ВВЕДЕНИЕ
В условиях пандемии новой коронавирусной инфекции (COVID-19), унесшей жизни более 6 млн человек [1], вакцинация показала высокую эффективность в снижении частоты госпитализации и смертности у вакцинированных, в отличие от невакцинированных [2–8].
Согласно ряду исследований, пациенты, вакцинированные от COVID-19, характеризуются более легким течением коронавирусной инфекции [9–14]. По данным Abhilash K. et al. [9], полная вакцинация значительно снижает частоту госпитализации (62,9 против 11,9%, р <0,001) и потребность в интенсивной терапии в реанимационном отделении (17,7 против 1%, р <0,001).
При этом в доступной нам литературе не найдено данных комплексной оценки течения COVID-19-ассоциированной пневмонии у пациентов, вакцинированных от COVID-19.
Цель исследования – комплексная клинико-инструментальная оценка течения COVID-19-ассоциированной пневмонии у пациентов, вакцинированных от COVID-19.
МАТЕРИАЛЫ И МЕТОДЫ
В проспективное одноцентровое исследование было включено 220 пациентов с COVID- 19-ассоциированной вирусной пневмонией (ПЦР COVID-19 «+»). В соответствии с дизайном исследования пациенты были разделены на 2 группы:
- основная группа (I) – пациенты, вакцинированные от COVID-19 (n=110, в том числе 57 мужчин; средний возраст 64,6±12,0 лет). Применявшиеся вакцины – «Спутник V» (n=78), «ЭпиВакКорона» (n=16), «КовиВак» (n=16);
- группа сравнения (II) – пациенты, не вакцинированные от COVID-19 (n=110, в том числе 56 мужчин; средний возраст 64,8±12,8 лет).
Критерии включения в исследование
1. Пациенты с COVID-19-ассоциированной пневмонией, подтвержденной мультиспиральной компьютерной томографией органов грудной клетки (МСКТ ОГК).
2. Развитие симптомов COVID-19 не ранее чем на 14±3-й день после полной вакцинации.
3. Подписанное информированное согласие.
Критерии исключения
1. Наличие вирусно-бактериальной пневмонии на момент госпитализации.
2. Развитие симптомов COVID-19 между введением двух компонентов вакцины (для «Спутник V» и «ЭпиВакКорона»).
3. Наличие тяжелой коморбидной патологии: состояния иммунодефицита (ВИЧ, иммуносупрессия в связи с трансплантацией органов и т.д.), онкологического заболевания в стадии обострения, выраженного неврологического дефицита.
Для оценки клинического статуса пациентов применялись шкалы NEWS2 и ШОКС-КОВИД. У всех участников проводились лабораторные исследования: общеклинический анализ крови, определение уровня маркеров синдрома системной воспалительной реакции (С-реактивного белка, ферритина, фибриногена), лактатдегидрогеназы (ЛДГ), аспартатаминотрансферазы (АСТ), аланинаминотрансферазы (АЛТ), Д-димера, иммуноглобулинов M и G COVID-19 (IgM и IgG COVID-19). Выполнялась МСКТ ОГК с определением объема поражения легочной паренхимы при помощи программного обеспечения MULTI-VOX.
Оценка клинических, лабораторных и инструментальных данных пациентов обеих групп проводилась дважды: на 10±3-й день от начала симптомов (группы I10, II10) и на 17±3-й день от начала симптомов (группы I17, II17).
Пациенты получали комбинированную лекарственную терапию в соответствии с Временными методическими рекомендациями Минздрава России «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Версия 14» [15].
Проведение исследования было одобрено Локальным этическим комитетом ФГАОУ ВО «РНИМУ им. Н.И. Пирогова» Минздрава России, протокол № 212 от 22.11.2021.
Статистическая обработка полученных данных осуществлялась с помощью пакета IBM SPSS 26 для Windows (США). Количественные показатели оценивались на предмет соответствия нормальному распределению с помощью критерия Шапиро–Уилка или критерия Колмогорова–Смирнова. Количественные показатели, имеющие нормальное распределение, описывались в виде средних арифметических величин (M) и стандартных отклонений (SD); в случае отсутствия нормального распределения количественные данные описывались посредством медианы (Me) и нижнего и верхнего квартилей [Q1; Q3]. Категориальные данные приводились с указанием абсолютных значений и процентных долей. Сравнение двух групп по количественным показателям выполнялось с помощью t-критерия Стьюдента или t-критерия Уэлча (при неравенстве дисперсий), U-критерия Манна–Уитни. Направление и теснота корреляционной связи между двумя количественными показателями оценивались через коэффициент ранговой корреляции Спирмена и коэффициент корреляции Пирсона. Различия считались достоверными при p <0,05.
