Хроническая болезнь почек (ХБП) – распространенная патология, объединяющая широкий спектр первичных и вторичных заболеваний почек, c тенденцией к ежегодному приросту [1]. По данным ряда эпидемиологических исследований, распространенность ХБП составляет около 13,4%, колеблясь в разных странах от 11,7 до 15,1%, а больных, получающих заместительную почечную терапию, насчитывается порядка 7,083 млн [2].
Механизмы повреждения почечной паренхимы и прогрессирования почечной недостаточности разнообразны: среди них выделяют такие, как гиперактивация ренин-ангиотензин-альдостероновой системы (РААС), гиперурикемия, гипергликемия, оксидативный стресс, активация эпителиально-мезенхимального перехода, аутофагии и апоптоза и многие другие [3, 4]. Особый интерес представляет развитие оксидативного стресса и изучение путей его влияния на развитие и прогрессирование подоцитопатии, тубулоинтерстициального фиброза, повреждения мезангиальных клеток, эпителия проксимальных канальцев [5]. Оксидативный стресс выступает неотъемлемой частью воспаления, наблюдающегося в почечной паренхиме при большинстве ХБП [6]. Изучение различных путей воздействия на оксидативный стресс при ХБП, в том числе медикаментозных, является перспективным направлением исследований в области нефрологии [7]. Именно это и стало целью предлагаемого исследования.
МАТЕРИАЛ И МЕТОДЫ
Нами проведено ретроспективное сравнительное неинтервенционное исследование 80 пациентов (34 мужчины и 46 женщин, средний возраст 58,8±13,2 лет) с ХБП С3А-5Д (ХБП С3А – 13,7%, 3Б – 20%, 4 – 12,5%, 5 – 3,8%, 5Д – 50%). Длительность ХБП составляла 8,68±9,3 лет (разброс 0,5–37 лет), длительность гемодиализа – 53,6±47,4 мес (разброс 4–148 мес). Все пациенты подразделялись на консервативную (n=40) и диализную группы (n=40).
Критерии включения: больные ХБП 3А-5Д стадий различного генеза; возраст от 20 до 80 лет; при ХБП С5Д терапия гемодиализом.
Критерии исключения: острая или обострение хронической инфекции; острые заболевания различной природы; беременность и кормление грудью; проведение иммунодепрессивной терапии; болезни накопления и другие наследственные заболевания; системные заболевания соединительной ткани.
В ходе исследования выполнялись опрос, сбор анамнеза заболевания и жизни, объективное обследование пациентов и ряд дополнительных методов исследования. Оценивалась скорость клубочковой фильтрации (СКФ) расчетным путем по формуле CKD-EPI (мл/мин/1,73м2).
Всем больным проводилось клинико-лабораторное исследование в соответствии со стандартным протоколом. Определялись сывороточные уровни малонового диальдегида (МДА), супероксиддисмутазы (СОД), асимметричного диметиларгинина (АДМА) методом количественного иммуноферментного анализа (ИФА) (Luminex MAGPIX, США). Медиана распределения СОД в группе составила 23,3 нг/мл, АДМА – 46,0 нг/мл, МДА – 6,1 нмоль/л.
Биоимпедансометрия выполнялась всем больным однократно с помощью анализатора водных секторов «Диамант АИСТ-мини» (Диамант, СПб.). Рассчитывались следующие параметры: жировая масса (ЖМ), безжировая масса (БЖМ), общая вода (ОВ), общий объем жидкости (ООЖ), объем внутриклеточной жидкости (ОвнутЖ), объем внеклеточной жидкости (ОвнекЖ).
В рамках статистического анализа мы определяли средние значения признака (средняя арифметическая), принимая во внимание стандартное отклонение или медиану с учетом первого и третьего квартилей распределения. Для сравнения двух групп при условии нормальности распределения применялcя критерий Стьюдента, при распределении, отличном от нормального, – критерий Манна–Уитни. В случае нормального распределения признаков оценивалась корреляционная связь Пирсона, при ненормальном распределении – Спирмена. Осуществлялся логит-регрессионный анализ.
В ходе ретроспективного анализа регистрировались виды терапии больных продолжительностью 12 мес и более до включения пациентов в протокол исследования (рис. 1).
