) значительно затрудняет научные исследования в этом направлении и ограничивает получение объективных данных о распространенности ПА. Так, при использовании в эпидемиологических исследованиях критериев Центра по контролю и профилактике заболеваний США (CDC, Fukuda K.С. et al., 1994) [17] ПА выявлялась в 5 раз чаще по сравнению с более новыми и строгими международными Критериями международного консенсуса (ICC) или Критериями канадского консенсуса (CCC) [18]. Сравнение основных критериев диагностики ПА приведено в таблице.
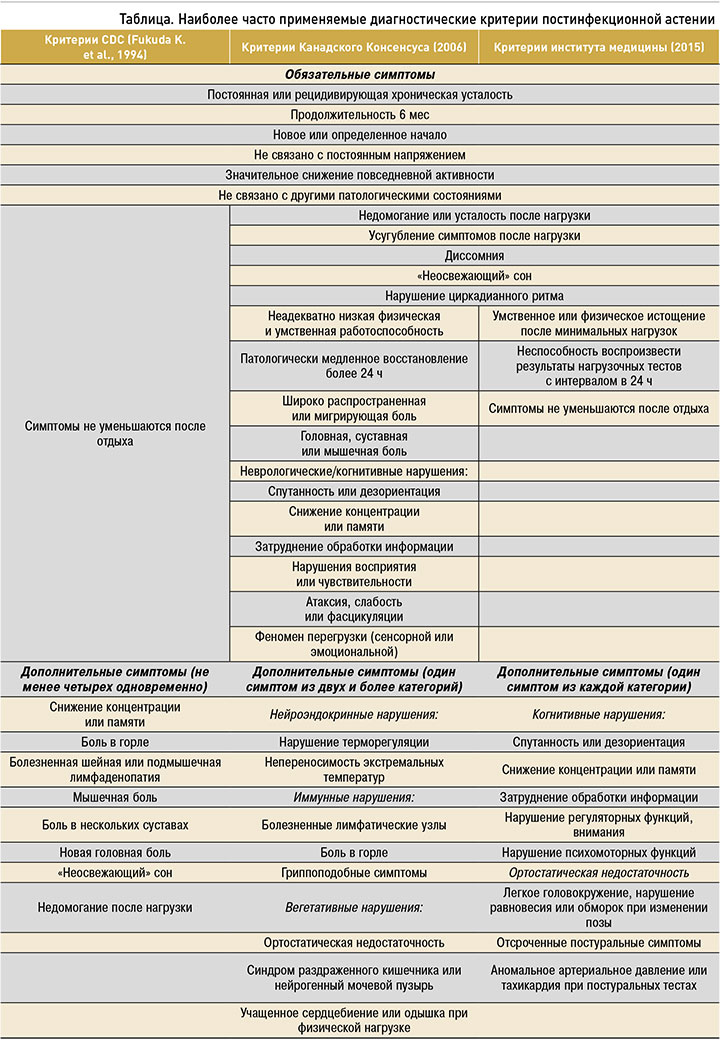
Наиболее современные диагностические критерии Института медицины США (IOM), опубликованные в 2015 г., характеризуют ПА как синдром, состоящий из пяти основных симптомов: усталости, недомогания после нагрузки, когнитивных изменений (расстройств памяти, концентрации, обработки информации), диссомнии (нерегулярного сна, нарушения циркадианного ритма) и ортостатической недостаточности [19]. При этом симптомы не должны регрессировать после отдыха и должны сохраняться более 6 мес при отсутствии каких-либо значимых клинических или лабораторных нарушений [12].
Широкий спектр вторичных симптомов ПА, такие как боль, сенсомоторные нарушения, артралгии, желудочно-кишечные симптомы (тошнота, вздутие живота, синдром раздраженного кишечника), нарушение функций мочевыделительной системы (увеличение частоты, неожиданные позывы к мочеиспусканию), боль в горле и лимфаденопатия (шейная и/или подмышечная), включены в некоторые критерии, но их наличие не является условием для постановки диагноза [19].
Недомогание после нагрузки (постнагрузочная истощаемость) проявляется в виде ухудшения основных симптомов ПА [20] и выступает особенно важной характеристикой ПА, что отличает ее от других хронических заболеваний, таких как фибромиалгия, соматическая депрессия или первичные нарушения сна [21]. Постнагрузочное истощение может провоцироваться не только физической активностью (физическими упражнениями и ортостатическими нагрузками), но и различной когнитивной и эмоциональной деятельностью (когнитивной и эмоциональной истощаемостью) [20].
