Болезнь Шегрена (БШ) – хроническое аутоиммунное заболевание, для которого характерна инфильтрация иммунными клетками ткани экзокринных желез с развитием сухого синдрома различных локализаций [1].
У 30% пациентов БШ протекает с системными (внежелезистыми) проявлениями и сопровождается высоким титром антинуклеарного фактора (АНФ), aнти-SSA, анти-SSB антител, ревматоидного фактора (РФ), снижением концентрации С3-, С4-фракций комплемента, гипергаммаглобулинемией, криоглобулинемией, которые выступают лабораторными предикторами неблагоприятного течения заболевания [1].
У этой группы пациентов, характеризующихся наличием внежелезистых проявлений БШ, наблюдается повышение риска развития В-клеточных лимфопролиферативных процессов, в частности MALT-лимфом.
Криоглобулинемия, снижение концентрации C4-фракции комплемента, лимфопения, анти-SSB антитела служат маркерами более тяжелого течения БШ, для них также была установлена ассоциация с повышенным риском развития В-клеточных неоплазий [2]. В частности, частота возникновения MALT-лимфомы при БШ составляет 5%, медиана времени от постановки диагноза БШ до формирования МАLT-лимфомы – 7,5 лет [3].
Важной особенностью, отличающей БШ от других системных аутоиммунных заболеваний, является закономерное, четко морфологически очерченное поражение малых слюнных желез (МСЖ). Поскольку биопсия МСЖ является рутинной, малоинвазивной медицинской процедурой [4, 5], ее результаты включены в число классификационных критериев этого заболевания [6]. Характерная гистологическая картина БШ – очаговый сиалоаденит с наличием фокусов, состоящих из ≥50 мононуклеарных клеток (преимущественно лимфоцитов), расположенных перидуктально и/или периваскулярно, при значении Focus Score (FS) ≥1.
FS – показатель, который соответствует количеству фокусов в 4 мм2 площади гистологического среза. При иммуногистохимическом исследовании в воспалительном инфильтрате преобладают CD3- и CD20-позитивные клетки [7, 8].
Существует возможность проведения повторных биопсий МСЖ в ходе экспериментальной терапии БШ с объективной оценкой динамики клеточных популяций (воспалительного процесса). Это существенно повышает информативность обследования и позволяет провести пилотные исследования на небольших контингентах больных. Вышесказанное делает БШ оптимальной моделью для отработки таргетной терапии диффузных заболеваний соединительной ткани.
Подходы к терапии БШ в настоящее время недостаточно разработаны. Согласно рекомендациям EULAR 2019 г. [9], рассматривается возможность использования классических базисных препаратов (аминохинолиновых препаратов, азатиоприна, метотрексаат, иммуносупрессивной и цитостатический терапии) и биологической терапии у пациентов с системными проявлениями заболевания. Однако уровень и класс доказательности рекомендаций остаются недостаточными.
АНТИ-В-КЛЕТОЧНАЯ ТЕРАПИЯ В ЛЕЧЕНИИ БОЛЕЗНИ ШЕГРЕНА
Активация и гиперреактивность В-лимфоцитов относятся к хорошо охарактеризованным аспектам патогенеза БШ [10], поэтому применение анти-В-клеточных препаратов представляется перспективным направлением терапии.
Ритуксимаб – химерное моноклональное антитело к рецептору СD20, специфичного для В-лимфоцитов и необходимого для их созревания. Данные научной литературы по применению ритуксимаба у пациентов с БШ противоречивы.
В нескольких исследованиях отмечены уменьшение выраженности сухого синдрома, общей слабости, положительная динамика по внежелезистым проявлениям [10–13]. Однако в рандомизированных плацебо-контролируемых клинических исследованиях TEARS и TRACTISS значимого влияния препарата на системные проявления и сухой синдром выявлено не было [14, 15].
Другое направление анти-В-клеточной терапии – ингибирование факторов созревания и выживания В-клеток. BAFF (B-cell activating factor) – один из цитокинов, необходимых для пролиферации и дифференцировки В-клеток [16]. Участие BAFF в патогенезе аутоиммунных заболеваний доказано в экспериментах с трансгенными мышами, у которых увеличение секреции BAFF сопровождалось повышением уровня зрелых Т- и В-лимфоцитов во вторичных лимфоидных органах, повышением уровня РФ, антител к двуспиральной ДНК, развитием иммунокомплексного гломерулонефрита [17]. У пациентов с БШ показано повышение концентрации BAFF в ткани слюнных желез [18, 19] и сыворотке крови [20, 21], что коррелирует c риском развития лимфопролиферативного заболевания и индексом ESSDAI [21]. На фоне терапии ритуксимабом сывороточная концентрация BAFF у пациентов с БШ сравнительно выше, чем до терапии [22, 23]. Этот факт, по мнению многих исследователей, лежит в основе недостаточной эффективности ритуксимаба при лечении диффузных заболеваний соединительной ткани, и в частности БШ [24, 25].