РЕЗУЛЬТАТЫ
Пациенты в обеих группах были сопоставимы по полу (p=1,000) и возрасту (p=0,888).
На 10±3-й день от начала симптомов вакцинированные пациенты характеризовались более легким клиническим статусом, о чем свидетельствовали достоверные различия по показателям шкалы ШОКС-КОВИД (4 [4; 6] баллов в группе I10 против 5 [4; 7] баллов в группе II10, p=0,042) и по уровню сатурации SpO2 (95±3% в I10 против 94±3% во II10, p=0,037). По температуре тела и баллам шкалы NEWS2 статистически значимых различий получено не было (табл.).
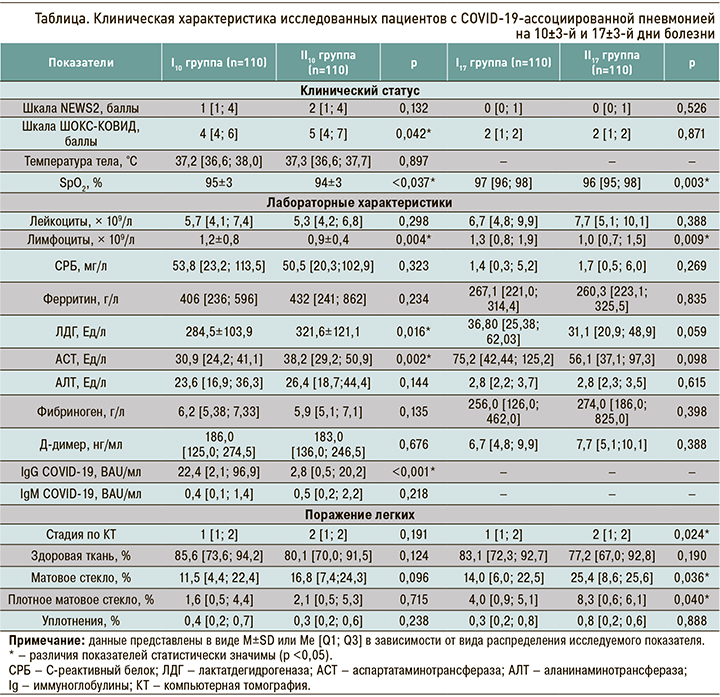
При оценке лабораторных данных в группе вакцинированных пациентов, в отличие от невакцинированных, был выше уровень лимфоцитов – 1,2±0,8 × 109/л против 0,9±0,4 × 109/л (р=0,004). По другим показателям общеклинического анализа крови достоверных различий выявлено не было (р >0,05).
По уровням маркеров синдрома системной воспалительной реакции и Д-димера достоверных различий между группами обнаружено не было (р >0,05). Обратим внимание, что в группе вакцинированных пациентов, в отличие от невакцинированных, отмечались более низкие уровни ЛДГ (284,5±103,9 против 321,6±121,1 Ед/л, р=0,016) и АСТ (30,9 [24,2; 41,1] против 38,2 [29,2; 50,9] Ед/л, р=0,002).
На 10±3-й день болезни уровень IgG COVID- 19, характеризующий наличие специфического иммунного ответа, оказался достоверно выше у вакцинированных пациентов в сравнении с невакцинированными – 22,4 [2,1; 96,9] против 2,8 [0,5; 20,2] BAU/мл, р <0,001. Уровень IgМ COVID-19 находился в пределах референсных значений в обеих группах (р >0,05).
По данным МСКТ ОГК объем поражения легочной паренхимы достоверно не отличался в обеих группах: КТ-стадия пневмонии 1 [1; 2] против КТ-2 [1; 2], (р=0,191). Объем здоровой ткани составил 85,6 [73,6; 94,2] и 80,1 [70,0; 91,5] % (р=0,124) у вакцинированных и невакцинированнных соответственно.