АСТЕНИЯ ПОСЛЕ ПЕРЕНЕСЕННОЙ ИНФЕКЦИИ
Вспышки заболевания, напоминающего ПА, наблюдались на протяжении всего XX в. после внутрибольничных и эпидемических инфекций [22]. Хотя в ту пору не существовало определенных критериев ПА, описанные в литературе симптомы отдаленного периода (усталость, вялость, недомогание, нарушение сна и снижение концентрации внимания, часто усугубляемые физической нагрузкой или стрессом) позволяют предположить, что и в этих случаях у пациентов развивалось состояние, которое сейчас мы расцениваем как синдром хронической усталости / миалгический энцефаломиелит / синдром ПА [23, 24]. Со временем стали появляться и постепенно дополняться определения понятия ПА, развивались и совершенствовались методы диагностики в микробиологии, что позволило выявить более четкую связь между инфекцией и ПА.
Чаще всего ПА связывают с инфекционным мононуклеозом, вызываемым вирусом Эпштейна–Барр (ВЭБ) [19]. Проспективное исследование 301 подростка, имеющих диагноз ВЭБ-инфекции с положительным Моноспот-тестом через 6 мес от острого периода, выявило ПА у 13% участников [25]. Аналогичные показатели ПА были зарегистрированы и после других герпесвирусных инфекций (вызванных цитомегаловирусом, герпесвирусами человека 6А и 6В) [20], лихорадки Ку и вирусной инфекции реки Росс (12% за 6 мес по данным Критериев CDC, 1994), лихорадки Западного Нила (около 20%) [23], а также после инфекций, вызванных вирусом Эбола, вирусом денге, Borrelia burgdorferi, энтеровирусами, человеческим парвовирусом В19, микоплазмой пневмонии, лямблиями, коксиеллой и некоторыми видами Candida [20]. У пациентов отмечались сходные симптомы ПА независимо от конкретного инфекционного триггера [26].
ПОСТИНФЕКЦИОННАЯ АСТЕНИЯ И ВИРУСНЫЕ ЭПИДЕМИИ
После пандемии гриппа 1918 г. у 40% выживших отмечалась усталость, вялость и нарушение концентрации внимания, выраженность которых увеличивалась после физических нагрузок [23]. Отсроченность развития симптомов, прогрессирующее течение и значительная инвалидизация пациентов позволили расценивать такое состояние как отдельную форму хронического прогрессирующего заболевания, однако тогда оно так и не получило своего названия. Аналогично в 2009 г. в Норвегии был выявлен рост заболеваемости ПА после пандемии гриппа H1N1 [27].
Помимо ассоциации с перенесенным гриппом, была отмечена связь ПА со вспышками коронавирусной инфекции в 2002 и 2012 гг. Так, у больных, перенесших тяжелый острый респираторный синдром (SARS, 2002) или ближневосточный респираторный синдром (MERS, 2012), наблюдались многочисленные стойкие симптомы, включая усталость, неспецифическую диффузную боль во всем теле, «неосвежающий» сон, недомогание после физической нагрузки и когнитивные нарушения. При этом критериям CDC удовлетворяло 27,1% пациентов спустя 41 мес после инфекции SARS [28].
Кроме постоянной усталости, после эпидемий гриппа и коронавируса у многих пациентов отмечались психические и нейрокогнитивные нарушения. Так, после эпидемии испанки в 1918 г. число первичных госпитализаций по поводу психических расстройств увеличилось в 7,2 раза в течение нескольких лет [29]. Кроме того, исследование 37 пациентов с острым респираторным дистресс-синдромом на фоне гриппа H1N1 выявило высокие показатели тревожности (50%) и депрессии (28%) через 1 год после инфекции [30]. Аналогично у пациентов, перенесших SARS или MERS, имела место высокая распространенность депрессии (14,9%) и тревоги (14,8%) по сравнению с показателями в общей популяции (около 7%) [31].
Таким образом, существующие данные свидетельствуют о временной взаимосвязи между вирусными эпидемиями и хроническими постинфекционными симптомами, которые соответствуют критериям ПА.
