Нестероидные противовоспалительные препараты (НПВП) – одна из наиболее используемых во всем мире группа лекарственных средств. Ежедневно в мире только по назначению врача НПВП принимают более 30 млн человек [1], однако, поскольку некоторые препараты этого класса отпускаются без рецепта, истинное количество их «потребителей» значительно выше.
Ацетилсалициловая кислота (АСК) относится к группе НПВП и, подобно другим препаратам этой группы, обладает противовоспалительным, анальгезирующим и жаропонижающим действием, но применяется главным образом как антиагрегант для первичной и вторичной профилактики сердечно-сосудистых заболеваний.
Известно, что НПВП могут оказывать специфическое негативное действие на различные органы и системы, в частности на желудочно-кишечный тракт (ЖКТ), приводящее к развитию опасных нежелательных явлений (НЯ) [2]. Так, НПВП-индуцированные НЯ со стороны ЖКТ включают НПВП-гастропатию, НПВП-ассоциированную диспепсию и НПВП-энтеропатию. Это серьезная медицинская и социальная проблема, поскольку большинство «потребителей» НПВП – люди пожилого и старческого возраста, имеющие, как правило, несколько хронических заболеваний. При этом пожилой (≥65 лет) возраст является одним из факторов риска НПВП-ассоциированных осложнений со стороны ЖКТ [2].
По нашим данным, среди 664 пациентов в возрасте ≥65 лет (диапазон 65–107 лет; средний возраст 79±9 лет; 25% мужчин) частота постоянного приема антиагрегантов (АСК в 92% случаев) составила 67%, НПВП – 51%; каждый третий (35%) пациент принимал антиагреганты в сочетании с НПВП. Учитывая, что данные препараты назначаются на длительный срок, зачастую пожизненно, проблема профилактики и лечения НПВП-индуцированных поражений ЖКТ чрезвычайно актуальна.
НПВП-ГАСТРОПАТИЯ
Клинические проявления НПВП-гастропатии включают образование эрозий и язв на слизистой оболочке желудка и/или двенадцатиперстной кишки (ДПК), а также кровотечение, перфорацию и нарушение проходимости (стриктуры) верхнего отдела ЖКТ. Риск возникновения подобных НЯ у пациентов, регулярно принимающих НПВП, более чем в 4 раза выше, чем в общей популяции, и составляет 0,5–1,0 случай на 100 пациенто-лет [3]. По данным Roth S.H. [4], при проведении эндоскопического исследования у 68% пациентов, принимающих НПВП не менее 6 нед, обнаруживают эрозии и геморрагии на слизистой оболочке желудка, а у 15% – язвы. На основании этих данных в 1986 г. Roth S.H. предложил термин «НПВП-гастропатия» для обозначения специфического поражения слизистой оболочки желудка и ДПК, возникающего при приеме НПВП и имеющего характерную клинико-эндоскопическую картину.
В течение последних десятилетий НПВП остаются препаратами первой линии для лечения боли и воспаления, поэтому полностью отказаться от их применения у большинства пациентов не представляется возможным. В связи с этим перед врачами стоит задача обеспечить адекватную защиту слизистой желудка и ДПК от повреждающего действия НПВП, необходимость которой была подтверждена результатами клинических исследований. В одной из таких работ [5] проанализировали ассоциации между приемом гастропротекторов и возникновением осложнений со стороны верхнего отдела ЖКТ у пациентов, получавших неселективные НПВП. Оказалось, что у больных, не принимавших гастропротекторы, по сравнению с пациентами, получавшими гастропротекторы, риск любых осложнений со стороны верхнего отдела ЖКТ был выше в 2,4 раза [относительный риск (ОР) 2,39; 95% доверительный интервал (ДИ) 1,66–3,44], а риск желудочно-кишечных кровотечений (ЖКК) из верхнего отдела ЖКТ – в 1,9 раз (ОР 1,89; 95% ДИ 1,09–3,28).
Согласно российским клиническим рекомендациям по профилактике и лечению эзофагогастроэнтероколопатий, индуцированных НПВП [6], наиболее эффективными препаратами для профилактики и лечения НПВП-гастропатии являются ингибиторы протонной помпы (ИПП). При этом эксперты предлагают врачам рассматривать необходимость профилактического применения ИПП на протяжении всего срока лечения НПВП. Эксперты отмечают, что «ИПП могут быть идеальным решением для пациентов, подверженных повышенному риску поражения ЖКТ», однако не упоминают о том, что долгосрочное применение этой группы лекарственных средств часто сопряжено с возникновением НЯ. Так, имеются данные [7], что у пациентов, длительно получающих ИПП, повышен риск развития некоторых хронических заболеваний и состояний: деменции (на 4–80%), переломов костей (на 30–400%), инфаркта миокарда (уровень риска не определен), инфекций (до 600%), дефицита микроэлементов (на 60–70%), дисбиоза кишечника (на 200–800%), хронических заболеваний почек (на 10–20%) и злокачественных опухолей ЖКТ (уровень риска не определен).
Помимо этого, в нескольких исследованиях [8–10] было показано, что длительное применение НПВП ассоциируется с повышением риска смерти. В когортном наблюдательном исследовании [8] с участием более 3 млн человек у пациентов, получавших ИПП в течение периода наблюдения (медиана 5,71 года), выявлено увеличение риска смерти на 15% (ОР 1,15; 95% ДИ 1,14–1,15) по сравнению с лицами, не принимавшими ИПП, и на 25% (ОР 1,25; 95% ДИ 1,23–1,28) – по сравнению с пациентами, леченными блокаторами H2-рецепторов гистамина.
В систематическом обзоре [9] были проанализированы результаты 37 исследований, в 5 из которых непосредственно изучали влияние ИПП на смертность и/или сердечно-сосудистую заболеваемость, при этом всего в анализ смертности включили 22 427 пациентов, а в анализ заболеваемости – 354 446 человек. У больных, принимавших ИПП, риск смерти от всех причин оказался выше на 68% (ОР 1,68; 95% ДИ 1,53–1,84; p <0,001), а риск неблагоприятного сердечно-сосудистого события (инфаркт миокарда, инсульт) – на 54% (ОР 1,54; 95% ДИ 1,11–2,13; p=0,01).
В этот анализ включили одно проспективное исследование [10] с участием пожилых (≥65 лет) пациентов (n=491; средний возраст 80±6 лет), в котором обнаружили зависимость риска смерти от всех причин в течение года после выписки из отделения неотложной помощи от дозы ИПП. Так, у всех больных терапия ИПП ассоциировалась с увеличением риска смерти от всех причин на 51% (ОР 1,51; 95% ДИ 1,03–2,77), тогда как у пациентов, леченных высокими дозами ИПП, риск смерти оказался выше в 2,6 раза (ОР 2,59; 95% ДИ 1,22–7,16). Необходимо отметить, что в этом исследовании лица, получавшие ИПП, имели более выраженные когнитивные нарушения, больше сопутствующих заболеваний и принимали большее количество лекарств. Однако, несмотря на эти ограничения, результаты данной работы указывают на то, что следует с осторожностью назначать высокие дозы ИПП пожилым пациентам и обязательно учитывать соотношение риск/польза длительного лечения.
