ВВЕДЕНИЕ
Пандемия новой коронавирусной инфекции (COVID-19) стала глобальным вызовом для мирового здравоохранения и показала масштабы социальных и экономическим последствий, связанных с резким ростом заболеваемости острыми респираторными инфекциями (ОРИ) в популяции [1, 2]. Несмотря на текущую распространенность COVID- 19, острые инфекции верхних и нижних дыхательных путей некоронавирусной этиологии остаются одной наиболее частых причин обращения за медицинской помощью [3]. ОРИ занимают первое место в мире по причинам временной утраты трудоспособности. В России до 90% страховых выплат по временной нетрудоспособности от общего числа всей заболеваемости приходится именно на долю ОРИ [4–6]. Ряд аналитических исследований свидетельствует не только о значительных экономических потерях, сопряженных с ОРИ, но и негативном их влиянии на состояние и прогноз здоровья работающего населения страны [4, 6].
Острый бронхит (ОБ) – одно из самых распространенных заболеваний, встречающихся в амбулаторной клинической практике. Согласно оценкам Центра по контролю и профилактике заболеваний США (CDC), до начала пандемии COVID-19 на долю ОБ приходилось около 10% приемов врача общей практики, или порядка 100 млн посещений в год [6]. Заболеваемость ОБ особенно высока поздней осенью и зимой, когда наблюдается пик передачи респираторных вирусов.
Наиболее часто ОБ возникает на фоне острой вирусной инфекции верхних дыхательных путей и реже представлен изолированным поражением бронхов. Распространенными возбудителями ОБ выступают респираторно-синцитиальный вирус, риновирус, вирусы парагриппа, гриппа А и В [7, 8]. Бактериальные ОБ, как правило, вызваны патогенами, характерными для внебольничное пневмонии, – пневмококком и гемофильной палочкой [9]. Среди факторов риска развития ОБ выделяют курение, значительное загрязнение воздуха, проживание на территории с высокой плотностью населения, бронхиальную астму с атопией, в случае которой триггером для развития заболевания могут служить аллергены и поллютанты окружающей среды [10].
Подобно новой коронавирусной инфекции COVID-19, большая часть пациентов переносит ОБ в легкой или умеренной форме, тогда как пожилые пациенты с одним или несколькими факторами риска имеют высокую вероятность тяжелого течения болезни [11, 12]. В частности, пациенты с бронхиальной астмой или хронической обструктивной болезнью легких (ХОБЛ) представляют отдельную группу больных, у которых дифференцировать обострение подлежащей патологии и первичный ОБ крайне затруднительно; это диктует необходимость проведения как адекватной терапии обострения хронического респираторного заболевания, так и купирования симптомов ОБ [11–13]. Разработка оптимальных алгоритмов профилактики и лечения ОБ остается в сфере интересов широкого круга специалистов, включая врачей-терапевтов, пульмонологов и инфекционистов [12]. У большинства пациентов с ОБ симптомы проходят самостоятельно в течение 1–3 нед. При этом симптоматическая и патогенетическая терапии остаются краеугольными камнями в лечении этого заболевания [13–15].
Инвазия и активность вирусного или бактериального патогена способствует развитию острого воспаления в стенке бронхов, что приводит к утолщению слизистой оболочки, десквамации эпителия и повреждению базальной мембраны. В связи с этим клиническая картина ОБ представлена преимущественно результатом продуктивного воспаления в виде скопления мокроты и развитием кашля – ведущего симптома ОБ, часто сопровождаемого затрудненным дыханием и хрипами [7, 11, 12].
Хотя острый кашель и считается самостоятельно разрешающимся симптомом, он в значительной степени ухудшает качество жизни пациентов с ОБ и может сохраняться в среднем до 25 дней [15]. Безрецептурные препараты от кашля служат средствами первой помощи и достаточно часто принимаются пациентами без назначений врача. Такие средства различаются по механизму действия в зависимости от активных компонентов и включают противокашлевые, антигистаминные и мукоактивные препараты. В отсутствие возможности этиотропной терапии ключевым моментом в лечении вирусного ОБ является назначение лекарственных средств, обладающих мукоактивным свойствами. Мукоактивные препараты изменяют вязкость слизи, усиливают мукоцилиарный клиренс и могут быть разделены на подгруппы в соответствии с механизмом действия: отхаркивающие средства, мукорегуляторы, муколитики и мукокинетики [16].