ВОЗМОЖНЫЕ МЕХАНИЗМЫ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
Учитывая вариабельность бактериальных и вирусных заболеваний, которые могут приводить к развитию ПА, а также схожесть ее клинических проявлений, несмотря на различных возбудителей, можно предположить, что первичный патоген выступает лишь в роли триггера и инициирующего звена дальнейшего патологического каскада [26]. Это подтверждается и тем фактом, что чаще всего симптомы развиваются несколько отсрочено и сохраняются длительное время, т.е. тогда, когда первичного возбудителя уже явно нет в организме, а диагностические тесты не выявляют практически никаких значимых отклонений от нормы [12]. Эти особенности ПА привели к развитию гипотезы, согласно которой в результате воздействия инфекционного «агента» у восприимчивых лиц развиваются стойкие нарушения регуляции основных иммунных, неврологических и метаболических путей с вовлечением множества органов и систем. И, несмотря на то что механизмы ПА до сих пор остаются недостаточно понятными, аутоиммунное поражение, а также центральная и гуморальная дизрегуляции считаются ведущими патогенетическими процессами, лежащими в основе формирования ПА [32].
ИММУННЫЕ И ВОСПАЛИТЕЛЬНЫЕ МЕХАНИЗМЫ РАЗВИТИЯ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
ПА часто рассматривается как воспалительное расстройство, при котором инфекционный патоген, выступая в роли триггера (стрессора), вызывает патологический системный иммунный ответ, сохраняющийся уже после разрешения инфекции [12]. Наряду с инфекционным патогеном в качестве серьезного физиологического стрессора, способного вызвать выраженную воспалительную реакцию, могут выступать многократные прививки и химические токсины [14].
Предполагается, что вирус SARS-CoV-2, как и другие инфекционные агенты, является несомненным триггером ПА. При этом важнейшей его мишенью может быть мозговой центр стресса – паравентрикулярное ядро гипоталамуса (ПВЯ) [33]. Гипоталамическое ПВЯ представляет собой сложный массив ядер и нейрональных цепочек и функционирует в качестве «интегратора» стресса, получая, обрабатывая и отвечая на широкий спектр физиологических реакций, контролируя нейроэндокринный и вегетативный ответ и обеспечивая его регуляцию. Входящие стрессовые сигналы передаются через медиаторы воспаления, такие как цитокины и хемокины, и сходятся на гипоталамическом ПВЯ через гуморальные и нейрональные пути [34]. Различные инфекции, в том числе вызываемая вирусом SARS-CoV-2, за счет длительной провоспалительной активации и постоянной выработки цитокинов и хемокинов могут переключать гипоталамическое ПВЯ в стойкий дисфункциональный режим. В качестве ведущего механизма неадаптивной реакции ПВЯ на различные воспалительные триггеры рассматривается нарушение чувствительности рецепторов с формированием резистентности к молекулам противовоспалительного ответа. В итоге это приводит к дисбалансу активирующих и подавляющих воспаление систем с персистирующими реакциями воспалительной активации. При этом чем более выражена иммуннотропность вируса и чем сильнее активация провоспалительного ответа, тем больше риск дисбаланса иммунного ответа и нарушения работы гипоталамического ПВЯ.
Кроме этого, существует и генетическая детерминированность особенностей иммунного ответа: так, у предрасположенных людей может быть снижен порог стрессовых реакций, вследствие чего даже короткодействующий и не очень интенсивный стрессор может запускать выраженный иммунный ответ и способствовать нарушению физиологической работы центральных регулирующих систем. По-видимому, чувствительность самого гипоталамического ПВЯ также очень вариабельна и зависит от индивидуальных особенностей человека: у кого-то дисфункциональный режим может быть инициирован в условиях минимальной воспалительной активности, а у кого-то, несмотря на грубые нарушения иммунноопосредованного ответа, реакция ядер гипоталамуса будет оставаться адекватной. Поэтому даже относительно легкая инфекция, вызванная SARS-CoV-2, с минимальными проявлениями острого периода способна вызывать у некоторых пациентов достаточно выраженный и интенсивный постковидный синдром с тяжелой астенией. Дополнительно этому может способствовать совокупное действие нескольких стрессоров (помимо самой инфекции в роли стрессора могут выступать психоэмоциональные реакции, различные кардиоваскулярные события и т.д.); каждый из них может быть недостаточен для запуска патофизиологического механизма, однако за счет кумулятивного эффекта при их сочетании достигается необходимый «порог», который и запускает дальнейший патологический каскад. Вероятно, по этой причине часть пациентов затрудняется определить конкретное событие, инициировавшее начало заболевания [14].