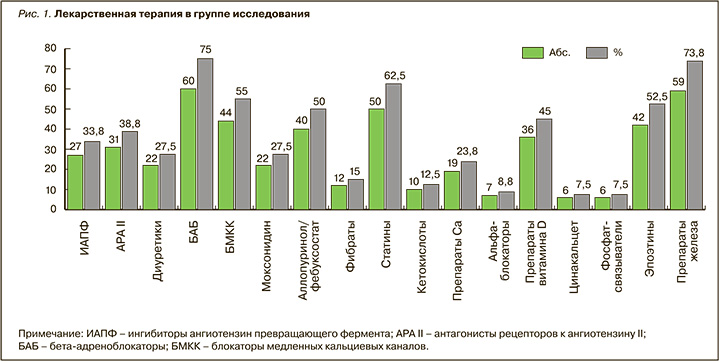
РЕЗУЛЬТАТЫ
Нами изучались данные о возможном влиянии медикаментозной терапии, а также особенностей диализной терапии на состояние оксидативного стресса и антиоксидантной защиты. Результаты логит-регрессионного анализа, в рамках которого был осуществлен анализ влияния различных вариантов лечения на вероятность повышения уровня МДА в крови, представлен в таблице 1.
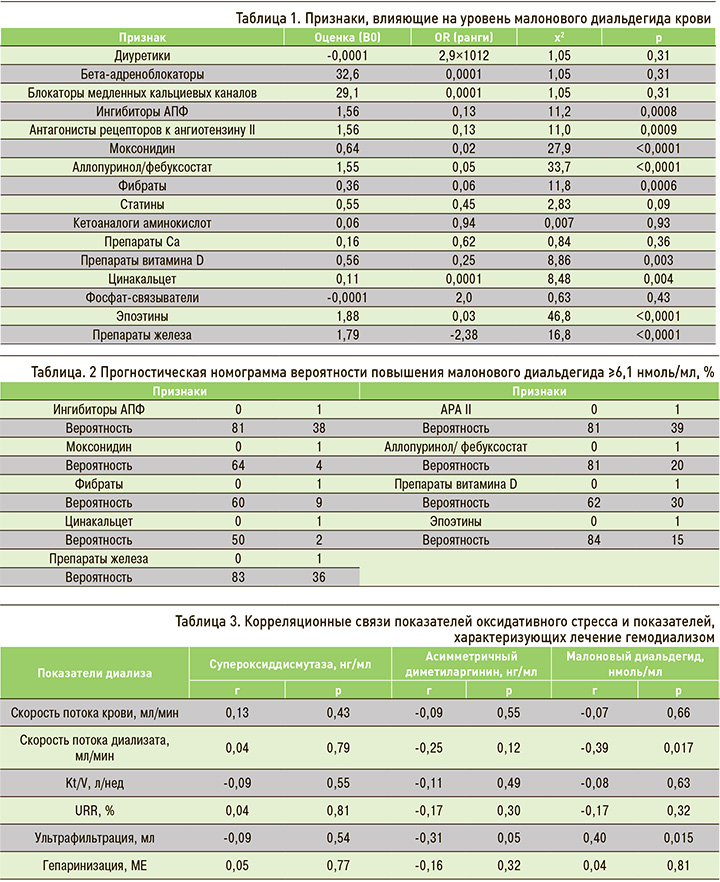
Было установлено, что среди антигипертензивных препаратов бета-блокаторы, блокаторы медленных кальциевых каналов и диуретики не оказывали влияния на уровень МДА, тогда как применение ингибиторов АПФ или антагонистов рецепторов к ангиотензину II сопровождалось снижением выраженности оксидативного стресса (рис. 2). Схожим эффектом обладал и моксонидин.