На 10±3-й день болезни была выявлена достоверная прямая слабой силы взаимосвязь между уровнем лимфоцитов и SpO2 (ρ=0,142, р=0,036), а также обратная слабой силы взаимосвязь между уровнем лимфоцитов и баллами по шкале ШОКС-КОВИД (ρ=-0,155, р=0,023).
Таким образом, на 10±3-й день с начала симптомов пациенты, вакцинированные против COVID- 19, в отличие от невакцинированных, имели менее тяжелый клинический статус, более высокий уровень лимфоцитов при сопоставимом объеме поражения легочной паренхимы. При этом установлено, что более высокий уровень лимфоцитов был связан с менее тяжелым клиническим течением COVID-19-ассоциированной пневмонии.
На 17±3-й день от начала симптомов в клиническом статусе обращало на себя внимание сохраняющееся различие между группами I17 и II17 по уровню SpO2 – 97 [96; 98] против 96 [95; 98] % соответственно (p=0,003). По шкалам NEWS2 и ШОКС-КОВИД достоверных различий между группами не наблюдалось (р >0,05).
При сравнении динамики лабораторных показателей у вакцинированных пациентов сохранялся более высокий уровень лимфоцитов – 1,3 [0,8; 1,9] × 109/л против 1,0 [0,7; 1,5] × 109/л у невакцинированных (р=0,009). Достоверных различий по другим лабораторным показателям получено не было.
На 17±3-й день было отмечено, что в группе пациентов, вакцинированных от COVID-19, стадия пневмонии по МСКТ ОГК была достоверно меньше, чем у невакцинированных – 1 [1; 2] против 2 [1; 2] (p=0,024). Анализ КТ-картины показал, что у невакцинированных пациентов, в сравнении с вакцинированными, объем поражения легких был достоверно больше за счет увеличения процента матового и плотного матового стекла: 25,4 [8,6; 25,6] против 14,0 [6,0; 22,5] % (р=0,036) и 8,3 [0,6; 6,1] против 4,0 [0,9; 5,1] % (р=0,04) соответственно.
На 17±3-й день болезни сохранялась слабой силы прямая взаимосвязь между уровнем лимфоцитов с уровнем SpO2 (ρ=0,241, р <0,001). Дополнительно была выявлена слабой силы обратная взаимосвязь между уровнем лимфоцитов и стадией пневмонии по МСКТ ОГК (ρ=-0,162, р=0,016).
Таким образом, на 17±3-й день заболевания у вакцинированных пациентов, в отличие от невакцинированных, сохранялся более легкий клинический статус, более высокий уровень лимфоцитов, и эти пациенты имели меньший объем поражения легочной паренхимы. При этом установлено, что высокий уровень лимфоцитов был связан с менее тяжелым клиническим течением и меньшим объемом поражения легочной паренхимы.
ОБСУЖДЕНИЕ
В представленном исследовании была проведена комплексная клинико-инструментальная оценка течения COVID-19-ассоциированной пневмонии у пациентов, вакцинированных от COVID-19. Результаты показали, что как на 10±3-й, так и на 17±3-й дни болезни пациенты, вакцинированные от COVID-19, характеризовались более легким клиническим течением заболевания, которое подтверждалось исходно более низкими баллами по шкале ШОКС-КОВИД и более высоким уровнем сатурации, сохранявшимся в динамике.
Аналогичные результаты приводятся и в ряде других работ. Так, Seo W.J. et al. [10] выполнили сравнительный анализ риска развития пневмонии и прогрессирования дыхательной недостаточности с потребностью в кислородной поддержке в группах вакцинированных и невакцинированных пациентов. В исследование были включены 387 госпитализированных пациентов с симптомной и бессимптомной коронавирусной инфекцией, из них 204 были полностью вакцинированы. У вакцинированных пациентов значительно реже развивалась пневмония (36,8 против 65,6%, р <0,001) и была ниже потребность в кислородной поддержке (15,7 против 29,0%, p=0,002).
В исследование Abhilash K.P.P. et al. [9] вошли 4183 пациента с симптомным COVID-19 (n=3234 – невакцинированные, n=403 – полностью вакцинированные, n=481 – частично вакцинированные, n=65 – с неопределенным прививочным статусом). В исследовании были получены данные, свидетельствующие о меньшей потребности полностью вакцинированных пациентов в сравнении с невакцинированными в кислородной поддержке (40,9 против 4,0%, р <0,001) и неинвазивной вентиляции легких (20,5 против 1%, р <0,001).