Данные, свидетельствующие о системном хроническом воспалении и патологической экспрессии провоспалительных цитокинов у пациентов с ПА, подтверждаются многочисленными исследованиями in vivo и in vitro. Например, у пациентов с синдромом хронической усталости / постинфекционной астении были обнаружены измененные уровни фактора некроза опухоли-альфа, трансформирующего фактора роста бета, интерлейкина-2 (ИЛ-2) и ИЛ-4 по сравнению со здоровыми контрольными группами [35]. Кроме того, в ряде работ показано, что при ПА может отмечаться патологический воспалительный ответ с изменением иммунных клеток. В частности, описан аттенуированный ответ клеток TH1/TH17, нарушение выработки естественных киллеров и Т-регуляторных клеток [24, 36]. Кроме того, повреждение тканей во время острой инфекции располагает к активации аутореактивных фоновых В-клеток и молекулярной мимикрии [32]. Так, при тяжелой инфекции COVID-19 было выявлено значительное повышение уровня антиядерных антител и ревматического фактора как маркеров патологического аутоиммуного ответа [37]. Также при ПА были обнаружены аутоантитела к мускариновым и адренергическим рецепторам, что коррелировало клинически с постуральными ортостатическими симптомами [36].
Интересно, что повышение уровней некоторых провоспалительных цитокинов, таких как интерферон гамма и ИЛ-7, связывают с нарушением функции глимфатической регуляции [38]. Последняя, по сути, представляет собой дренажный механизм, обеспечивающий элиминацию фрагментов отработанных белков, продуктов перекисного окисления липидов и другого метаболического «мусора» из ЦНС. Нарушение клиренса различных молекул и реактивных частиц вследствие активации провоспалительного ответа будет способствовать формированию окислительного стресса и дополнительному риску отсроченной нейродегенерации за счет накопления белков с измененной конформационной структурой. Таким образом, воздействие инфекционного стрессора в этом случае не просто будет выходить за пределы острого периода: отдаленные последствия могут растянуться даже не на недели и месяцы, как, например, в случае лонг-ковида, а на годы и десятилетия [36].
ВОВЛЕЧЕНИЕ ЦЕНТРАЛЬНОЙ НЕРВНОЙ СИСТЕМЫ В РАЗВИТИЕ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
Несколько основных симптомов ПА (нарушение когнитивных функций, нарушение сна) и некоторые дополнительные симптомы (явления сенсорной перегрузки, двигательные симптомы) отражают нарушение функции центральной нервной системы (ЦНС) при ПА [39]. Известно, что многие вирусы, включая некоторые коронавирусы, обладают нейроинвазивным потенциалом и могут вызывать воспалительное повреждение тканей ЦНС [40]. Показано, что при SARS, MERS и COVID-19 повышена угроза церебральной ишемии и микроангиопатии [41]. Согласно данным нейровизуализации, при ПА чаще наблюдаются нарушение мозгового кровотока, структурные изменения коры, очаговое воспаление и изменения функциональных связей по сравнению со здоровой группой контроля [39].
Остается неясным, возникает ли какой-то специфический нейрокогнитивный дефицит в результате прямой инвазии в ЦНС, опосредован ли он аутоиммунными механизмами повреждения или же связан с системным нарушением функции эндотелия, что в том числе отражается и на церебральных сосудах.
В качестве одной из причин когнитивного дефицита в рамках ПА обсуждается распространенный воспалительный ответ, который начинается с «эпицентра» в гипоталамическом ПВЯ и за счет активации микроглии и астроцитов может распространяться на весь гипоталамус и тесно связанную с ним лимбическую систему. Действительно, активированная микроглия и астроциты были обнаружены с помощью позитронно-эмиссионной (ПЭТ) и магнитно-резонансной томографии (МРТ) в лимбической системе (поясной извилине, гиппокампе, миндалине и таламусе) пациентов с ПА в 2014 г. [42]. Трудности запоминания новой информации, выраженная тревога могут отражать непосредственное вовлечение гиппокампа и миндалины в патологический воспалительный процесс. Более того, часть воспалительных цитокинов, обладая непосредственным нейротоксическим действием, могут способствовать нарушению функции основных скоплений нейронов (ядер нейромедиаторных систем) и вызывать значительные нейротрансмиттерные нарушения, которые также создают условия для формирования нейропсихиатрических симптомов. Дисфункция гипоталамуса и лимбической системы, по-видимому, объясняет большинство симптомов ПА [26].