С учетом вышеизложенного рассматривать ИПП как «идеальные» препараты для лечения и профилактики НПВП-гастропатии, по всей видимости, неправомерно. Российские эксперты, отдавая ИПП безусловное предпочтение, все же отмечают, что в качестве альтернативы можно использовать блокаторы H2-рецепторов гистамина или продуценты простагландина Е2 (Pg E2) [6].
РЕБАМИПИД В ЛЕЧЕНИИ НПВП-ГАСТРОПАТИИ
Одним из таких препаратов является гастроэнтеропротектор ребамипид. Он повышает уровень Pg E2 в слизистой оболочке желудка и Pg E2 и I2 в содержимом желудочного сока, а также оказывает цитопротективное действие в отношении слизистой желудка при повреждающем воздействии этанола, кислот, щелочей и АСК. Препарат способствует активации энзимов, ускоряющих биосинтез высокомолекулярных гликопротеинов, и повышает содержание слизи на поверхности стенки желудка; улучшает кровоснабжение слизистой желудка и активизирует ее барьерную функцию; активизирует щелочную секрецию желудка; усиливает пролиферацию и обмен эпителиальных клеток желудка; очищает слизистую от гидроксильных радикалов и подавляет супероксиды, продуцируемые полиморфно-ядерными нейтрофилами в присутствии Helicobacter pylori; защищает слизистую желудка от поражения бактериями.
Ребамипид был разработан и синтезирован в Японии фирмой Otsuka Pharmaceutical Company. С 1990 г. препарат используется для лечения язвенной болезни, с 1994 г. – хронического гастрита. Одна таблетка содержит 100 мг активного вещества; рекомендованная доза составляет 100 мг 3 раза в день. В настоящее время ребамипид широко применяется в клинической практике под торговым названием Mucosta® в Японии, Корее, Китае, Филиппинах, Таиланде, Индонезии, Камбодже, Вьетнаме, Малайзии и Египте. С недавних пор препарат доступен и в нашей стране под торговым названием Ребагит®.
Имеются данные об использовании ребамипида у здоровых добровольцев с НПВП-индуцированным повреждением слизистой оболочки желудка. Так, была продемонстрирована эффективность ребамипида в предотвращении либо уменьшении проявлений НПВП-гастропатии у здоровых лиц при приеме индометацина [11–12], ибупрофена [13] и низких доз АСК [14]. В одной из этих работ [12] было показано, что ребамипид и блокатор H2-рецепторов гистамина фамотидин одинаково эффективны в профилактике гастропатии, вызванной применением индометацина, на основании чего было рекомендовано использовать ребамипид (по 100 мг 3 раза в день) или фамотидин (по 10 мг 2 раза в день) для профилактики НПВП-гастропатии у пациентов без факторов риска.
Эффективность ребамипида для профилактики и лечения НПВП-гастропатии у пациентов с различными заболеваниями показана в серии рандомизированных контролируемых исследований (РКИ) [15–17]. В исследовании GLORIA [15] участвовало 65 пациентов (средний возраст 67±13 лет; 16 мужчин) с ревматоидным артритом, остеоартритом и болью в нижней части спины, которые были рандомизированы на 2 группы: монотерапии НПВП (целекоксиб по 100 мг 2 раза/сут; n=34) и комбинированной терапии (целекоксиб по 100 мг 2 раза/сут + ребамипид по 100 мг 3 раза/сут; n=31). Через 3 мес лечения частота выявления НПВП-гастропатии при эндоскопическом исследовании составила 17,6% в группе монотерапии НПВП и 0% – в группе комбинированной терапии (р=0,0252).
В исследование STORM [16] включили 332 пациента с ревматоидным артритом, остеоартритом, анкилозирующим спондилитом или другим заболеванием, требующим постоянной терапии НПВП, которых рандомизировали на 2 группы: первая в дополнение к НПВП получала ребамипид (по 100 мг 3 раза/сут; n=176), вторая – мизопростол (по 200 мг 3 раза/сут; n=156). Длительность лечения составила 12 нед. Эффективность ребамипида и мизопростола в отношении профилактики НПВП-гастропатии оказалась практически идентичной: частота выявления язвенного поражения верхнего отдела ЖКТ составила 4,5% в группе ребамипида и 4,4% – в группе мизопростола [отношение шансов (ОШ) 0,98; р=0,9796].
Еще в одном РКИ [17] ребамипид также сравнили с мизопростолом. В него включили 479 пациентов, нуждающихся в постоянном приеме НПВП, которые в течение 12 нед принимали либо ребамипид по 300 мг/сут (n=242), либо мизопростол по 600 мг/сут (n=237). Как и в исследовании STORM, ребамипид по своей эффективности был сопоставим с мизопростолом: частота возникновения язв желудка составила 20,3 и 21,9%соответственно (p=0,6497), однако тяжесть желудочно-кишечных симптомов (p=0,0002) и частота сопутствующего применения антацидов (p=0,0258) оказались значительно ниже в группе ребамипида.
В 2014 г. опубликованы результаты японского двойного-слепого плацебо-контролируемого РКИ [18], в котором впервые изучили возможности ребамипида для профилактики гастропатии у пациентов, получающих двойную антитромбоцитарную терапию низкими дозами АСК в сочетании с клопидогрелом. В нем участвовали 32 здоровых добровольца, которых рандомизировали на 4 группы: в группе А (n=8) назначали АСК по 100 мг/сут и плацебо, в группе Б – АСК по 100 мг/сут и ребамипид по 300 мг/сут, в группе В – АСК по 100 мг/ сут, клопидогрел по 75 мг/сут и плацебо, в группе Г – АСК по 100 мг/сут, клопидогрел по 75 мг/сут и ребамипид по 300 мг/сут. Исходно и через 14 дней лечения оценивали наличие и выраженность желудочно-кишечных расстройств при помощи японской версии опросника Gastrointestinal Symptom Rating Scale (GSRS), проводили эзофагогастродуоденоскопию (ЭГДС) и определяли уровень гемоглобина в крови. Степень повреждения слизистой оболочки желудка оценивали при помощи модифицированной шкалы Lanza.
Через 14 дней у пациентов, получавших монотерапию АСК (группы А и Б), оценка по шкале Lanza оказалась ниже в группе ребамипида по сравнению с плацебо (0–3 в группе Б против 0–4 балла в группе А; p <0,05). Аналогичный эффект отмечен и у больных, получавших двойную антитромбоцитарную терапию (группы В и Г): оценка по шкале Lanza составила 0–3 в группе ребамипида и 3–4 в группе плацебо (p <0,01). У пациентов, получавших плацебо (группы А и В), в динамике было отмечено увеличение балла по шкале Lanza: 0–3 против 0–4 (p <0,05) в группе А и 0–3 против 3–4 (p <0,01) в группе В, тогда как у пациентов, получавших ребамипид (группы Б и Г), оценка по шкале Lanza существенно не менялась. Таким образом, прием ребамипида уменьшал или предотвращал повреждающее действие антиагрегантов на слизистую оболочку желудка.
