Применение многих лекарственных средств (ЛС) ассоциировано с поражением различных отделов желудочно-кишечного тракта (ЖКТ). При этом подобные осложнения характеризуются отсутствием специфической симптоматики, которая может клинически манифестировать спустя некоторое время. Все это может значительно затруднять постановку диагноза и определение причинно-следственной связи между нежелательной реакцией (НР) и приемом ЛС [1–3]. Информирование специалистов практического здравоохранения о возможных рисках и осложнениях фармакотерапии со стороны органов пищеварения будет способствовать повышению безопасности пациентов и эффективности проводимого лечения.
ИНГИБИТОРЫ ПРОТОННОЙ ПОМПЫ
Несмотря на то что ЛС этого класса являются основными в лечении и профилактике заболеваний ЖКТ, их прием может быть ассоциирован с риском развития гастроинтестинальных поражений. Во многих исследованиях была показана связь между приемом ингибиторов протонной помпы (ИПП) и развитием инфекционных осложнений, вызванных Clostridium difficile [4]. Так, Kwok C.S. et al. [4], проведя анализ 39 исследований, выявили значительное повышение риска развития клостридиальной инфекции у пациентов, принимающих ИПП (отношение шансов (ОШ) 1,74; 95% доверительный интервал (ДИ) 1,47–2,85; p <0,001), по сравнению с лицами, не получающими эти препараты [5]. В других исследованиях риск варьировал в диапазоне ОШ 1,2 (95% ДИ 1,03–1,5) – 5,0 (95% ДИ 1,3–19,4) [6].
В основе патогенеза развития инфекционных осложнений на фоне приема ИПП, по всей видимости, лежит увеличение количества жизнеспособных клостридиальных спор и вегетативных форм, которые достигают и колонизируют кишечник вследствие снижения секреции соляной кислоты в желудке, а также изменения микробиоты кишечника [4, 7–13]. Например, в исследовании Hojo M. et al. [14] изучалось влияние приема ИПП в течение 4 и 8 нед на состав микробиоты кишечника в образцах кала. Было установлено, что общее количество бактериальных клеток до начала приема, через 4 и 8 нед терапии ИПП составило 10,6±0,6 log10 клеток/г кала, 10,5±0,5 и 10,5 ± 0,4 соответственно. Таким образом, авторами не было выявлено значительных изменений в общем количестве бактерий на фоне лечения ИПП, но были обнаружены статистически значимые (р=0,011 через 4 нед приема ИПП, р=0,002 через 8 нед) качественные изменения микробиоты (дисбиоз), в частности изменения содержания лактобактерий (Lactobacillus, L). Так, было отмечено увеличение количества L. gasseri (р=0,031 через 4 нед приема ИПП, р=0,002 через 8 нед), L. fermentum (р=0,002 через 4 и 8 нед), L. reuteri (р=0,001 через 4 и 8 нед), L. ruminis (р=0,022 через 4 нед, р=0,011через 8 нед) и L. brevis (р=0,025 через 4 нед). Количество факультативных анаэробов рода Streptococcus также значительно возрастало как через 4, так и 8 нед от начала терапии (р=0,005 и p <0,0001 соответственно). Также статистически значимо (р=0,003) через 8 нед терапии ИПП увеличивалось количество микробов семейства Enterobacteriaceae и Staphylococcus (р=0,002) [14].
Подобный микробиологический пейзаж у пациентов, принимающих ИПП, можно объяснить транслокацией бактерий из ротовой полости, глотки, носа и кожи в ЖКТ, что было показано, в частности, в работе Jackson M.A. et al. [15]. В экспериментальной работе Schubert A.M. et al. [16], выполненной на животных (мышах), было показано, что избыточное количество микроорганизмов семейства Streptococcus выступает фактором риска развития C. difficile-ассоциированной инфекции [16]. Таким образом, наблюдаемые в исследовании Hojo M. et al. изменения микробиоты действительно могут быть предпосылкой к развитию C. difficile-ассоциированной инфекции, вызванной применением ИПП. При этом ЖКТ сам становится потенциальным резервуаром для транслокации инфекции в другие органы и ткани, например в легкие, что повышает угрозу развития пневмоний, которые также могут быть ассоциированы с применением ИПП [17].
Другие НР, ассоциированные с длительным приемом ИПП, – новообразования и рак желудка. В большом исследовании типа «случай–контроль» Jalwing M. et al. [18] было установлено, что вероятность развития полипов желудка в 2 раза выше при приеме ИПП в течение 1–5 лет (ОШ 2,2; 95% ДИ 1,3–3,8) и в 4 раза – при приеме дольше 5 лет (ОШ 3,8; 95% ДИ 2,2–6,7). В то же время использование ИПП сроком менее 1 года не приводило к повышению риска (ОШ 1,0; 95% ДИ 0,5–1,8) [18].