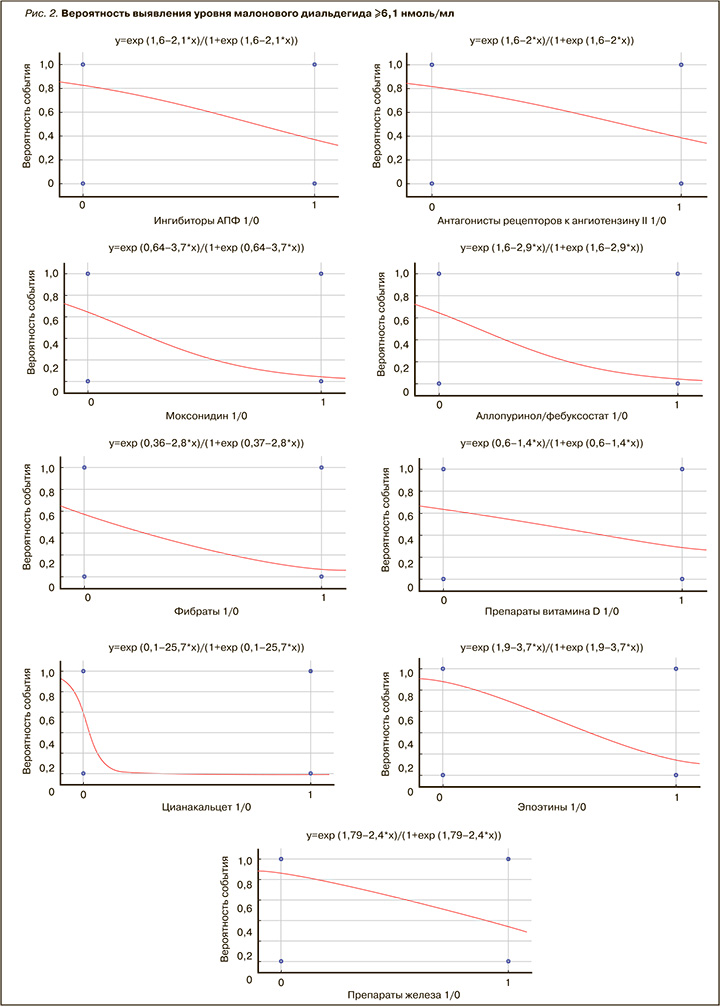
Было обнаружено позитивное влияние на оксидативный стресс уратснижающих средств (аллопуринол или фебуксостат) и фибратов. Применение препаратов витамина D, эпоэтинов и препаратов железа также сопровождалось антиоксидантным эффектом в виде снижения уровня МДА в крови.
Таким образом, применение препаратов, обладающих способностью снижать уровень МДА в крови, отражает их плейотропные свойства в виде антиоксидантной активности. Нами была сформирована прогностическая номограмма, с помощью которой представляется возможным оценивать риск повышения уровня МДА в крови (табл. 2).
Также нами были изучены данные о влиянии параметров проводимого гемодиализа на показатели оксидативного стресса и антиоксидантной защиты (табл. 3). Установлено, что по мере увеличения продолжительности гемодиализа происходило постепенное снижение уровня АДМА, что свидетельствует, вероятно, о постепенной адаптации эндотелия сосудов к проведению процедур на фоне регулярной аппаратной детоксикации. Вместе с тем низкая скорость потока диализата, приводящая к менее эффективной детоксикации в процессе процедуры, сопровождается ростом уровня МДА в крови, т.е. активацией оксидативного стресса. Еще одним важным фактором, способным активировать оксидативный стресс, является высокий уровень ультрафильтрации. Он обычно устанавливается тем больным, которые имеют выраженные явления гипергидратации из-за несоблюдения ограничений по приему жидкости в междиализный период.
Подтверждением этой гипотезы служит тот факт, что уровень МДА был высок у больных с явлениями внеклеточной гипергидратации по данным биоимпедансометрии (r=0,34, p=0,003).
Таким образом, высокая ультрафильтрация вследствие чрезмерного потребления жидкости в междиализный период и низкая скорость потока диализата относятся к факторам активации оксидативного стресса.