Еще в одном РКИ [19], результаты которого опубликованы в 2019 г., у 83 больных, получавших двойную антитромбоцитарную терапию в течение ≥1 года и не имевших указаний на ЖКК и перфорацию пептической язвы в анамнезе, эффективность ребамипида (300 мг/сут) также сравнили с плацебо. Пациентов, принимавших ИПП, в исследование не включали. Первичной конечной точкой были «свежие» эрозии и язвы слизистой желудка, обнаруженные при проведении ЭГДС через 3 или 12 мес от начала лечения. Вторичными конечными точками служили снижение гематокрита по сравнению с исходным уровнем, ЖКК и боли в грудной клетке.
Через 12 мес у 13 больных (43%), принимавших ребамипид, и у 19 (66%), получавших плацебо, были обнаружены эрозивные поражения слизистой оболочки желудка (р=0,07); у 2 пациентов (6,7%) в группе ребамипида и у 8 (27,6%) в группе плацебо наблюдались пептические язвы диаметром ≥5 мм (р=0,03). Значения гематокрита практически не различались между двумя группами. Эпизодов ЖКК и болей в груди отмечено не было. Эта работа продемонстрировала эффективность ребамипида в предотвращении пептических язв диаметром ≥5 мм у пациентов, получающих двойную антитромбоцитарную терапию на протяжении ≥1 года, а также подтвердила его безопасность при длительном (в течение 1 года) лечении.
В японском ретроспективном исследовании [20] оценили частоту гастропатии у 530 пациентов, получавших низкие дозы АСК в течение ≥1 мес, и изучили эффективность различных гастропротекторов, в том числе и ребамипида. Эрозивно-язвенные поражения желудка были выявлены у 192 (36,2%) пациентов, у 25 (3,7%) человек имело место ЖКК. У пациентов, принимавших любые гастропротекторы, была отмечена значительно более низкая частота эрозивно-язвенного поражения желудка и кровотечений (p <0,01 для обоих сравнений) по сравнению с больными, не получавшими эти препараты. Аналогично монотерапия ИПП ассоциировалась с меньшей частотой ЖКК и эрозивно-язвенных повреждений желудка, чем отсутствие терапии гастропротекторами (p <0,01 для обоих сравнений). Вообще, назначение ИПП оказалось единственным фактором, ассоциированным со снижением риска ЖКК на 66% (ОР 0,34; 95% ДИ 0,12–0,94; р <0,05). Сочетанное применение ИПП или блокатора Н2-рецепторов гистамина с ребамипидом также было связано с меньшей частотой ЖКК и повреждений слизистой желудка, чем их комбинация с другими гастропротекторами. Нужно отметить, что в этом исследовании ребамипид получали всего 27 пациентов, из них 22 – в сочетании с ИПП и 5 – с блокатором Н2-рецепторов гистамина. Среди этих больных не было выявлено ни одного случая ЖКК, а частота повреждений слизистой оболочки желудка составила всего 14,8%.
Способность ребамипида предотвращать желудочное кровотечение, индуцированное воздействием на слизистую желудка АСК в сочетании с клопидогрелом, продемонстрирована в экспериментальном исследовании [21]: в нем крысам посредством внутривенной инфузии гистамина (8 мг/кг/ч) стимулировали секрецию соляной кислоты, а желудок перфузировали AСК (25 ммоль/л) со скоростью 0,4 мл/мин. Выраженность желудочного кровотечения оценивали, определяя концентрацию гемоглобина в перфузате. Клопидогрел (30 мг/кг) вводили перорально за 24 ч до начала перфузии АСК. Ребамипид (3–30 мг/кг) или другие антисекреторные препараты вводили внутривенно перед перфузией АСК.
При перфузии желудка крыс АСК на фоне стимуляции секреции соляной кислоты возникало небольшое желудочное кровотечение, интенсивность которого значительно усиливалась в присутствии клопидогрела. Антисекреторные препараты (омепразол и фамотидин) подавляли секрецию соляной кислоты и предупреждали возникновение кровотечения при перфузии АСК в сочетании с клопидогрелом. Ребамипид не оказывал никакого влияния на секрецию соляной кислоты, но дозозависимо предотвращал желудочное кровотечение при перфузии АСК с клопидогрелом, причем степень ингибирования была эквивалентна таковой антисекреторных препаратов. Ребамипид также уменьшал тяжесть повреждений слизистой оболочки желудка. Эти данные свидетельствуют о том, что клопидогрел увеличивает интенсивность ЖКК, индуцируемого АСК на фоне стимуляции секреции соляной кислоты, а эффективность ребамипида в предотвращении ЖКК не уступает таковой антисекреторных препаратов.
Таким образом, результаты многих исследований подтверждают эффективность ребамипида в лечении и профилактике НПВП-гастропатии в сравнении как с плацебо, так и другими противоязвенными препаратами (ИПП, блокаторами Н2-рецепторов гистамина, мизопростолом). В российских рекомендациях «Коморбидная патология в клинической практике. Алгоритмы диагностики и лечения» [2] эксперты рассматривают профилактический прием ребамипида (по 100 мг 3 раза/ сут курсами по 2 мес) наряду с использованием ИПП и эрадикацией H. pylori в качестве мероприятий, направленных на снижение риска НПВП-гастропатии.
НПВП-ЭНТЕРОПАТИЯ
НПВП-энтеропатия – это осложнение, связанное с повышением кишечной проницаемости из-за повреждающего действия НПВП на эпителиоциты кишечника, приводящее к транслокации бактерий и их компонентов в толщу слизистой оболочки, подслизистый слой и далее в кровеносное русло, в результате чего развивается хроническое вялотекущее воспаление [22]. При этом повреждение слизистой оболочки тонкого кишечника сопровождается малозаметной кровопотерей, которая влечет за собой железодефицитную анемию. Сочетание железодефицитной анемии и гипоальбуминемии при отсутствии признаков НПВП-гастропатии при эндоскопическом исследовании считаются типичными клиническими проявлениями НПВП-энтеропатии. В некоторых случаях НПВП-энтеропатия может вызывать профузное кишечное кровотечение, перфорацию стенки кишки и появление характерных кольцевидных стриктур (так называемых мембран), приводящих к кишечной непроходимости.
Распространенность НПВП-энтеропатии аналогична таковой НПВП-гастропатии (0,5–1,0 случай на 100 пациенто-лет) [2], а частота кровотечений из нижнего отдела ЖКТ составляет не менее 30–50 % всех эпизодов ЖКК, ассоциированных с приемом НПВП [23–24]. Необходимо также отметить общее для НПВП-гастропатии и НПВП-энтеропатии звено патогенеза – блокаду циклооксигеназы-1 и подавление синтеза Pg в слизистой оболочке ЖКТ. При этом в патогенезе НПВП-энтеропатии, в отличие от гастропатии, важнейшую роль также играет повышение кишечной проницаемости и развитие воспаления, связанного с транслокацией бактерий. В то же время одним из звеньев патогенеза НПВП-гастропатии, не характерных для энтеропатии, выступает уменьшение защитного потенциала слизистой оболочки желудка и ее повреждение кислотой желудочного сока [2]. Именно этим объясняется эффективность ИПП, подавляющих секрецию соляной кислоты, при НПВП-гастропатии и соответственно их неэффективность при НПВП-энтеропатии.