Амброксол относится к группе мукоактивных лекарственных средств и широко используется для лечения острых и хронических респираторных заболеваний с конца 1978 г. [17]. Являясь производным бромгексина, он обладает мукокинетическим и мукоцилиарным действием, что было показано в клинических исследованиях с участием взрослых пациентов с респираторными заболеваниями [18, 19]. Структурные модификации придали амброксолу дополнительные фармакологические свойства, включая стимуляцию выработки сурфактанта, противовоспалительную активность, антиоксидантное действие, а также местный анальгезирующий эффект, в связи с чем этот препарат в форме пастилок или спреев для полости рта также показан для симптоматического лечения фарингита или острой боли в горле при ОРИ верхних дыхательных путей [16, 18].
Цель настоящего обзора – провести анализ клинических данных по применению амброксола в реальной медицинской практике у пациентов с ОБ.
ДЕЙСТВИЕ АМБРОКСОЛА НА ПАТОФИЗИОЛОГИЧЕСКИЕ МЕХАНИЗМЫ РАЗВИТИЯ ОСТРОГО БРОНХИТА
Основной механизм действия амброксола включает стимуляцию синтеза сурфактанта, что способствует очищению слизистых оболочек дыхательных путей, улучшению отхождения мокроты и облегчению продуктивного кашля. Сурфактант уменьшает вязкость слизистой за счет деполимеризации кислых полисахаридных волокон в бронхиальном секрете и стимуляции выработки нейтральных полисахаридов железистыми клетками, что влечет за собой разделение слоев слизистого секрета в дыхательных путях и уменьшение адгезии слизи к эпителию дыхательных путей [17, 18]. Экспериментальные модели на животных показывают способность амброксола усиливать мукоцилиарный клиренс путем увеличения частоты биения ресничек мерцательного эпителия бронхов [19, 20].
Противокашлевое действие амброксола реализуется предположительно через ряд взаимосвязанных процессов, которые включают утилизацию реактивных форм кислорода, ингибирование выработки провоспалительных медиаторов лейкоцитами и блокаду натриевых каналов [19–21]. Последнее считается одним из возможных механизмов, обеспечивающих местноанестезирующее действие препарата с антитуссивным эффектом. Также установлен защитный эффект амброксола против вирусных и бактериальных инфекций дыхательных путей, который связан с повышением выработки сурфактант-специфичных белков, иммуноглобулинов A и G, способных подавлять вирусную репликацию, и формированием бактериальной биопленки [22–24].
КЛИНИЧЕСКАЯ ЭФФЕКТИВНОСТЬ АМБРОКСОЛА
Первоначальные клинические исследования по эффективности и безопасности применения амброксола были проведены рамках профилактики и лечения больных с обострением ХОБЛ. Позднее были представлены результаты наиболее крупного рандомизированного двойного слепого плацебо-контролируемого клинического исследования, изучавшего эффективность и переносимость амброксола у взрослых пациентов с ОБ и объемом форсированного выдоха за первую секунду (ОФВ1) >75% [25]. Данное исследование включало четыре когорты пациентов, рандомизированных в соотношении 1:1:1:1 для приема фитотерапевтического экстракта, цефуроксима, амброксола или плацебо в течение 14 дней. Конечные точки исследования включали оценку доли респондентов в каждой группе, ОФВ1, данные о ночном кашле и приступах кашля в течение дня. Частота клинических ответов через 2 нед составила 63,4% в группе плацебо, 88,2% – в группе фитотерапии, 83,6% – в группе цефуроксима и 82,2% – в группе амброксола. По сравнению с плацебо в группе амброксола меньшая доля пациентов испытывала приступы кашля по ночам (около 70 против 50%) и в дневное время (50 против 30%). Данных в пользу бронхоконстрикции или рецидива во всех группах лечения через 2 нед получено не было [25].
Превосходство амброксола и других активных препаратов по сравнению с плацебо при незначительной разнице между активными методами лечения было подтверждено по всем дальнейшим критериям оценки, таким как жалобы и симптомы ОБ, отсутствие рецидива, общее самочувствие больных. Авторы указывают на более быстрое и полное разрешение ОБ у пациентов, принимавших амброксол. В дальнейшем два наблюдательных когортных исследования подтвердили эффективность этого лекарственного средства в реальной клинической практике по лечению острого кашля и респираторных симптомов [26, 27].