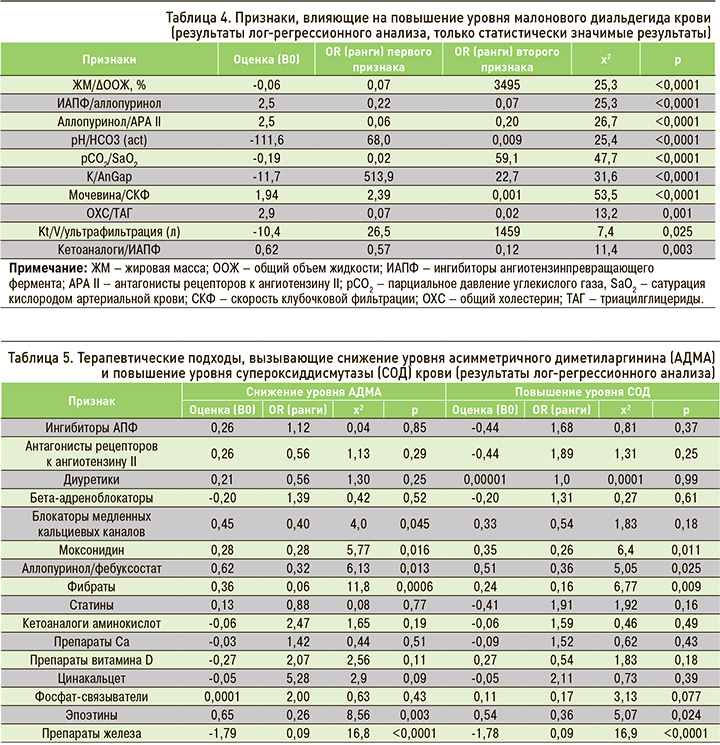
Далее нами анализировались влияния комбинаций факторов на уровень МДА (табл. 4). Было показано, что применение ингибиторов АПФ в сочетании с уратснижающей терапией (аллопуринол или фебуксостат) сопровождается снижением риска оксидативного стресса (повышение МДА) за счет воздействия каждого компонента лечебной схемы. Аналогичное влияние было продемонстрировано при комбинации препарата из группы антагонистов рецепторов к ангиотензину II с уратснижающей терапией.
Самостоятельного профилактического влияния кетоаналогов аминокислот на вероятность развития оксидативного стресса нами выявлено не было. Однако в случае, когда терапия кетоаналогами аминокислот сочеталась с терапией ингибиторами АПФ, такое влияние становилось статистически значимым.
Максимальное подавление оксидативного стресса наблюдалось при комбинированном применении кетоаналогов аминокислот и ингибиторов АПФ в сравнении с применением одной из групп препаратов в отдельности.
Далее нами анализировалось влияние терапии на уровень АДМА в крови, а значит, и на проявления эндотелиальной дисфункции и оксидативного стресса в эндотелии (табл. 5). Применение блокаторов медленных кальциевых каналов и моксонидина сопровождалось снижением уровня АДМА в крови; это свидетельствовало об их эндотелиопротективных эффектах и способности подавлять оксидативный стресс в эндотелиальной клетке. Помимо этого, подобные свойства продемонстрировали фибраты, эпоэтины, препараты железа и уратснижающая терапия.
Аналогичным образом нами было изучено влияние медикаментозной терапии на уровень СОД в крови у больных с ХБП С3А-5Д (см. табл. 5). Повышение продукции СОД с возрастанием ее концентрации в крови наблюдалось при терапии моксонидином, уратснижающими препаратами, эпоэтинами и фибратами. Другие классы препаратов не обладали подобными свойствами.