Согласно результатам РКИ [25–26], при проведении капсульной эндоскопии эрозии и язвы в тонком кишечнике выявляют у 30–50 % лиц, принимавших неселективные НПВП в течение 2 нед. С помощью капсульной эндоскопии также было установлено, что низкие дозы АСК в кишечнорастворимой оболочке часто повреждают слизистую оболочку тонкого кишечника [27]. В это исследование включили 11 пациентов, у которых язвы желудка возникли на фоне приема низких доз АСК в кишечнорастворимой оболочке. Пациенты продолжали принимать АСК, одновременно получая ИПП для лечения язвы желудка в течение 8 нед, после чего вместо ИПП назначали мизопростол по 200 мкг 4 раза/сут еще на протяжении 8 нед. Через 8 нед терапии ИПП при проведении капсульной эндоскопии обнаружили петехии и повреждения слизистой оболочки тонкого кишечника у 100 (11/11) и 90,9% (10/11) больных соответственно, что подтверждает неэффективность ИПП в профилактике энтеропатии. Мизопростол, напротив, оказался действенным препаратом для последующего лечения АСК-индуцированной энтеропатии: у 7 пациентов количество петехий и повреждений слизистой оболочки значительно уменьшилось, у 4 было обнаружено полное заживление слизистой кишечника. В этой работе продемонстрировано, что кишечнорастворимая оболочка не всегда защищает желудок от ульцерогенного действия АСК, но при этом практически в 100% случаев возникают повреждения тонкого кишечника, т.е. АСК-индуцированная гастропатия почти всегда сочетается с энтеропатией, что необходимо учитывать при назначении лечения таким пациентам.
Частоту, локализацию и характер поражения тонкого кишечника оценили в японском ретроспективном исследовании [28] у пациентов с положительным результатом анализа кала на скрытую кровь или перенесших неясное ЖКК (n=181). Неясным считали ЖКК неизвестной этиологии, которое сохранялось или рецидивировало после исключения патологии ЖКТ при проведении ЭГДС, колоноскопии и рентгенологического исследования тонкого кишечника. Были проанализированы клинические данные и результаты лабораторно-инструментального обследования, включая капсульную эндоскопию.
Эрозивно-язвенные поражения тонкого кишечника были выявлены у 45 (25%) человек, 27 (60%) из которых принимали низкие дозы АСК или НПВП (7 – только НПВП, 9 – только АСК, 9 – АСК + производное тиенопиридина, 2 – АСК + варфарин). Частота выявления эрозивно-язвенных поражений кишечника составила 64, 75 и 80% у пациентов, принимавших только АСК, только НПВП и АСК + производное тиенопиридина соответственно. Эрозивные поражения наблюдали преимущественно у больных, получавших монотерапию АСК, а язвенные, напротив, чаще выявляли у лиц, принимавших НПВП. Однако при одновременном применении АСК и производного тиенопиридина (например, клопидогрела) возрастала доля язвенных поражений. Эрозии обнаруживали на всем протяжении тонкого кишечника, а язвы были локализованы в основном в подвздошной кишке (р <0,05).
Это исследование подтвердило, что длительное применение АСК и НПВП приводит к различным видам и локализации повреждений слизистой оболочки тонкого кишечника, а сочетанное использование АСК с другими антиагрегантами или антикоагулянтами может усиливать повреждающее действие на слизистую и приводить к более тяжелому поражению – язвам.
Следует подчеркнуть, что применение НПВП может вызывать не только эрозивно-язвенное поражение, но и воспаление слизистой кишечника, связанное с транслокацией бактерий в результате повышения проницаемости кишечной стенки. Это было продемонстрировано в работе Maiden L. et al. [29]. В исследование вошли 40 здоровых добровольцев, принимавших диклофенак по 75 мг 2 раза/сут в комбинации с омепразолом по 30 мг 2 раза/сут в течение 2 нед. Исходно и через 2 нед всем участникам выполнили капсульную эндоскопию и анализ на фекальный кальпротектин – кальций-связывающий белок, служащий маркером нейтрофильного кишечного воспаления.
Через 2 нед приема диклофенака у 30 (75%) обследуемых было выявлено повышение уровня фекального кальпротектина, который во всех случаях превышал верхнюю границу нормы по сравнению с таковым до лечения. При выполнении капсульной эндоскопии «новые» повреждения тонкого кишечника были обнаружены у 27 (68%) человек. Такими «находками» оказались разрывы слизистой оболочки – у 16 (40%) человек (у 2 из них проявились в виде кровотечения); гиперемированные складки – у 14 (35%); петехии или очаговая гиперемия – у 13 (33%); атрофия слизистой – у 8 (20%); кровь в просвете кишечника без визуализированного источника – у 3 (8%) больных. У 15 (56%) из 27 человек единовременно имелось более одного поражения слизистой. Помимо подтверждения взаимосвязи между применением НПВП и воспалительными изменениями слизистой кишечника, в данном исследовании у здоровых лиц была установлена достаточно высокая (68–75%) частота НПВП-энтеропатии после 2-недельного приема диклофенака, что указывает на необходимость назначения энтеропротекторов даже при непродолжительном лечении НПВП.
РЕБАМИПИД В ЛЕЧЕНИИ НПВП-ЭНТЕРОПАТИИ
В настоящее время единственным энтеропротектором является ребамипид, который обладает одновременно и гастро-, и энтеропротективным действием. Напомним, что гастропротективный эффект ребамипида обусловлен стимуляцией синтеза Pg Е2 в слизистой оболочке желудка и Pg Е2 и I2 в содержимом желудочного сока, тогда как его энтерпротективное действие связано с устранением повышенной проницаемости кишечной стенки и восстановлением целостности кишечного барьера посредством увеличения количества бокаловидных клеток и стимуляции их пролиферации, усиления (восстановления) плотных межклеточных контактов и подавления воспалительных реакций. Так, в экспериментальном исследовании [30] было показано, что введение ребамипида в дозе 320 мг/кг/сут в течение 5 дней мышам с АСК-индуцированным повреждением слизистой оболочки кишечника приводит к значительному улучшению плотных контактов между эпителиоцитами, повышению экспрессии циклооксигеназы-2 и белка адгезивных межклеточных контактов β-катенина, увеличению концентрации Pg Е2 и уменьшению выраженности АСК-индуцированного воспаления в слизистой кишечника.
Возможности использования ребамипида для профилактики и лечения НПВП-энтеропатии изучены в нескольких РКИ, в каждом из которых препарат сравнивался с плацебо. Например, в японском плацебо-контролируемом двойном-слепом перекрестном РКИ [31] оценили влияние низких доз АСК на кровоток в тонком кишечнике и повреждения слизистой оболочки, а также эффективность ребамипида при АСК-индуцированной энтеропатии у 10 здоровых добровольцев в возрасте 29±5 лет, которых рандомизировали на 2 группы: в группе плацебо (n=5) назначали АСК по 100 мг/сут и плацебо, в группе ребамипида (n=5) – АСК по 100 мг/сут и ребамипид по 300 мг/сут. Длительность лечения в каждой группе составила 14 дней, затем следовал как минимум 2-недельный «отмывочный» период, после чего лечение меняли на противоположное в течение еще 14 дней. До и после каждого периода лечения всем пациентам выполняли капсульную эндоскопию и ультразвуковое исследование (УЗИ) тонкого кишечника с усилением контрастным веществом. Первичной конечной точкой была оценка изменения АСК-индуцированного кровотока в тонком кишечнике, который измеряли при помощи УЗИ с усилением контрастным веществом. На основании полученных ультразвуковых изображений строили кривую интенсивности времени сигнала, рассчитывали площадь под кривой и пиковое значение интенсивности времени сигнала. Эти показатели использовали для оценки кровотока в слизистой оболочке тонкого кишечника. Вторичной конечной точкой была оценка профилактического эффекта ребамипида в отношении развития АСК-индуцированной энтеропатии.