В метаанализе 11 обсервационных исследований было показано увеличение риска развития рака желудка на фоне препаратов, снижающих секрецию соляной кислоты в целом и ИПП в частности (скорректированное ОШ=1,39; 95% ДИ 1,19–1,64). Однако следует отметить, что авторами не уточняется, учитывалось ли при этом наличие инфекции H. pylori, которая сама по себе служит мощным фактором риска возникновения онкологических заболеваний ЖКТ [19]. Другими типами новообразований, которые потенциально могут возникать на фоне ИПП, являются рак толстого кишечника и аденокарцинома пищевода [20, 21]. К примеру, когортное исследование типа «случай–контроль», выполненное Yang Y.-X. et al. [20], показало, что у пациентов 50 лет и старше, которые принимали ИПП на протяжении 5 и более лет, скорректированный относительный риск колоректального рака составил 1,1 (95% ДИ 0,7–1,9; р=0,7). Среди пациентов, получавших ИПП в высоких дозах (>1,5 установленных суточных доз/сут), с возрастанием длительности применения этих средств наблюдался статистически недостоверный тренд на увеличение риска колоректального рака (р=0,2) [20]. Потенциальным механизмом развития злокачественных новообразований ЖКТ на фоне приема ИПП может быть гипергастринемия, которая, по данным ряда работ [22–24], усиливает пролиферацию эпителиальных клеток, локализованных в слизистой оболочке поджелудочной железы, желудка и толстого кишечника. Трофические эффекты гастрина могут способствовать повышению риска возникновения спонтанных мутаций в нормальных клетках, потенцировать пролиферативные процессы и тем самым приводить к развитию новообразований [20]. В экспериментах на животных было показано, что именно гипергастринемия ассоциирована с риском развития колоректальных опухолей [25–33].
Кроме этого, согласно результатам популяционного когортного национального исследования, выполненного Brusselaers N. et al. (796 492 взрослых жителя Швеции) [21], рак поджелудочной железы был выявлен у 1733 лиц, длительно принимавших ИПП [21]. Стандартизованный коэффициент заболеваемости (СКЗ) для пациентов, получающих ИПП, составил 2,22 (95% ДИ 2,12–2,32) для всех возрастных подгрупп с наибольшей распространенностью среди лиц младше 40 лет (СКЗ=8,90; 95% ДИ 4,26–16,37), а также пациентов с инфекцией H. pylori в анамнезе (СКЗ=2,99; 95% ДИ 2,54–3,49). После первого года включения пациентов в исследование, в течение которого прием ИПП мог быть обусловлен ранними симптомами рака поджелудочной железы, риск увеличивался пропорционально времени приема препаратов (после 5 лет терапии СКЗ=1,57; 95% ДИ 1,38–1,76) [21]. Авторами был сделан вывод о том, что длительный прием ИПП ассоциирован с повышенным риском развития рака поджелудочной железы, особенно в молодом и среднем возрасте [21].
Помимо инфекционных осложнений и новообразований, ИПП, традиционно считающиеся относительно безопасной группой ЛС (некоторые препараты даже продаются без рецепта), могут приводить к развитию других поражений ЖКТ, о которых специалисты практического здравоохранения знают меньше или не связывают их возникновение с приемом ИПП. Так, для ИПП ОШ развития микроскопического колита составляет 19,7 (прием ИПП в анамнезе) и 9,8 (текущий прием ИПП), а скорректированное ОШ – 7,2 для пациентов, получавших ИПП в течение предшествующего года [34]. В работе Bonderup O.K. et al. [35] было продемонстрировано, что у пациентов на фоне ИПП повышен риск развития как коллагенозного (скорректированное ОШ 7,04; 95% ДИ 6,55–7,56), так и лимфоцитарного колита (скорректированное ОШ 3,37; 95% ДИ 3,08–3,69), хотя и в меньшей степени [34–36].