Фармацевтическое исследование Kardos P. et al. было выполнено в аптечных сетях Германии и включало анонимный опрос среди людей, самостоятельно принимавших амброксол в одной из 4 лекарственных форм (капсулы ретард, сироп для взрослых, сироп для детей, мягкие пастилки). 965 участников заполнили опросник для оценки шкалы тяжести бронхита, степени ухудшения самочувствия из-за острого кашля, времени до начала облегчения симптомов и общей продолжительности лечения. Временной интервал до начала облегчения симптомов находился в пределах 1–15 мин у 12,2% пациентов, 15–30 мин – у 38,4%, 30–60 мин – у 37,2% и >60 мин – у 12,1%. При использовании всех лекарственных форм амброксола оценка по шкале тяжести бронхита на момент окончания лечения снизилась в среднем на 5,5 баллов (59%). Максимальное улучшение было зарегистрировано в отношении боли в груди при кашле (-1,2; 75%), хрипов (-1,2; 71%), одышки (-0,9; 69%) и кашля (-1,6; 57%), тогда как жалобы на отделение мокроты снизились меньше всего (-0,8; 40%) [27].
Также у пациентов заметно уменьшалась частота ночных и дневных приступов кашля. Среди всех участников после лечения 82,1% сообщили о 0–2 эпизодах кашля в час, 15,0% – о 3–4 и только 2,9% – о более 4. Примечательно, что 21,2% участников с исходной частотой кашля >4 эпизодов в час к концу лечения имели 3–4 таких эпизода и 74,3% – 0–2. Аналогично, у 87,5% участников с исходными 3–4 приступами кашлями в час частота кашля снизилась до 0–2 эпизодов. Количество ночных пробуждений из-за кашля также заметно уменьшалось при применении всех форм амброксола: у 31,6% пациентов с исходной частотой пробуждений ≥4 за ночь этот показатель снизился до 2, у 33,0% – до 1 и у 23,1% – до 0. В свою очередь, у 15,8% пациентов с исходной частотой пробуждений от кашля 3 раза за ночь эта частота уменьшилась до 2, у 56,4% – до 1 и у 23,9% – до 0 пробуждений. Сдвиг частоты дневного кашля и ночных пробуждений был сопоставим для всех форм амброксола [27].
В целом лечение амброксолом значимо улучшало общее состояние пациентов, связанное с симптомами ОБ. Так, у 51,4% участников исследования, сообщивших до начала лечения о нарушении способности засыпать из-за кашля, по окончании терапии таких нарушений не было. Аналогичным образом 45,1% пациентов, изначально жаловавшихся на полное снижение работоспособности и повышенную утомляемость, 55,1% пациентов, исходно указавших на полное снижение способности к концентрации внимания, а также 60% пациентов, сообщивших о полном снижении способности к выполнению повседневных задач, после лечения не имели указанных расстройств. При этом авторы исследования констатировали равноценную эффективность всех лекарственных форм амброксола в рамках симптоматической терапии ОБ, в частности в отношении кашля и боли в грудной клетке [27].
В другом когортном исследовании пациенты, принимавшие амброксол, заполнили анкету, в которой предоставили данные о переносимости, самооценке эффективности и характере использования этого препарата. Оценке подлежали 2664 анкеты, заполненные пациентами в возрасте 12–95 лет [26]. В целом участники соблюдали рекомендованную дозировку и способ применения амброксола – только 0,7% превысили максимальную суточную дозу [26]. До 92% пациентов субъективно оценили эффективность амброксола как «очень хорошую» (29%) или «хорошую» (63%) [26].
ПРИМЕНЕНИЕ АМБРОКСОЛА ПРИ COVID-19
Несмотря на отсутствие прямых доказательства эффективности применения амброксола при COVID-19, ряд зарубежных авторов приводят данные о его потенциальной противовирусной активности в отношении SARS-CoV-2 и возможных точках приложения для терапии COVID-19 в целом. В качестве одного из вариантов рассматривается совместное применение экзогенного легочного сурфактанта и амброксола: оно может обеспечить синергетический эффект, обусловленный пополнением и покрытием эпителия сурфактантом, а также компенсацией различных нарушений синтеза сурфактанта, его рециркуляцией и снижением ROS-индуцированного окислительного стресса под влиянием амброксола. Авторы указывают на значительный потенциал использования такого подхода в реальной клинической практике и рекомендуют рассмотреть его как объект для скорейшего клинического исследования, посвященного лечению COVID-19 [28, 29].