Прием низких доз АСК приводил к снижению кровоснабжения слизистой оболочки тонкого кишечника, о чем свидетельствовало уменьшение площади под кривой и пикового значения кривой интенсивности времени. Абсолютная разность между значениями этих показателей до и после приема АСК представлена в таблице.
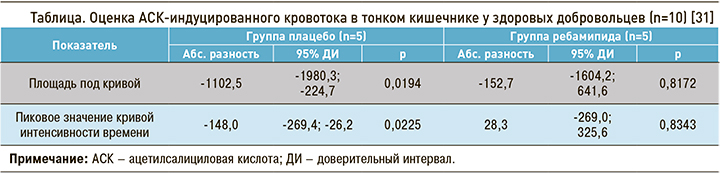
Как видно из таблицы, выраженная разница значений изученных показателей на фоне приема АСК в группе плацебо оказалась статистически значимой: р для площади под кривой = 0,0194; р для пикового значения кривой интенсивности времени = 0,0225. Это указывает на значительное уменьшение кровотока в слизистой тонкого кишечника. В группе ребамипида, напротив, существенной динамики данных показателей на фоне приема АСК отмечено не было (р для площади под кривой = 0,8172; р для пикового значения кривой интенсивности времени = 0,8343), т.е. применение препарата оказывает защитный эффект и препятствует ухудшению кровоснабжения слизистой кишечника под действием АСК.
При проведении капсульной эндоскопии были обнаружены поражения слизистой оболочки тонкого кишечника только в группе плацебо. Не было отмечено образования язв, эпизодов кровотечений или перфорации стенки кишечника. В группе плацебо у 2 пациентов выявили разрывы слизистой оболочки подвздошной кишки, тогда как в группе ребамипида таких случаев не было. Полученные результаты подтверждают гипотезу, что нарушение кровотока является одним из механизмов НПВП-индуцированных поражений тонкого кишечника и демонстрируют эффективность ребамипида в уменьшении и предупреждении АСК-индуцированной энтеропатии у здоровых добровольцев.
В другое японское одноцентровое двойное-слепое плацебо-контролируемое перекрестное РКИ [32] включили 11 здоровых мужчин в возрасте от 24 до 43 лет (медиана 27 лет), которых рандомизировали на 2 группы. Участники из группы А (плацебо) получали АСК по 100 мг/сут, омепразол по 20 мг/ сут и плацебо; участники из группы В (ребамипид) – АСК по 100 мг/сут, омепразол по 20 мг/ сут и ребамипид по 300 мг/сут. Длительность лечения в каждой группе составила 4 нед, затем следовал 4-недельный «отмывочный» период, после которого лечение меняли на противоположное на протяжении еще 4 нед. Для оценки повреждений тонкого кишечника всем обследуемым выполнили капсульную эндоскопию через 1 и 4 нед от начала лечения.
В тощей кишке повреждения слизистой оболочки обнаруживали значительно реже, чем в подвздошной. Через 1 нед повреждения слизистой оболочки подвздошной кишки возникли у 1 человека в группе ребамипида и у 6 – в группе плацебо (р=0,0173), через 4 нед – у 2 человек в группе ребамипида и у 7 – в группе плацебо (р=0,0266). Через 4 нед в группе ребамипида среднее количество петехий и эрозий в подвздошной кишке было в 3 раза меньше, чем в группе плацебо: 15,7 против 48,4 (р=0,0354) и 0,6 против 2,0 (р=0,0362) соответственно. Как и предыдущее РКИ, эта работа подтверждает эффективность ребамипида в профилактике АСК-индуцированной энтеропатии по сравнению с плацебо.
Еще в одном японском многоцентровом двойном-слепом плацебо-контролируемом РКИ [33] участвовали пациенты, принимавшие АСК или НПВП в течение ≥3 мес. Больных с НПВП-энтеропатией (n=61) рандомизировали в группы ребамипида (по 100 мг 3 раза/сут) и плацебо. Длительность лечения составила 4 нед. Капсульную эндоскопию выполнили исходно и через 4 нед. Оценивали количество мелких язв и эрозий в тонком кишечнике. В качестве маркера трофического статуса определяли содержание общего белка в крови.
Динамика количества эрозий составила -2,5±3,4 в группе ребамипида и 2,1±3,9 в группе плацебо (р <0,0001), т.е. в группе ребамипида количество эрозий уменьшилось в среднем на 2,5, а в группе плацебо, напротив, увеличилось на 2,1. Динамика количества язв составила -0,5±1,6 и 0,1±0,7 соответственно (р=0,024). Изменение концентрации общего белка в крови составило 0,06±0,36 г/дл в группе ребамипида и -0,27±0,34 г/дл в группе плацебо (р=0,0005). Полная ремиссия была отмечена у 9 больных (37,5%) в группе ребамипида и только у одного (5,3%) в группе плацебо. Таким образом, по сравнению с плацебо ребамипид не только оказывал лечебный эффект при НПВП-энтеропатии, но и улучшал трофический статус.
Представляют несомненный интерес результаты еще одного японского многоцентрового двойного-слепого плацебо-контролируемого РКИ [34], особенностью которого стало применение тройной дозы ребамипида при АСК-индуцированной энтеропатии умеренной и тяжелой степени. В него включали пациентов, принимавших кишечнорастворимую форму АСК в дозе 100 мг/сут не менее 3 мес, у которых при капсульной эндоскопии было обнаружено более 3 повреждений (эрозий или язв) слизистой оболочки тонкого кишечника. Пациентов рандомизировали в группы ребамипида (по 300 мг 3 раза/сут) или плацебо в соотношении 2:1. Длительность лечения составила 8 нед. Исходно и через 8 нед проводили капсульную эндоскопию. Первичной конечной точкой считали изменение количества повреждений слизистой оболочки тонкого кишечника на фоне лечения. Вторичные конечные точки включали полное заживление повреждений слизистой оболочки и изменение балла Lewis (балл, определяемый при эндоскопической оценке и отражающий тяжесть повреждения слизистой) через 8 нед.
Исследование завершили 38 из 43 пациентов (25 человек в группе ребамипида; 13 – в группе плацебо). Через 8 нед терапии количество повреждений слизистой оболочки в группе ребамипида значительно уменьшилось (с 4 до 2; p=0,046), тогда как в группе плацебо была отмечена лишь тенденция к снижению (с 6 до 3; р=0,08). Частота полного заживления повреждений слизистой оболочки в группе ребамипида оказалась в 4,2 раза выше (32 %, 8/25), чем в группе плацебо (7,7%, 1/13), хотя различия между группами не были статистически значимыми (p=0,13), вероятно, из-за небольшой численности групп. Лечение ребамипидом также значительно уменьшало тяжесть поражения слизистой тонкого кишечника по оценке Lewis (p=0,02), тогда как в группе плацебо она существенно не изменилась (р=0,32). Ни в одной из групп не было выявлено значительных изменений уровней гемоглобина и сывороточного альбумина через 8 нед лечения. Таким образом, применение тройной (900 мг/сут) дозы ребамипида в течение 8 нед у пациентов с умеренной и тяжелой АСК-индуцированной энтеропатией оказалось эффективным и безопасным.