АНТИКОАГУЛЯНТЫ
В силу основного фармакологического действия антикоагулянтов их применение ассоциировано с повышенным риском кровотечений различной локализации, в том числе из ЖКТ [37–39]. Последние относятся к серьезным (в том числе с высоким риском летального исхода) осложнениям лекарственной терапии. Частота развития желудочно-кишечных кровотечений (ЖКК) ежегодно оценивается у 2–3% всех пациентов, принимающих прямые оральные антикоагулянты (дабигатрана этексилат, апиксабан, ривароксабан, эдоксабан) [40]. Источники кровотечения при этом могут локализоваться в различных отделах ЖКТ. Например, прием ингибитора II фактора дабигатрана этексилата ассоциирован с риском развития больших кровотечений как из верхних, так и нижних отделов пищеварительного тракта с небольшим (53% случаев) превалированием вторых, что может быть связано с наличием в составе оболочки таблеток препарата винной кислоты, неполной абсорбцией ЛС в тонком кишечнике и наличием бессимптомного поражения слизистой оболочки толстого кишечника (эрозии, онкология и др.) [40, 41].
Применительно к антагонистам Ха фактора ривароксабану и апиксабану, наоборот, сообщается о преобладании вероятности кровотечения из верхних отделов ЖКТ, а вот у эдоксабана риски кровотечений из верхних и нижних отделов кишечника были сопоставимы [39, 40, 42]. В то же время, по данным FDA, частота кровотечений из верхних отделов ЖКТ при приеме этого ЛС была несколько выше [43].
В двойном слепом рандомизированном контролируемом исследовании (РКИ) ROCKET-AF [44] (14 264 пациента с неклапанной фибрилляцией предсердий) было показано, что частота ЖКК составила 3,2% в группе ривароксабана и 2,2% в группе варфарина (р <0,001) [147, 148].
В двойном слепом РКИ ARISTOTLE [42] риск большого кровотечения в группе апиксабана составил 2,13% в год (из них ЖКК 0,76% в год), что было статистически значимо ниже (ОШ 0,69; 95% ДИ 0,60–0,80; р <0,001) в сравнении с группой варфарина, где этот показатель составил 3,09% в год (из них ЖКК 0,86% в год) [42, 45, 46].
В исследовании RE-LY [47] риск ЖКК на фоне приема варфарина составил 1,02% в год, тогда как для дабигатрана этексилата аналогичное значение варьировало в зависимости от дозы препарата (1,12% в год для дозы 110 мг 2 раза/cут и 1,51% для 150 мг 2 раза/cут), но при этом было статистически значимо выше для второй, более высокой дозировки (относительный риск 1,10; 95% ДИ 0,86–1,41; р=0,43 для дозы 110 мг 2 раза/сут vs 1,5; 95% ДИ 1,19–1,89; р <0,001 для 150 мг 2 раза/сут) [45, 47].
С целью профилактики кровотечений из ЖКТ перед началом терапии прямыми оральными антикоагулянтами (ПОАК) пациентам рекомендуется выполнение диагностической эзофагогастродуоденоскопии и колоноскопии для своевременного выявления патологических состояний, которые потенциально способны повысить риск кровотечений (эрозии, язвенные дефекты и др.) [39]. Применение ИПП может также способствовать уменьшению риска кровотечений из верхних отделов ЖКТ, ассоциированных с терапией ПОАК [39]. Однако, как было показано выше, длительный прием этих препаратов ассоциирован с развитием многих вариантов гастроинтестинальных поражений; кроме того, ИПП в силу их механизма действия не эффективны в профилактике кровотечений из нижних отделов ЖКТ, в связи с чем крайне актуален поиск других стратегий защиты слизистой оболочки ЖКТ. В последние годы, в частности, активно изучаются протекторные возможности ребамипида, что будет подробно рассмотрено далее.
К редким, но имеющим тенденцию к увеличению распространенности осложнениям оральной антикоагулянтной терапии относится нетравматическая интрамуральная гематома кишечника (НИГК) [48–52]. Она является потенциально жизнеугрожающим состоянием, возникающим либо в случае тупой травмы живота, либо на фоне коагулопатий, которые могут быть следствием приема ПОАК.
Классической триадой симптомов, характерных для НИГК, считают боль в животе, обструкцию тонкого кишечника и кровотечения различной локализации (гематурия, экхимозы, мелена, кровавая рвота, кровь в стуле) [48–52]. Для подтверждения диагноза используют лучевые методы исследования органов брюшной полости или диагностическую лапаратомию [48–52]. Помимо прочего, на фоне НИГК возможно развитие транслокации микробиоты, сопровождающееся интоксикационным синдромом, что в отсутствие своевременного и адекватного лечения может привести к летальному исходу. Осложнениями НИГК также могут быть сдавление органов брюшной полости, нагноение гематомы, сепсис и перитонит [48–52].