МИТОХОНДРИАЛЬНАЯ ДИСФУНКЦИЯ И УСТАЛОСТЬ ПРИ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
Усталость выступает главным симптомом как ПА, так и первичных митохондриальных расстройств. Это обусловило большой объем исследований, посвященных изучению связи функций митохондрий и ПА [43].
Изменения структуры, метаболизма и синтеза энергии в митохондриях в мышечных клетках могут быть связаны с усталостью и недомоганием после физических нагрузок при ПА [32]. Так, исследование образцов мышечной биопсии 50 пациентов с ПА выявило выраженные структурные нарушения и митохондриальную дегенерацию в 80% случаев по сравнению с небольшими структурными изменениями у 52% здоровых лиц из группы контроля [44]. Наряду с этим при ПА обнаруживаются изменения уровня ферментов, связанных с окислением (оксид азота, активные формы кислорода), метаболизмом жирных кислот и синтезом энергии [45]. Принимая во внимание их участие в периферической вазодилатации и вегетативной регуляции сердечно-сосудистой системы, можно предположить, что нарушение функции митохондрий может быть одним из механизмов развития ортостатической нетолерантности и недомогания после физических нагрузок [46].
Несмотря на то что к настоящему моменту явно недостаточно данных, чтобы классифицировать ПА как митохондриальное расстройство, нарушение энергетических и метаболических процессов в рамках данного состояния не вызывает сомнений.
COVID-19 И ПОСТИНФЕКЦИОННАЯ АСТЕНИЯ
Постковидный синдром представляет собой персистирующее состояние после перенесенной инфекции COVID-19, длящееся не менее 3 нед от начала заболевания. При этом некоторые пациенты сообщают о сохранении симптомов дольше чем в течение 4 мес [16]. Симптомы постковидного синдрома включают усталость (75%), недомогание после нагрузки (69%), когнитивные нарушения (52%), расстройства сна (26%), тревогу или депрессию (23%), одышку в покое и при движении, головную боль напряжения, мигренеподобную головную боль, нарушение циркадианных ритмов, прогрессирующую ангедонию, диарею, тахикардию, нарушения зрения, агевзию или гипогевзию, аносмию или гипосмию, кашель, головокружение, боль в суставах, миалгию, лихорадку [47]. Пациенты могут предъявлять жалобы на нехватку энергии, «туман» в голове, снижение повседневной активности, выраженную усталость и ослабление когнитивных функций, которые усугубляются при физической активности или стрессе [48].
Хотя перечисленные симптомы аналогичны тем, которые наблюдаются при ПА, данные, указывающие на COVID-19 как инфекционный триггер ПА, ограниченны. Распространенность и средняя длительность симптомов после острой COVID-19 в настоящее время изучаются. Имеются сообщения о наличии у 75% пациентов по крайней мере одного постоянного симптома в течение 7–12 нед после перенесенного COVID-19 [49]. При этом усталость, одышка и аносмия относятся к наиболее часто регистрируемым симптомам, длящимся более 3 нед [50].
В ряду многочисленных симптомов постковидного синдрома усталость является одним из наиболее стойких и обременительных. Так, в крупном проспективном когортном исследовании 1733 пациентов с COVID-19 усталость/миалгия были выявлены в 63% случаев спустя 6 мес после выздоровления [51]. В свою очередь Rudroff T. et al. (2020) определяют постковидную усталость как снижение физической и/или умственной работоспособности, которое возникает из-за изменений центральных, психологических и/или периферических факторов, вызванных инфекцией COVID- 19. Авторы предложили модель для объяснения потенциальных факторов, способствующих развитию усталости после перенесенного COVID- 19. Согласно этой модели усталость зависит от условных и физиологических факторов: первые включают работу, окружающую среду, физические и умственные способности пациентов, ко вторым относят центральные, психологические и периферические составляющие [52].