По данным метаанализа 15 РКИ [35], в сравнении с плацебо ребамипид – более эффективное средство для заживления эрозий и язв при НПВП-энтеропатии (ОШ 2,70; 95% ДИ 1,02–7,16; р=0,045).
Ребамипид упоминается в нескольких российских регламентирующих документах [2, 6, 22, 36] как препарат для профилактики и лечения НПВП-энтеропатии. Формулировки рекомендаций, естественно, несколько различаются, но общее мнение экспертов сводится к тому, что ребамипид может быть использован при НПВП-индуцированных повреждениях слизистых оболочек желудка и тонкого кишечника. При этом в рекомендациях по профилактике и лечению эзофагогастроэнтероколопатий, индуцированных НПВП [6], эксперты делают акцент на том, что «ребамипид является средством выбора при НПВП-индуцированной энтеро- или колопатии». В рекомендациях «Коморбидная патология в клинической практике. Алгоритмы диагностики и лечения» [2] эксперты рассматривают профилактический прием ребамипида (по 100 мг 3 раза/сут курсами по 2 мес) в качестве единственной меры, направленной на снижение риска НПВП-энтеропатии. В этом же документе [2] эксперты отмечают, что «эффективным методом профилактики развития эрозивно-язвенных изменений слизистой оболочки верхних отделов ЖКТ, тонкой и толстой кишки может быть назначение ребамипида, что доказано в серии рандомизированных контролируемых испытаний».
Сочетание гастро- и энтеропротективного действия является безусловным преимуществом ребамипида перед другими гастропротекторами, что позволяет использовать его как при гастро-, так и при энтеропатии. Напомним, что АСК-индуцированная гастропатия, которая развивается несмотря на применение кишечнорастворимой формы АСК, призванной защитить желудок от ее повреждающего действия, в 100% случаев сочетается с энтеропатией, поэтому назначение ребамипида может быть эффективным решением у таких пациентов.
Результаты нескольких РКИ [27, 29, 32] подтверждают, что применение ИПП при НПВП-энтеропатии не оказывает ни лечебного, ни профилактического эффекта. Более того, есть данные [37–39], что использование ИПП повышает риск развития НПВП-энтеропатии, может усиливать повреждения слизистой тонкого кишечника и ухудшать течение НПВП-энтеропатии при совместном применении с НПВП. Очевидно, что пациентам с НПВП-энтеропатией не следует назначать ИПП даже с целью гастропротекции. В таких ситуациях оптимальным вариантом также будет назначение ребамипида.
Заметим, что синтетический аналог Pg Е1 мизопростол является гастро-, а не энтеропротектором, хотя он и продемонстрировал эффективность в лечении АСК-индуцированной энтеропатии в одном из исследований [27]. Однако мизопростол воздействует лишь на одно из звеньев патогенеза НПВП-энтеропатии – подавление синтеза простагландинов в слизистой оболочке кишечника и, в отличие от ребамипида, абсолютно не влияет на повышенную проницаемость кишечной стенки. Кроме того, российские эксперты [6] констатируют, что «в российской практике препарат мизопростол используется крайне редко из-за большого количества побочных эффектов». Ребамипид же не только устраняет повышенную кишечную проницаемость, но и стимулирует синтез простагландинов в слизистой ЖКТ.
В соответствии с инструкцией к препарату, одним из показаний для назначения ребамипида является «профилактика возникновения повреждений слизистой оболочки на фоне приема НПВП». С этой целью ребамипид применяют по 100 мг 3 раза/сут до 8 нед курсами 2 раза в год.
РИСК ЖКК И ВОЗМОЖНОСТИ ГАСТРОПРОТЕКЦИИ У ПАЦИЕНТОВ, ПОЛУЧАЮЩИХ ПЕРОРАЛЬНЫЕ АНТИКОАГУЛЯНТЫ ПРЯМОГО ДЕЙСТВИЯ
В последние годы в кардиологической практике все шире применяются пероральные антикоагулянты прямого действия (ППОАК), к которым относятся ингибитор тромбина дабигатрана этексилат (далее – дабигатран) и ингибиторы Ха-фактора ривароксабан, апиксабан и эдоксабан. Основные показания для их назначения – профилактика кардиоэмболического инсульта при неклапанной фибрилляции предсердий (ФП), лечение острых эпизодов и вторичная профилактика венозных тромбоэмболических осложнений, профилактика тромбоэмболических осложнений при эндопротезировании крупных суставов. Подобно другим антитромботическим препаратам, главной проблемой при использовании ППОАК являются геморрагические осложнения, включая ЖКК.
Результаты как РКИ, так и наблюдательных исследований реальной клинической практики у пациентов с неклапанной ФП свидетельствуют о том, что по сравнению с варфарином применение ривароксабана, дабигатрана в дозе 150 мг 2 раза/ сут и эдоксабана в дозе 60 мг 1 раз/сут ассоциируется с более высоким риском ЖКК. Так, в РКИ с применением ППОАК риск большого ЖКК у пациентов, получавших дабигатран по 150 мг 2 раза/ сут, был выше на 48% (ОР 1,48; 95% ДИ 1,19–1,86; р <0,001) [40], ривароксабан – на 61% (ОР 1,61; 95% ДИ 1,30–1,99; р <0,001) [41], эдоксабан по 60 мг 1 раз/сут – на 23% (ОР 1,23; 95% ДИ 1,02–1,50; р=0,03) [42] по сравнению с варфарином. В метаанализе всех РКИ [43], объединившем более 70 000 пациентов с ФП, из которых 29 272 человека получали варфарин, а 42 411 – один из четырех ППОАК (пациентов, получавших дабигатран по 110 мг 2 раза/сут и эдоксабан по 30 мг 1 раз/сут, из анализа исключили; проанализировали эффект только высоких доз дабигатрана и эдоксабана), было показано, что в целом применение ППОАК увеличивает риск большого ЖКК на 25% (ОР 1,25; 95% ДИ 1,01–1,55; р=0,04) по сравнению с варфарином.
Терапия ППОАК может приводить к кровотечениям как из верхнего, так и нижнего отдела ЖКТ. Например, в исследовании RE-LY [44] 53% больших ЖКК возникли в нижнем отделе ЖКТ. Интересно, что частота ЖКК из верхнего отдела ЖКТ практически не различалась между группами дабигатрана 110 и 150 мг и варфарина, тогда как ЖКК из нижнего отдела ЖКТ значительно чаще возникали у леченных дабигатраном. Так, по сравнению с варфарином риск большого ЖКК из нижнего отдела ЖКТ у пациентов, принимавших дабигатран по 150 мг 2 раза/сут, был выше в 2,2 раза (ОР 2,23; 95% ДИ 1,47–3,38), а у пациентов, получавших дабигатран по 110 мг 2 раза/сут, – на 78% (ОР 1,78; 95% ДИ 1,16–2,75). Предполагается, что это связано с неполной абсорбцией активного дабигатрана в верхнем отделе ЖКТ. Это приводит к увеличению биодоступности препарата в нижнем отделе ЖКТ, который оказывает местное повреждающее воздействие на слизистую оболочку, приводящее к ЖКК, особенно при наличии уже существующей патологии ЖКТ, такой как ангиодисплазии и эрозии.