Данных об эффективности анти-BAFF-терапии при БШ недостаточно. В ходе второй фазы клинического исследования BELISS терапия белимумабом при БШ приводила к уменьшению индексов ESSDAI, ESSPRI, достижению конечных точек у 60% пациентов [26, 27].
Таким образом, комбинированная терапия белимумабом и ритуксимабом при БШ может оказаться эффективной, учитывая современные представления о патогенезе заболевания, данные литературы [28] и эффективность такой терапии у пациентов с системной красной волчанкой [24, 29].
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ
Пациентка К., 55 лет (1963 г.р.), с 1998 г. отмечала появление умеренно выраженной сухости, дискомфорта и чувства инородного тела в глазах.
В 2001 г. была проконсультирована офтальмологом: тот диагностировал синдром сухого глаза, по поводу чего пациентка использовала препараты «искусственной слезы» со слабым положительным клиническим эффектом. С 2006 г. появились сухость в ротовой полости, частые рецидивы хронического синусита (до 4–6 раз в год); проводилась симптоматическая и антибактериальная терапия с временным положительным эффектом.
В 2015 г. пациентка была обследована ревматологом, который с учетом сухого синдрома заподозрил диагноз болезни Шегрена. Терапия была дополнена глазными каплями с циклоспорином А, а также приемом гидроксихлорохина в дозе 200 мг/сут.
Рекомендованную терапию пациентка получала регулярно, отмечала незначительную положительную динамику относительно сухого синдрома.
В 2016 г. пациентка стала отмечать выраженную общую слабость, повышение температуры тела до субфебрильных значений, артралгии в коленных суставах и суставах кистей, у нее усилился сухой синдром, была выявлена шейная лимфаденопатия, по поводу чего она неоднократно обращалась за медицинской помощью. При обследовании в стационаре была проведена оценка сухого синдрома: проба Ширмера 0/0 мм, нестимулированная сиалометрия 1,0 мл. По результатам лабораторных исследований выявлен антинуклеарный фактор (АНФ) 1:102 40 (норма менее 1:160), в иммуноблоте антинуклеарных антител обнаруживались анти-SSA 3+, анти-SSB 3+, гаммаглобулины 19,3 г/л (норма 5,5–15 г/л), РФ 96 МЕ/мл (норма менее 20 МЕ/мл), скорость оседания эритроцитов (СОЭ) 13 мм/ч (норма 0–15 мм/ч), С-реактивный белок (СРБ) 14 мг/л (норма менее 5 мг/л). Согласно критериям ACR/EULAR 2016 г., у пациентки был верифицирован диагноз болезни Шегрена. Активность заболевания расценивалась как умеренная (ESSPRI 6, ЕSSDAI 12) [6].
Пациентке была выполнена биопсия МСЖ. Фиксация материала осуществлялась в 10% нейтральном забуференном формалине. Затем кусочки подвергались стандартной проводке с последующей заливкой в парафин. Из полученных блоков готовили срезы 2–3 мкм, окрашивали гематоксилином и эозином, пикрофуксином по ван Гизону, реактивом Шиффа. Иммуногистохимическое исследование проводили на парафиновых срезах. Использовалась следующая панель антител: CD3 (polyclonal rabbit, фирма DAKO), CD4 (monoclonal rabbit, clone SP35, фирма CELL MARQUE), CD8 (monoclonal mouse, clone CB/1448, фирма DAKO), CD20 (monoclonal mouse, clone L26, фирма DAKO), CD21 (monoclonal mouse, clone 2G9, фирма Immuologic), легкие цепи иммуноглобулинов (лямбда/каппа), IgG/IgG4, CD68 (monoclonal mouse, clone KP1, фирма DAKO), CD138 (monoclonal mouse, clone M15, фирма DAKO); система визуализации Envision FLEX (фирма DAKO). Препараты докрашивали гематоксилином и заключали под покровное стекло. Оценка результатов (рис. 1–12) проводилась с помощью светооптического микроскопа OLYMPUS BX-46. Морфометрическое исследование препаратов проводили следующим образом: после обзорного морфологического изучения микропрепарата с учетом окрашенных дополнительными гистохимическими методиками параллельных срезов находили гистотопографически удаленные друг от друга поля зрения для исключения «перекрывания» зон подсчета, и при увеличении микроскопа в 400 раз производили подсчет абсолютного числа клеточных элементов в 10 полях зрения. При подсчете скопления клеточных элементов старались поместить в центр поля зрения. Для этого в окуляры устанавливали измерительную шкалу с ценой деления 100 мкм (0,1 мм). С учетом технических характеристик микроскопа при данном увеличении диаметр поля зрения составляет 5,0 мм, соответственно радиус подсчета от изучаемой структуры составлял 2,5 мм (Sп/зр=0,20 мм2).