На текущий момент доступные данные по применению амброксола при COVID-19 представлены преимущественно результатами исследований in vitro клеточных линий, инфицированных SARS- CoV- 2. В исследовании Bradfute S.B. et al. комбинация амброксола и ципрофлоксацина показала мощную ингибиторную активность против SARS-CoV-2 в концентрациях, которые являются клинически значимыми и находятся в пределах известных профилей безопасности этих лекарств для человека. В связи с этим они могут стать потенциальным объектами для дальнейших доклинических и клинических исследований, связанных с COVID-19 [30].
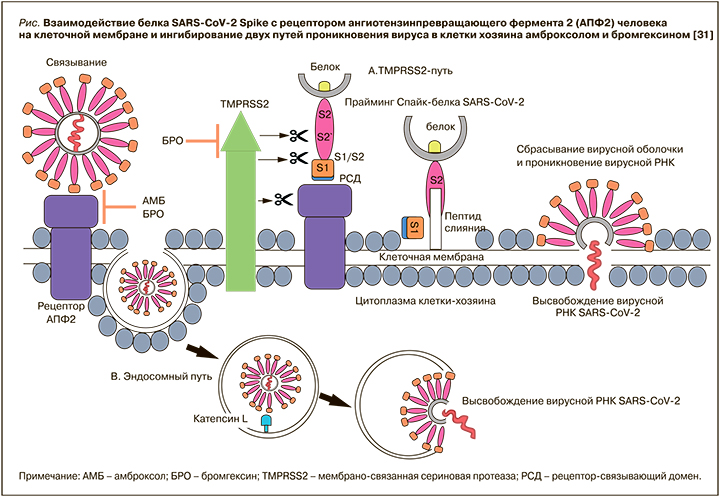
Имеются предварительные данные о влиянии амброксола и его предшественника бромгексина на взаимодействие между доменом связывания рецептора S-белка SARS-CoV-2 и рецептором ангиотензинпревращающего фермента 2 (рАПФ2) [31]. Так, в работе Olaleye O.A. et al. было установлено, что оба этих лекарственных средства ингибировали вызванный инфекцией SARS-CoV-2 цитопатический эффект в микромолярных концентрациях. Также впервые было доложено о способности амброксола воздействовать и модулировать еще одно ключевое белок-белковое взаимодействие, необходимое для двух известных путей проникновения SARS-CoV-2 в клетки хозяина (рис.) [31]. Эффективность, благоприятный фармакологический профиль безопасности и доступность делает амброксол перспективным кандидатом для использования в качестве возможного варианта профилактики и/или лечения COVID-19.
Весьма интересны и результаты исследования Carpinteiro А. et al., в котором оценивалась способность к инвазии псевдовирусных частиц SARS- CoV-2 (pp-VSV-SARS-CoV-2), имитирующих инвазию этого вируса в клетках эпителия носовой полости у здоровых людей, до и после введения аэрозольной формы амброксола. Ингаляция амброксола снизила активность кислой сфингомиелиназы в эпителиоцитах, а также предотвратила вызванную шипом pp-VSV-SARS-CoV-2 активацию кислой сфингомиелиназы, высвобождение церамида и проникновение псевдочастиц pp-VSV-SARS- CoV-2 ex vivo [32]. Таким образом, амброксол обладает рядом свойств ингибитора вирусной инвазии и может быть рассмотрен как фармакологическая опция для клинических исследований, посвященных профилактике COVID-19.
ЗАКЛЮЧЕНИЕ
Проведенный анализ различных исследований предоставляет сравнительно убедительные данные об эффективности амброксола в качестве компонента симптоматической и патогенетической терапии ОБ. Вследствие многонаправленного механизма действия амброксол может рассматриваться и как потенциальный фармакологический агент для профилактики и лечения COVID-19. При этом необходимы дальнейшие клинические исследования для подтверждения эффективности амброксола в различных клинических ситуациях и анализ результатов исходов пациентов в рамках реальной клинической практики.