В отличие от RE-LY, в исследовании ROCKET-AF [45] у больных ФП преобладали кровотечения из верхнего отдела ЖКТ, на долю которых пришлось 48% всех эпизодов ЖКК, в то время как частота ЖКК из нижнего отдела ЖКТ составила 22%, из прямой кишки – 30%. При этом частота ЖКК из верхнего и нижнего отделов ЖКТ была практически одинаковой в группах ривароксабана и варфарина.
По данным крупного метаанализа [46], включившего 43 исследования и более 160 000 пациентов, получавших ППОАК, частота ЖКК из верхнего отдела ЖКТ все же несколько выше, чем из нижнего: 1,5 против 1,0% в год.
Известны несколько механизмов, посредством которых ППОАК вызывают ЖКК [47]:
- локальный (топический) антикоагулянтный эффект, связанный с неполной абсорбцией препарата из ЖКТ (в отличие от ППОАК, варфарин не оказывает местного повреждающего воздействия на слизистую ЖКТ даже в случае неполной абсорбции; у пациентов, получающих варфарин, ЖКК возникают в результате системного антикоагулянтного эффекта препарата);
- ингибирование заживления слизистой оболочки ЖКТ;
- прямое повреждающее действие винной кислоты, присутствующей в капсуле с дабигатраном (это вспомогательное вещество снижает рН желудка и необходимо для улучшения всасывания лекарственного средства).
Риск ЖКК и эффективность гастропротекторов в отношении профилактики ЖКК у пациентов, получающих ППОАК, оценили в ряде исследований. В гонконгском ретроспективном когортном популяционном исследовании [48] участвовали 5041 пациент, принимавших дабигатран. Частота ЖКК составила 2,5% (4,2/100 пациенто-лет). Анализ подгрупп показал, что у больных в возрасте ≥75 лет риск ЖКК был выше в 2,5 раза (ОР 2,47; 95% ДИ 1,66–3,68); у пациентов с язвенной болезнью или ЖКК в анамнезе – в 2,3 раза (ОР 2,31; 95% ДИ 1,54–3,46); у больных, принимавших АСК в сочетании с дабигатраном, – в 1,5 раза (ОР 1,52; 95% ДИ 1,03–2,24). Сопутствующее применение гастропротекторов было связано со снижением риска ЖКК на 48% (ОР 0,52; 95% ДИ 0,35–0,77). При этом использование ИПП снижало риск ЖКК на 47% (ОР 0,53; 95% ДИ 0,31–0,91), а блокаторов Н2-рецепторов гистамина – на 39% (ОР 0,61; 95% ДИ 0,40–0,94). Дальнейший анализ показал, что прием гастропротекторов снижал риск ЖКК только из верхнего отдела ЖКТ (ОР 0,29; 95% ДИ 0,15–0,54) и только у пациентов с язвенной болезнью или ЖКК в анамнезе (ОР 0,14; 95% ДИ 0,06–0,30). Таким образом, в гонконгской популяции использование гастропротекторов ассоциировалось со снижением риска ЖКК у пациентов, принимавших дабигатран, однако они были эффективны для снижения риска ЖКК только из верхнего отдела ЖКТ и у пациентов с язвенной болезнью или ЖКК в анамнезе.
В японском ретроспективном исследовании [49] участвовали 658 пациентов (средний возраст 72 года; 68% мужчин), получавших ППОАК (дабигатран, n=220; ривароксабан, n=283; апиксабан, n=155). Частота всех ЖКК составила 2,0% в год, в том числе больших ЖКК – 0,9 % в год. Особенностью этого исследования оказалась более высокая частота ЖКК из нижнего отдела ЖКТ – 1,3 против 0,7% в год, т.е. такие кровотечения возникли у двух из трех пациентов. По данным однофакторного анализа, сопутствующее применение низких доз АСК увеличивало риск ЖКК из нижнего отдела ЖКТ в 3 раза (ОР 3,03; 95% ДИ 1,19–7,68; р=0,02), двойной антитромбоцитарной терапии – в 7 раз (ОР 7,19; 95% ДИ 2,34–22,10; р <0,01), НПВП – в 10 раз (ОР 10,19; 95% ДИ 2,70–38,40; р <0,01). Язвенная болезнь в анамнезе ассоциировалась с повышением риска ЖКК из верхнего отдела ЖКТ почти в 17 раз (ОР 16,80; 95% ДИ 4,50–62,60; р <0,01), а вот ЖКК в анамнезе, напротив, увеличивало риск ЖКК из нижнего отдела ЖКТ в 16 раз (ОР 15,90; 95% ДИ 3,60–70,00; р <0,01). Многофакторный анализ идентифицировал 2 независимых предиктора ЖКК из верхнего отдела ЖКТ: применение ИПП, которое ассоциировалось со снижением риска ЖКК до 0 (ОР 0; 95% ДИ 0–2Е+134; р <0,001), поскольку среди пациентов с ЖКК из верхнего отдела ЖКТ никто не получал ИПП, и язвенная болезнь в анамнезе, наличие которой увеличивало риск ЖКК в 29 раз (ОР 29,11; 95% ДИ 7,27–116,68; р <0,001). Независимыми предикторами ЖКК из нижнего отдела ЖКТ оказались сопутствующее применение НПВП (ОР 12,60; 95% ДИ 3,20–49610; р <0,001) и двойной антитромбоцитарной терапии (ОР 8,60; 95% ДИ 2,70–27,10; р <0,001), а также ЖКК в анамнезе (ОР 15,10; 95% ДИ 3,20–72,00; р=0,001) и женский пол.
Еще в одном ретроспективном исследовании [50] оценили эффективность гастропротекторов в снижении риска ЖКК из верхнего отдела ЖКТ у 2076 пациентов, получавших ППОАК, среди которых только 360 человек (17%) принимали гастропротекторы. Частота ЖКК из верхнего отдела ЖКТ составила 0,3% (1/360; 0,7 на 100 пациенто-лет) у больных, получавших гастропротекторы, и 1,7% (29/1716; 2,8 на 100 пациенто-лет) у пациентов, не получавших таковых (p=0,189). Многофакторный анализ показал, что возникновение ЖКК из верхнего отдела ЖКТ было ассоциировано с пожилым возрастом (ОР 1,04; р=0,048), язвенной болезнью или ЖКК из верхнего отдела ЖКТ в анамнезе (ОР 5,93; р <0,001) и сопутствующим использованием антитромбоцитарных препаратов (ОР 3,12; p=0,014). Прием гастропротекторов не снижал риск ЖКК из верхнего отдела ЖКТ (p=0,289). Однако анализ подгруппы пациентов с сопутствующим применением антитромбоцитарных препаратов или язвенной болезнью/ЖКК из верхнего отдела ЖКТ в анамнезе (n=225) обнаружил тенденцию к снижению риска ЖКК из верхнего отдела ЖКТ у больных, получавших гастропротекторы (0 на 100 пациенто-лет), по сравнению с пациентами, не получавшими таковых (11,3 на 100 пациенто-лет) (p=0,065).