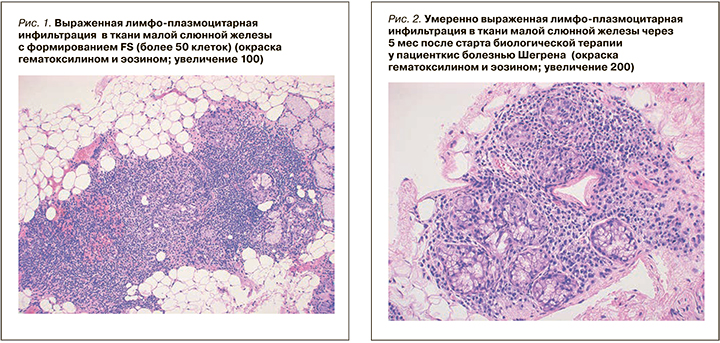
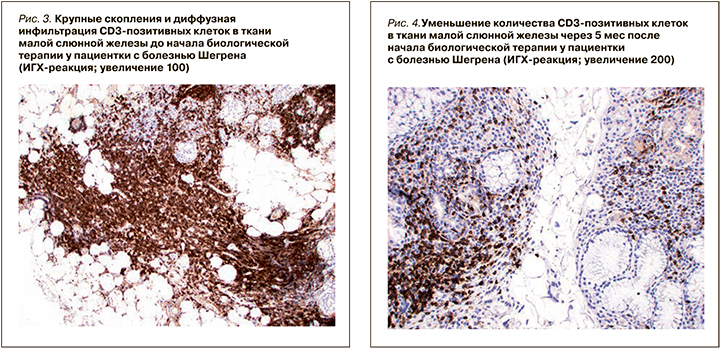
В дальнейшем полученные данные морфометрических исследований подвергались статистическому анализу.
В МСЖ определялся диффузный лимфоплазмацитарный инфильтрат (концентрация более 50 клеток в 4 мм2). Лимфоидные клетки инфильтрировали отдельные железы и формировали единичные, мелкие лимфоэпителиальные повреждения с разрушением эпителия, отмечалось распространение инфильтрата вне дольки, умеренно выраженный перидуктальный и периваскулярный склероз. Показатель FS (Focus score) – 5, по классификации Chisholm–Mason – 4 стадия (см. рис. 1).
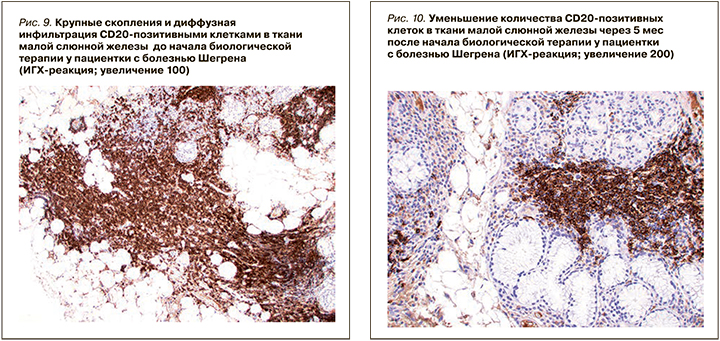
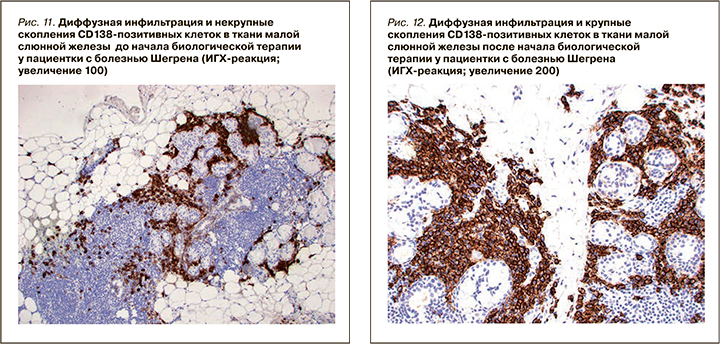
При ИГХ-исследовании в инфильтрате преобладали Т- и В-лимфоциты (80%), доля CD138-позитивных клеток составляла 20% (см. рис. 11). В лимфоидном инфильтрате преобладали СD20-позитивные В-лимфоциты – 70% (см. рис. 9), CD3-позитивные Т-лимфоциты составляли около 30% (см. рис. 3). CD8-позитивных клеток значительно меньше (см. рис. 7), чем CD4 позитивных Т-лимфоцитов (см. рис. 5).