Лечение гематом тонкого кишечника преимущественно консервативное: оно включает дренаж содержимого кишечника через назогастральный зонд, рациональную антибиотикотерапию (при необходимости), коррекцию интоксикационного синдрома и т.д. [53]. При неэффективности этих мер пациентам выполняется оперативное вмешательство, преимущественно лапароскопическим доступом [54]. Прогноз в случае своевременной диагностики и лечения благоприятный.
НЕСТЕРОИДНЫЕ ПРОТИВОВОСПАЛИТЕЛЬНЫЕ СРЕДСТВА И АЦЕТИЛСАЛИЦИЛОВАЯ КИСЛОТА
Применение нестероидных противовоспалительных средств (НПВС) может быть ассоциировано с риском поражения любого отдела ЖКТ. Так, клинические симптомы НПВС-ассоциированных эзофагитов (изжога, заброс кислого содержимого желудка в пищевод, боли за грудиной, дисфагия и др.), по результатам исследования Ruszniewskiy P. et al. [55], наблюдались у 68% пациентов, принимавших эти препараты. По данным А.Е. Каратеева с соавт., симптоматическое поражение пищевода отмечалось у 35% обследованных пациентов, получавших НПВС по поводу различных ревматических заболеваний; у 2,2% из этих пациентов был выявлен эрозивный эзофагит [56].
НПВС-ассоциированная гастропатия является хорошо известным, изученным и довольно частым осложнением фармакотерапии, в то время как НПВС-ассоциированная энтеропатия известна практикующим врачам в меньшей степени, хотя встречается тоже достаточно часто даже при приеме небольших доз НПВС в независимости от длительности терапии [57]. К примеру, согласно исследованиям MUCOSA [58] и VIGOR [59], в тонком кишечнике локализуется до 40% НПВС-ассоциированных повреждений ЖКТ, а по данным видеокапсульной эндоскопии, этот показатель и вовсе достигает 75% (!) [60, 61].
Частота поражений тонкой кишки вследствие приема ацетилсалициловой кислоты (АСК) в кишечнорастворимой форме в дозе 100 мг/сут достигает 50% [62]. Smecuol E. et al., которые проводили видеокапсульное эндоскопическое исследование тонкого кишечника, исследовали кал на кальпротектин и оценивали проницаемость кишечной стенки (экскреция сахарозы с мочой и соотношение лактулоза/маннитол) до и после приема кишечнорастворимой АСК 100 мг/сут в течение 14 дней, установили следующее: у половины пациентов наблюдались поражения слизистой оболочки (петехии, эрозии и язвенные дефекты, в том числе с признаками кровотечения), которые отсутствовали на момент включения их в исследование [62]. Также была отмечена тенденция к повышению отношения лактулоза/маннитол относительно величины этого показателя в момент включения пациентов в исследование, причем у половины испытуемых после приема АСК оно было выше верхней границы нормы (>0,025; р <0,02). Средние значения фекального кальпротектина и экскреция сахарозы с мочой статистически значимо повысились после приема АСК [62]. Перечисленные изменения свидетельствуют о повышении тонкокишечной и гастродуоденальной проницаемости, а также активации воспалительного процесса на фоне приема АСК [62, 63].
Имеющиеся данные свидетельствуют, что НПВС-индуцированная энтеропатия встречается даже чаще, чем НПВС-гастропатия, но реже диагностируется из-за малосимптомного течения и редкого применения видеокапсульной эндоскопии [64]. Патогенез этой НР сложный: он включает прямое токсическое воздействие ЛС на слизистые оболочки, нарушение функции митохондрий, окислительного фосфорилирования, плотных межклеточных контактов, энтерогепатической рециркуляции желчи, содержащей токсичные для клеток кишечника метаболиты ЛС (в том числе НПВС), а также изменение микробиоты кишечника [65, 50, 63, 64]. При этом все перечисленные звенья патологического процесса тесно взаимосвязаны с друг другом. Так, многие НПВС, включая АСК, представляют собой слабые кислоты, а следовательно, могут оказывать повреждающее действие при контакте со слизистой кишечника, вызывая воспаление [66, 67]. После всасывания из просвета кишечника НПВС попадают в систему воротной вены и далее в печень, где подвергаются глюкуронизации с последующей секрецией с желчью в тонкий кишечник. Продукты метаболизма НПВС, находящиеся в составе желчи, токсичны для эпителиальных клеток кишечника и усиливают имеющееся воспаление, обусловленное химическим воздействием. Ферменты (β-глюкуронидазы) бактерий, населяющих кишечник, усиливают цитотоксичность желчи, так как под их действием происходит отщепление глюкуронида, повторное всасывание и рециркуляция НПВС [65]. Также прием НПВС способствует активной колонизации кишечной флорой начальных отделов тонкой кишки [68] и активации грамотрицательными микроорганизмами толл-подобного рецептора 4 (TLR-4), что является одним путей активации повреждения энтероцитов [69].