Необходимо добавить, что в обсуждаемые выше исследования включались пациенты с симптомным или бессимптомным течением COVID-19 вне зависимости от наличия или отсутствия пневмонии, тогда как в проведенное нами исследование вошли пациенты только с COVID-19-ассоциированной пневмонией.
При оценке лабораторных показателей в рамках нашего исследования в группе пациентов, вакцинированных от СOVID-19, в сравнении с невакцинированными, наблюдались более высокий уровень лимфоцитов как на 10±3-й, так и на 17±3-й дни болезни и исходно более низкие уровни ЛДГ и АСТ. Следует отметить, что достоверных различий по уровню маркеров синдрома системной воспалительной реакции (С-реактивный белок, ферритин) между группами нами обнаружено не было.
В контексте вышеизложенного интересны результаты работы Teran-Tinedo J.R. et al. [17] с участием 1888 пациентов с COVID- ассоциированной пневмонией. В нем был сделан сравнительный анализ лабораторных показателей между группами невакцинированных (n=1327) и полностью (n=209) или частично (n=352) вакцинированных пациентов. Согласно результатам этой работы, достоверных различий между группами по уровню лимфоцитов выявлено не было. В отличие от нашего исследования, Teran-Tinedo J.R. et al. получили данные, согласно которым полностью вакцинированные пациенты, в сравнении с невакцинированными, имели более низкий уровень ферритина (367,0 [182,0; 731,0] против 527,0 [237,2; 1083,5] нг/мл, р <0,0001) и более высокий уровень – С-реактивного белка (48,9 [21,7; 102,9] против 31,8 [14,1; 68,7] мг/л, р <0,0001). Авторы данной работы интерпретировали высокий уровень С-реактивного белка у вакцинированных пациентов пожилым возрастом и наличием большего количества сопутствующих заболеваний. Кроме этого, в исследовании обсуждалось, что уровень С-реактивного белка не влиял на течение и исход заболевания. В этом же исследовании было установлено, что у вакцинированных пациентов, по сравнению с невакцинированными, уровень ЛДГ был ниже (269,0 [218,5; 330,5] против 313,0 [258,0; 386,0] Ед/л, р <0,0001), что совпадает с результатами нашей работы.
Одной из важных задач нашего исследования была оценка объема поражения легочной паренхимы. На 10±3-й день от начала симптомов этот показатель как у вакцинированных, так у невакцинированных пациентов достоверно не различался. Однако на 17±3-й день болезни у пациентов, вакцинированных от COVID-19, объем поражения легочной паренхимы был меньше, чем у невакцинированных.
В рамках этого безусловный интерес представляет работа отечественных ученых. По результатам исследования Ю.П. Линец с соавт. [18], при первичной оценке объема поражения легочной паренхимы в группах вакцинированных (n=209) и невакцинированных (n=475) пациентов статистически значимых различий выявлено не было. При этом в группе невакцинированных пациентов, по данным МСКТ ОГК, в динамике отмечалось прогрессирование поражения легочной ткани до 3–4 стадии.
ЗАКЛЮЧЕНИЕ
Результаты настоящего исследования показали, что у пациентов, полностью вакцинированных от COVID-19, течение COVID-19-ассоциированной пневмонии отличалось менее тяжелыми клиническими проявлениями, отсутствием лимфопении и в динамике меньшим объемом поражения легочной паренхимы. Сохранение уровня лимфоцитов в пределах референсных значений ассоциировано с менее тяжелым клиническим течением COVID-19-ассоциированной пневмонии и меньшим объемом поражения легочной паренхимы.
Ограничения исследования
Интерпретация результатов нашего исследования имеет следующие ограничения: небольшая выборка, включавшая пациентов, госпитализированных только в ГБУЗ «Городская клиническая больница № 52 Департамента здравоохранения г. Москвы», ограниченный период наблюдения за пациентами. Следует отметить, что формирование выборки проводилось во время 4-й волны COVID-19 с преобладанием омикрон-штамма вируса SARS-CoV-2.