В очагах, где преобладает инфильтрация плазматическими клетками, обнаружена монотипическая экспрессия каппа легких цепей иммуноглобулинов (каппа > лямбда). В очаге с выраженной лимфоидной инфильтрацией и лимфоэпителиальными повреждениями в плазматических клетках отмечена монотипическая экспрессия легких цепей иммуноглобулинов (лямбда >каппа).

В значительной части плазматических клеток обнаружена выраженная экспрессия IgG, экспрессия IgG4 обнаружена в единичных клетках. Антитела к панцитокератину (AE1/AE3) маркировали лимфоэпителиальные повреждения. Аберрантной экспрессии циклина D1 и MNDA не выявлено. CD21 маркировал расширенную сеть фолликулярных дендритических клеток. Пациентке проведено молекулярно-генетическое исследование (методом ПЦР, капиллярный электрофорез, протокол BIOMED2), по результатам которого моноклональных реаранжировок генов тяжелых и легких цепей иммуноглобулинов (IgH и IgK) не обнаружено. Полученные результаты гистологического, ИГХ и молекулярно-биологического исследований МСЖ не позволили провести окончательный дифференциальный диагноз между болезнью Шегрена и начальными проявлениями, наблюдаемыми при MALT-лимфоме. Пациентка была проконсультирована гематологом; учитывая отсутствие в исследуемом материале моноклональной реаранжировки генов тяжелых и легких целей иммуноглобулинов, убедительных данных, подтверждающих диагноз MALT-лимфомы, не было.
В связи с выраженной ксерофтальмией, общей слабостью, активностью БШ, высокими рисками злокачественной В-клеточной трансформации было принято решение о проведении комбинированной биологической терапии ритуксимабом и белимумабом. С 28.12.2016 начата терапия БМ в режиме 400 мг в 0, 14 и 28 дни лечения, с 03.01 по 17.02.2017 проводился курс терапии ритуксимабом («Ацеллбия») в режиме 500 мг в 0, 7, 14 и 28 дни.
В дальнейшем была продолжена терапия белимумабом в режиме 120 мг 1 раз в месяц в течение 2017 г.
РЕЗУЛЬТАТЫ
Через 5 мес после начала биологической терапии ритуксимабом и белимумабом проводилось клинико-лабораторное и иммунологическое обследование, оценка активности заболевания. Пациентка отмечала уменьшение сухости в глазах, общей слабости, нормализацию температуры тела, регресс шейной лимфаденопатии. Проведена повторная оценка сухого синдрома: тест Ширмера 2/2 мм, нестимулированная сиалометрия 1,0 мл. Индекс ESSDAI 0, индекс ESSPRI 2,7 (табл. 1).
Лабораторно было отмечено уменьшение титра АНФ 1:10240 – > 1:160, исчезновение SSB антител, снижение концентрации гамма-глобулинов 19,3 – >15 г/л, РФ 96 – > 74 МЕ/мл (табл. 2).
В мае 2017 г. была выполнена повторная биопсия малой слюнной железы с последующим гистологическим и ИГХ-исследованиями. При обзорной микроскопии выявлено два фрагмента малой слюнной железы (площадь среза более 4 мм2) с очаговой и диффузной плазмоцитарной инфильтрацией (плотность более 50 клеток в 4 мм2), лимфоэпителиальные повреждения отсутствовали, распространение инфильтрата вне долек не выявлено. Отмечался умеренно выраженный перидуктальный и периваскулярный склероз. Показатель FS – 4, по классификации Chisholm–Mason – 4 стадия (см. рис. 1–2).