Таким образом, в совокупности результаты вышеупомянутых исследований указывают на то, что профилактическое применение гастропротекторов может снизить риск ЖКК из верхнего отдела ЖКТ у пациентов, получающих ППОАК и имеющих факторы риска ЖКК, такие как сопутствующее применение НПВП и/или антитромбоцитарных препаратов, язвенная болезнь и/или ЖКК в анамнезе. Рутинное же назначение гастропротекторов всем пациентам, принимающим ППОАК, нецелесообразно.
ПЕРСПЕКТИВЫ ПРИЕМА РЕБАМИПИДА НА ФОНЕ ИСПОЛЬЗОВАНИЯ ПЕРОРАЛЬНЫХ АНТИКОАГУЛЯНТОВ ПРЯМОГО ДЕЙСТВИЯ
К сожалению, доказательная база по применению ребамипида для защиты слизистой ЖКТ и профилактики ЖКК на фоне приема ППОАК пока отсутствует, однако такие исследования абсолютно необходимы, поскольку данный препарат обладает несомненным потенциалом в этом отношении. Учитывая особенности его гастро- и энтеропротективного действия, разумно ожидать, что ребамипид способен воздействовать на все вышеописанные механизмы, посредством которых ППОАК провоцируют развитие ЖКК, поэтому сможет и защитить слизистую желудка от повреждающего воздействия ППОАК в случае неполной абсорбции (в том числе от винной кислоты, присутствующей в капсуле с дабигатраном), и усилить заживление слизистой оболочки ЖКТ, которое ингибируется на фоне приема ППОАК. В настоящее время имеются результаты всего одного исследования [51], в котором изучили возможности применения ребамипида для лечения диспепсии, связанной с терапией дабигатраном у пациентов с неклапанной ФП.
Диспепсия, типичными клиническими проявлениями которой выступают гастралгии, тяжесть в эпигастральной области и тошнота, достаточно часто ассоциируется с приемом антитромботических препаратов и НПВП, но может возникать и на фоне приема антикоагулянтов. Дабигатран чаще других ППОАК вызывает диспепсию из-за наличия в его капсуле винной кислоты, оказывающей раздражающее действие на слизистую желудка. В исследовании RE-LY [40] частота диспепсии была значимо выше у пациентов, получавших дабигатран (16,9 против 9,4% в группе варфарина; ОР 1,81; 95% ДИ 1,66–1,97; р <0,001), но при этом не зависела от его дозы. Bytzer P. et al. [52] проанализировали НЯ со стороны ЖКТ (исключая ЖКК) у 18 113 пациентов с неклапанной ФП, участвовавших в исследовании RE-LY. Все НЯ исследователи разделили на 4 группы: гастроэзофагеальный рефлюкс, диспепсия, нарушение моторики верхнего отдела ЖКТ и повреждение слизистой оболочки желудка и/или ДПК.
В группе дабигатрана среди пациентов с любым НЯ со стороны ЖКТ (n=2045) симптомы были расценены как легкие, средние и тяжелые в 46,3; 44,8 и 8,9% случаев соответственно; это соотношение было аналогично таковому в группе варфарина. Наиболее частым среди четырех групп НЯ был гастроэзофагеальный рефлюкс (ОР 3,71; 95% ДИ 2,98–4,62; р <0,001). Частота прекращения антикоагулянтной терапии из-за НЯ со стороны ЖКТ оказалась выше в группе дабигатрана и составила 4 против 1,7% в группе варфарина (ОР 2,34; 95% ДИ 1,90–2,88; р <0,001). Возникновение НЯ со стороны ЖКТ у леченных дабигатраном ассоциировалось с более высокой частотой большого ЖКК (6,8 против 2,3% в группе варфарина; р <0,001), т.е. диспепсия, возникающая на фоне приема дабигатрана, может предшествовать развитию большого ЖКК. Эти результаты указывают на необходимость назначения гастропротекторов пациентам, получающим дабигатран, с целью купирования симптомов диспепсии, повышения приверженности к лечению и профилактики ЖКК.
В 2014 г. были опубликованы результаты многоцентрового проспективного открытого сравнительного РКИ в параллельных группах [51], в котором оценивались возможности использования ИПП, блокатора Н2-рецепторов гистамина или ребамипида для лечения диспепсии у 309 пациентов с неклапанной ФП, впервые начавших терапию дабигатраном. НЯ со стороны ЖКТ оценивали при помощи 7-балльного опросника Global Overall Severity (GOS). Пациентов с оценкой GOS ≥3 баллов рандомизировали на 3 группы для лечения ИПП, блокатором Н2-рецепторов гистамина или ребамипидом в течение 4 нед.
Частота симптомов диспепсии составила 17,2% (53/309), причем 77% НЯ были зафиксированы в первые 10 дней от начала лечения. Из-за НЯ прием дабигатрана прекратили 5 пациентов. Через 4 нед лечения средний балл по шкале GOS составил 3,5±1,7, при этом доля больных с GOS ≥3 баллов уменьшилась со 100 до 11,3%. Многофакторный регрессионный анализ не обнаружил ни одного фактора, значимо влияющего на частоту или тяжесть симптомов диспепсии. Большинство пациентов (83–100%) отметило уменьшение выраженности симптомов на фоне лечения (GOS ≤2 баллов), при этом ИПП, блокаторы Н2-рецепторов гистамина и ребамипид были одинаково эффективны для купирования симптомов диспепсии, связанной с приемом дабигатрана.
По данным клинических исследований, факторами риска ЖКК, ассоциированных с терапией ППОАК, служат пожилой возраст, нарушение функции почек, инфицирование H. pylori, язвенная болезнь/ЖКК в анамнезе и сопутствующее применение препаратов с ульцерогенным действием (НПВП, антиагреганты, глюкокортикостероиды). Профилактика ЖКК включает тщательный отбор пациентов для терапии ППОАК с учетом абсолютных и относительных противопоказаний для антикоагулянтной терапии, выявление и коррекцию модифицируемых факторов риска кровотечений, использование более низких доз некоторых ППОАК у пациентов с высоким риском ЖКК и нарушением функции почек (в соответствии с клиническими рекомендациями), назначение гастропротекторов.
В настоящее время в нашей стране отсутствуют рекомендации по использованию гастропротекторов у пациентов, получающих ППОАК. Однако результаты клинических исследований позволяют рекомендовать врачам рассмотреть вопрос о назначении гастропротекторов как минимум у пациентов с высоким риском ЖКК и в обязательном порядке у пациентов, получающих пероральные антикоагулянты (в том числе ППОАК) в сочетании с антиагрегантами (двойная или тройная антитромботическая терапия) или НПВП. Учитывая имеющуюся на данный момент доказательную базу, пациентам с высоким риском ЖКК, особенно из верхнего отдела ЖКТ, можно рекомендовать профилактический прием ИПП, а пациентам, получающим АСК, НПВП или двойную антитромбоцитарную терапию одновременно с ППОАК, целесообразно назначать ребамипид – уникальный гастроэнтеропротектор, подтвердивший высокую эффективность и безопасность для лечения и профилактики НПВП-гастро- и энтеропатий в серии РКИ.