ЦЕНТРАЛЬНЫЕ НЕВРОЛОГИЧЕСКИЕ И ПСИХОЛОГИЧЕСКИЕ ФАКТОРЫ РАЗВИТИЯ ПОСТИНФЕКЦИОННОЙ АСТЕНИИ
На сегодняшний день неизвестно, является ли COVID-19 нейроинвазивной инфекцией. Однако предполагается, что другие аналогичные коронавирусы человека проникают в ЦНС через гематоэнцефалический барьер (ГЭБ), а также посредством нейрональной диссеминации. Таким образом, центральные факторы, влияющие на постковидную усталость, могут быть результатом проникновения вируса в ЦНС [53]. Нарушение нейротрансмиттерных систем (дофамина, серотонина и ацетилхолина) [54], изменение возбудимости нейронов, воспалительный процесс, демиелинизация со снижением скорости распространения импульса по аксонам являются наиболее частыми патологическими процессами, обсуждаемыми в аспекте постковидной усталости [52].
Участие центральных факторов в развитии астении после COVID-19 подтверждается данными нейровизуализации. Так, Delorme C. et al. (2020) использовали ПЭТ с введением 18F-фтордезоксиглюкозы (18F-ФДГ) для измерения метаболизма глюкозы в мозге у пациентов с постковидной усталостью. Они обнаружили гипометаболизм в лобных долях и гиперметаболизм в мозжечке, которые коррелировали со степенью утомляемости и нетолерантности к нагрузкам [55].
В качестве дополнительного условно «центрального» фактора ПА можно назвать нарушение возбудимости мотонейронов, которое, скорее всего, становится следствием значительного ограничения физической активности в период длительных локдаунов [56]. Непосредственное вовлечение мотонейрона, а также показанное в ряде работ снижение проводимости двигательных единиц в результате инфицирования SARS-CoV-2 может быть еще одним источником усталости и астенических расстройств [57].
У многих пациентов постковидная усталость развивалась в обстановке стресса, тревоги, депрессии и страха. Многие меры, используемые для борьбы с пандемией, такие как карантин, социальная дистанция и изоляция, показали свою эффективность в замедлении распространения вируса, однако имели негативные психологические последствия, включая симптомы посттравматического стресса, раздражительность, тревогу, спутанность и депрессию у выздоравливающих после COVID-19 пациентов. В совокупности эти психологические факторы могут служить весомым фактором развития усталости [58].
ПЕРИФЕРИЧЕСКИЕ И СРЕДОВЫЕ ФАКТОРЫ
Постковидная усталость также может быть связана с поражением скелетных мышц путем прямого заражения резидентных типов клеток, богатых ангиотензинпревращающим ферментом (АПФ) 2-го типа [59], и/или косвенно в результате высвобождения цитокинов, включая ИЛ-6. Системное повышение уровня ИЛ-6 может нарушить метаболический гомеостаз мышц, усугубив потерю мышечной массы [60]. Боль и слабость скелетных мышц являются главными периферическими факторами развития постковидной усталости.
Средовыми факторами ПА считаются работа, окружающая среда, физические и умственные способности пациентов. Предполагается, что на усталость оказывает влияние тип выполняемых пациентом когнитивных и двигательных задач на своей работе. Факторы окружающей среды, например температура и влажность, также могут сказываться на физических способностях пациентов [61]. Кроме того, к развитию усталости может привести переживание тревоги и беспокойства по поводу пандемии при одновременном отсутствии физической активности в условиях карантина [62]. Средовые факторы оказывают воздействие на физиологические факторы (центральные, психологические и периферические), способствуя таким образом развитию постковидной усталости.
Однако одного только наличия хронической усталости недостаточно для диагностики у пациента ПА, которая является более комплексным и мультисистемным заболеванием. Работ по изучению частоты встречаемости ПА после COVID-19 с учетом современных критериев и подходов к диагностике не так много.