При ИГХ-исследовании в инфильтрате преобладали СD20 позитивные В-лимфоциты (20%) и CD138 позитивные клетки (70%) (см. рис. 4–12). Обращало внимание увеличение количества плазматических клеток в воспалительном инфильтрате до 14000 CD138 позитивных клеток и фолликуллярных дендиритических клеток, маркированы СD21, до 1500 (табл. 3).
Через 10 мес после начала комбинированной биологической терапии проводилось повторное клинико-лабораторное и иммунологическое обследование, оценка активности БШ в динамике. Проба Ширмера 1/2, нестимулированная сиалометрия – 0,8 мл. При лабораторном контроле: гаммаглобулины 14,7 г/л, РФ 76 МЕ/мл, АНФ 1:160, антитела анти-SSA 3+; анти-SSB не выявлены. Индексы ESSDAI 0, ESSPRI 3, активность заболевания расценивалась как низкая (см. табл. 2).
ОБСУЖДЕНИЕ
Мы наблюдали пациентку с БШ, имеющую высокий риск развития злокачественной В-клеточной трансформации. Полученные данные гистологического и ИГХ-исследования МСЖ не позволяли провести окончательный дифференциальный диагноз между БШ и начальными проявлениями MALT-лимфомы.
Комбинированная терапия ритуксимабом и белимумабом в представленном клиническом случае приводила к уменьшению ксерофтальмии, положительной динамике лабораторных и иммунологических показателей, снижению активности заболевания. Особенно важной представлялась возможность уменьшения проявлений сухого синдрома, что, как правило, не могло быть достигнуто при терапии стандартными базисными препаратами (метотрексатом, азатиоприном).
По результатам гистологического исследования в динамике отмечалось уменьшение удельного количества B- и T-лимфоцитов в воспалительных инфильтратах малых слюнных желез. Обращало на себя внимание увеличение количества CD138-позитивных клеток (с 20 до 70%), а также СD21-позитивных клеток. Увеличение пула плазматических клеток на фоне комбинированной анти-CD20 и анти-BAFF терапии, вероятно, отражало преимущественное влияние препаратов на В-лимфоциты. Однако изменение профиля антинуклеарных антител (снижение концентрации АНФ, негативация по ROSSB аутоантителам) предполагало воздействие комбинированной терапии также и на пул аутореактивных плазматических клеток. Регуляция распределения плазматических клеток в тканях осуществляется с помощью адгезионных молекул и хемокинов [30], изменение продукции которых, возможно, является причиной повышения содержания плазмобластов в биоптате на фоне терапии в данном клиническом случае. Необходимо отметить, что монотерапия ритуксимабом у пациентов БШ не приводила к значимому изменению серологического профиля, в частности RoSSa или LaSSB аутоантител [31].
В литературе имеются единичные описания успешного применения комбинированной терапии ритуксимабом и белимумабом у больных системной красной волчанкой и БШ, в том числе в случаях предшествовавшего неэффективного применения этих препаратов [24], что сопоставимо с полученными нами результатами. Однако авторы опубликованных ранее работ не проводили морфологического исследования биоптатов МСЖ в динамике, что не позволяло судить о механизмах полученного терапевтического эффекта. В 2017 г. у компании GlaxoSmithKline стартовало многоцентровое, двойное слепое, рандомизированное, плацебо-контролируемое исследование по оценке эффективности и безопасности комбинированной терапии белимумабом и ритуксимабом у пациентов с БШ. Протокол исследования включает гистологическое исследование МСЖ до и через 24 нед после начала терапии.
ЗАКЛЮЧЕНИЕ
Комбинированная биологическая терапия ритуксимабом и белимумабом у пациентки с БШ в представленном клиническом наблюдении приводила к уменьшению выраженности сухого синдрома, общей слабости, снижению активности заболевания, положительной лабораторной динамике, уменьшению лимфоцитарной инфильтрации, герминативноподобных и лимфоэпителиальных поражений в ткани МСЖ. Применение выбранного режима комбинированной терапии ритуксимабом и белимумабом вызвало формирование устойчивого и долгосрочного положительного эффекта. Полученные результаты могут служить дополнительным обоснованием для инициации клинических исследований, посвященных комбинированному применению анти BAFF и анти CD20 препаратов у пациентов БШ, прежде всего при резистентности заболевания к стандартной терапии, а также при наличии неблагоприятных прогностических факторов [17].