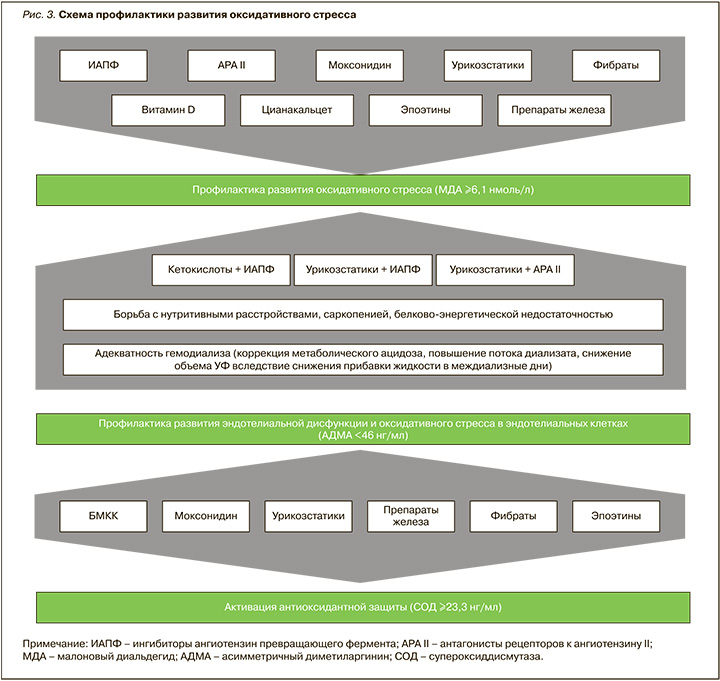
Таким образом, терапевтическое влияние на оксидативный стресс при ХБП С3А-5Д осуществлялось рядом лекарственных препаратов по-разному. Способность подавлять оксидативный стресс в условиях ХБП путем активации антиоксидантной защиты (СОД) или посредством нормализации эндотелиальной функции и подавления оксидативного стресса в эндотелиальных клетках определяет возможности дифференцированного подхода к контролю за патологическим процессом. Схема, отражающая влияние различных лечебных подходов на активность оксидативного стресса, представлена на рисунке 3.
Нами были разработаны прогностические номограммы, на основании которых можно прогнозировать влияние на эндотелий сосудов (уровень АДМА) ряда лекарственных препаратов, а также осуществлять прогнозирование активности антиоксидантной системы (СОД).
ОБСУЖДЕНИЕ
Представленные результаты ретроспективного анализа продемонстрировали возможности влияния ряда медикаментозных и технических (процедура диализа) подходов на активность оксидативного стресса. Поскольку для исследованных групп препаратов антиоксидантный эффект является дополнительным к основным фармакологическим свойствам, его следует рассматривать в качестве плейотропного. Антиоксидантные эффекты блокаторов РААС описывались и ранее на других моделях патологических процессов. В частности, в исследовании Li G. et al. [8] было показано антиоксидантное действие ингибиторов АПФ за счет их способности повышать выработку оксида азота эндотелиальными клетками легочных капилляров. Также показана способность этой группы лекарственных средств вызывать антиоксидантный эффект за счет регуляции NF-κB-, Nrf2- и miR155-5p-путей [9, 10]. Кроме этого, есть работы, описывающие антиоксидантные эффекты уратснижающей терапии, терапии статинами, витамином D [11–13]. Однако эти эффекты описаны преимущественно либо на моделях лабораторных животных, либо в случае развития патологии органов и систем, не имеющей отношения к мочевыделительной системе.
В нашем исследовании была установлена не только способность ряда препаратов инактивировать оксидативный стресс, но и раскрыты некоторые механизмы такого влияния. В частности, продемонстрирована способность контролировать оксидативный стресс за счет воздействия на эндотелий сосудов и подавление оксидативного стресса в эндотелиальных клетках. Также показана способность ряда вариантов терапии активировать процессы синтеза антиоксидантного фермента СОД.
ЗАКЛЮЧЕНИЕ
1. Комплексная терапия больных ХБП С3А-5Д с использованием ингибиторов АПФ, антагонистов рецепторов к ангиотензину II, моксонидина, урикозстатиков, фибратов, витамина D, цинакальцета, эпоэтинов и препаратов железа сопровождается плейотропным эффектом в виде снижения активности оксидативного стресса (МДА в крови). Комбинация урикозстатиков с антагонистами рецепторов к ангиотензину II или ингибиторами АПФ, а также кетоаналогов аминокислот с ингибиторами АПФ сопровождается снижением активности оксидативного стресса.
2. Блокаторы медленных кальциевых каналов, моксонидин, урикозстатики, фибраты, эпоэтины и препараты железа обладают плеойтропным эффектом в виде ингибирования явлений эндотелиальной дисфункции, в том числе оксидативного стресса (снижение АДМА) и повышения уровня антиоксидантной защиты (увеличение СОД).
3. Коррекция схемы лечения гемодиализом в виде повышения скорости потока диализата, снижения объема ультрафильтрации вследствие снижения прибавки жидкости в междиализные дни способствует снижению активности оксидативного стресса.