Исследователи из Германии Kedor C. et al. (2021) обследовали 42 пациента, перенесших COVID- 19, в возрасте от 22 до 62 лет, у которых спустя 6 мес после первоначального инфицирования SARS-CoV-2 наблюдалась хроническая усталость. Критериями включения в данное исследование стало легкое или среднетяжелое течение COVID- 19, т.е. было исключено влияние на хроническую усталость симптомов, длительно сохраняющихся в тяжелых случаях новой коронавирусной инфекции. У 41 из 42 пациентов отмечалось также недомогание после физической нагрузки, у 40 – когнитивные нарушения, у 38 – головная боль, у 35 – мышечная боль. С помощью критериев Канадского консенсуса (2003) авторы диагностировали ПА у 19 из 42 пациентов выборки. У остальных 23 участников было выявлено большое количество симптомов из этих Критериев, однако они были недостаточно выражены для диагностики ПА (к примеру, длительность восстановления после физической нагрузки составляла не 14, а 2–10 ч). В этом случае авторы вместо термина «постинфекционная астения» предложили использовать «хронический постковидный синдром» [63]. В роли факторов риска постковидного синдрома обсуждаются сопутствующие хронические заболевания, возраст, тяжесть перенесенной коронавирусной инфекции и женский пол [51].
Fernandez-de-las-Penas С. et al. (2021) исследовали данные 1100 пациентов, перенесших COVID- 19, и установили, что астения значительно чаще развивалась и сохранялась на протяжении 7 мес у больных, имевших головную боль в остром периоде заболевания, по сравнению с теми, у кого головная боль отсутствовала [47]. Наличие связи постковидного синдрома с другими возможными факторами риска, включая этническую принадлежность, психическое состояние, количество сопутствующих заболеваний или ожирение, во многих исследованиях обнаружено не было [64].
Как и в случае ПА, при постковидном синдроме не было обнаружено каких-либо специфических биомаркеров (общий анализ крови, количество лимфоцитов, количество нейтрофилов, количество моноцитов, D-димер, С-реактивный белок, лактатдегидрогеназа, интерлейкин-6, CD-25, функциональные тесты печени или креатинин) [65], однако исследования данного состояния только начинают набирать обороты.
ПОДХОД К ЛЕЧЕНИЮ
Лечение пациентов с ПА, как правило, строится на междисциплинарной терапии отдельных симптомов специалистами узкого профиля. Необходимо проводить дополнительные исследования пациентов для исключения других органических причин этих симптомов. Больным с постковидным синдромом следует выполнять визуализацию легких, а также скрининг и лечение сопутствующих психических заболеваний [2].
Daynes E. et al. (2021) показали выраженный положительный эффект комплексной реабилитационной программы у 32 пациентов с постковидным синдромом, включающим хроническую усталость и длящимся более 3 мес. По мнению авторов, при подборе программы реабилитации больным с хронической усталостью необходимо учитывать все симптомы постковидного синдрома. В проведенном исследовании реабилитационная программа длилась 6 нед и состояла из аэробных упражнений (прогулка, беговая дорожка), силовых тренировок верхних и нижних конечностей, а также образовательных дискуссий на основе интернет-ресурсов, в ходе которых с пациентами обсуждались такие темы, как одышка, кашель, усталость, страх и тревога, память и концентрация внимания, вкусы и запахи, правильное питание, возобновление двигательной активности, нормализация сна, выполнение повседневных дел и возвращение к работе [66]. Авторы не исключали, что реабилитационная программа, в частности физические упражнения, может усугубить постинфекционную астению. Однако в процессе такой реабилитационной программы никаких серьезных побочных явлений не было отмечено. У большинства пациентов уменьшилась выраженность усталости по Функциональной оценочной шкале усталости при лечении заболеваний (FACIT), увеличилась выносливость при физических нагрузках, уменьшилась одышка и улучшились когнитивные функции. Таким образом, согласно выводам исследователей, программы реабилитации должны быть направлены на обеспечение междисциплинарного подхода к ведению пациентов с постковидным синдромом. При этом лечащему врачу следует стремиться к индивидуализации программ и своевременно выявлять побочные явления и симптомы [66].
Для ускорения регресса симптомов постковидной астении Ferraro F. et al. (2020) предложили реабилитационную программу из комплекса физических упражнений, индивидуально скорректированных для каждого пациента. Реабилитационные мероприятия проводились ежедневно, 1–2 раза в сутки, по 30 мин каждое, в течение 6 нед; физические упражнения проводились с постепенным увеличением интенсивности нагрузки. Они включали: 1) изменение позы (положение лежа, сидя или полувертикально) для улучшения системной оксигенации; 2) контроль дыхания с привлечением диафрагмы и упражнениями для координации грудной клетки и живота; 3) пассивную мобилизацию верхних и нижних конечностей; 4) пассивное растяжение мышц; 5) упражнения для укрепления мышц верхних и нижних конечностей, туловища и ягодичных мышц, нацеленные на достижение контроля положения сидя и стоя; 6) упражнения на равновесие и координацию (например, в положении на одной ноге, упражнение «пятка–носок», тренировка ходьбы с увеличением дистанции). В выполненном авторами исследовании с участием 7 пациентов упражнения привели к выраженному эффекту с регрессом симптомов постковидной астении [67].
Современные рекомендации Национального института здравоохранения и медицинской помощи Великобритании (NICE) оговаривают использование ступенчатой лечебной физкультуры и когнитивно-поведенческой терапии у пациентов, перенесших COVID-19 [68].
В настоящий момент существует достаточно большое количество препаратов с заявленным антиастеническим эффектом. Однако в комплексных реабилитационных программах пациентов с ПА, в том числе перенесших COVID-19, следует использовать лишь средства с доказанным направленным противоастеническим действием. К числу таковых относится препарат Энерион [69], который представляет собой синтетическое производное тиамина – сульбутиамин. Обладая липофильными свойствами, Энерион лучше проникает через ГЭБ, позволяя увеличивать уровень тиамина и его производных в головном мозге за более короткий период, чем при использовании водорастворимых форм [70].
В неврологии тиамин наиболее часто применяется при тиамин-дефицитных состояниях – энцефалопатии Гая–Вернике и Корсаковском амнестическом синдроме. В той или иной степени у каждого из пациентов с этими заболеваниями нарушаются внимание и память, появляются аффективные симптомы, нарушение циклов сна и бодрствования, расстройства движения. В патогенезе их клинических проявлений лежат сложные нейромедиаторные расстройства с наибольшим вовлечением серотонинергических и холинергических систем, тиамин-зависимых процессов, а также нарушением функции ядер таламуса, сосцевидных тел, мозжечка и других структур головного мозга [70]. Как можно более раннее назначение и использование достаточных доз тиамина у этой группы пациентов позволяет как радикально восстановить, так и частично улучшить состояние.
Спектр психоневрологических нарушений, возникающих у лиц, перенесших COVID-19, позволяет провести аналогию с тиамин-дефицитными состояниями. Вероятно, в основе развития так называемой постковидной астении также лежит дисбаланс нейромедиаторных процессов с нарушением работы тиамин-зависимых релейных структур головного мозга. Назначение препаратов, имеющих патогенетический механизм действия (Энерион), в отличие от средств с общими стимулирующими свойствами, обоснованно позволяет ожидать более выраженного терапевтического эффекта.
Положительное действие Энериона было продемонстрировано у пациентов с хронической усталостью, постинфекционной астенией, а также у лиц, перенесших COVID-19. Так, 65% пациентов с психовегетативным синдромом и выраженными астеническими симптомами отметили значительное улучшение состояния уже через 1 нед терапии этим препаратом (в режиме 2 табл./сут). После окончания полного курса положительная динамика была отмечена у всех пациентов, а 75% прямо отметили высокую эффективность препарата. При этом чем раньше назначался Энерион, тем быстрее удавалось достичь клинического эффекта [71].
В качестве одного из механизмов антиастенического действия сульбутиамина обсуждается его непосредственное влияние на цикл Кребса, позволяющее улучшить энергообмен в митохондриях. Кроме того, Энерион способствует коррекции нейротрансмиттерных нарушений: он улучшает серотонинергическую передачу, тем самым уменьшая выраженность аффективных расстройств и нарушений циркадных ритмов, необходимых для нормального функционирования всех жизненных процессов в организме, а также работы цикла Кребса. При этом действие Энериона направлено на активизацию серотонинергической активности, что дополнительно способствует восстановлению циркадных ритмов [72].
В целом использование препарата Энерион у лиц с астеническим синдромом показало достаточно быстрый положительный результат, что определяет целесообразность его применения при астении как органического, так и функционального генеза [73], особенно у пациентов, переболевших COVID-19. Хорошая переносимость препарата позволяет назначать его как в рамках монотерапии, так и в комбинации с другими лекарственными средствами для повышения эффективности проводимого лечения.