Известно, что наиболее распространенный подход к уменьшению частоты развития НПВС-гастропатии – снижение секреции cоляной кислоты обычно с помощью ИПП или H2-блокаторов [70]. Однако данная стратегия не подходит для пациентов с лекарственно-индуцированной энтеропатией, поскольку использование ингибиторов секреции соляной кислоты может усугублять течение НПВС-индуцированной энтеропатии за счет изменения микробиоты кишечника [71–73]. При этом в отличие от НПВС-гастропатии циклооксигеназа (ЦОГ) не играет ключевой роли в патогенезе повреждения тонкой кишки [74], хотя и приводит к повышению чувствительности кишечной стенки к действию повреждающих факторов и замедлению репаративных процессов [75].
Интересно, что, по данным видеокапсульной эндоскопии, совместное применение ИПП и НПВС ассоциировано даже с большей частотой развития поражений слизистой оболочки тонкого отдела кишечника (язвы) по сравнению с приемом только НПВС без ИПП [76–78]. У большинства (80–100%) пациентов, получающих одновременно НПВС в малых дозах и ИПП, острые повреждения слизистого слоя тонкого кишечника развиваются уже после 2 нед лечения [78].
Аналогичные данные получены и в отношении малых доз АСК в кишечнорастворимой оболочке. В одном из исследований, посвященных этой проблеме, наличие язвенных дефектов в тонком кишечнике было выявлено у всех больных, которые принимали совместно АСК в кишечнорастворимой оболочке и ИПП [77]. В проспективном исследовании Endo H. et al. [79] было показано, что АСК, покрытая кишечнорастворимой оболочкой, и ИПП были двумя основными факторами риска развития повреждения стенки тонкого кишечника [79–80].
Кроме того, следует понимать, что в случае НПВС-индуцированного поражения кишечника прием ИПП с профилактической целью не эффективен, поскольку они не способствуют профилактике повреждений отделов ЖКТ, расположенных дальше связки Трейтца [71–73].
Поражение толстого кишечника на фоне приема НПВС и АСК – еще одна серьезная, потенциально жизнеугрожающая НР, которая обнаруживается у 30–70% пациентов и сопровождается морфологическими признаками воспаления, повышения кишечной проницаемости, а в ряде случаев появлением эрозий, изъязвлений и кровотечений [81]. Патогенез лекарственно-индуцированных поражений толстого кишечника на фоне приема НПВС напоминает таковой при НПВС-индуцированной энтеропатии. Токсическое действие ЛС или их метаболитов, активация образования свободных радикалов приводят к разрушению митохондрий и саркоплазматического ретикулума, снижению барьерной функции кишечника и повышению проницаемости слизистой кишечника для бактериальных токсинов, стимулирующих в дальнейшем TLR4-рецепторы макрофагов, выделению провоспалительных интерлейкинов и запуску локального воспалительного процесса (второе повреждение) [82]. Большое (n=135 780) проспективное исследование Chan S.S. et al. [83] продемонстрировало, что регулярный прием АСК был ассоциирован с 6-кратным увеличением риска развития болезни Крона (ОШ=6,14; 95% ДИ=1,76–21,35); статистически значимой связи с риском развития язвенного колита выявлено не было (ОШ=1,29; 95% ДИ=0,67–2,46) [83]. В другом исследовании [84] модели «случай–контроль», включившем 200 пациентов с впервые выявленными язвенным колитом и болезнью Крона и контрольную группу из 1198 пациентов без воспалительных заболеваний кишечника (ВЗК), было выявлено наличие положительной ассоциации как текущего, так и предшествующего приема НПВС с ВЗК (ОШ=2,96; 95% ДИ 1,32–6,64 и ОШ=2,51; 95% ДИ 1,13–5,55) [84]. НПВС могут также провоцировать обострение уже имеющихся ВЗК [85].
Селективные ингибиторы ЦОГ-2 потенциально могут быть более безопасны с точки зрения риска развития ВЗК: по данным плацебо-контролируемого исследования безопасности целекоксиба у пациентов с язвенным колитом в стадии ремиссии, проведенного Sandborn W.J. et al. [86], было показано, что частота развития обострений заболевания на фоне кратковременного приема (2 нед) этого НПВС статистически значимо не отличалась от таковой на фоне плацебо (р=0,719) [86].
Другими типами поражения ЖКТ на фоне приема НПВС являются микроскопический колит (все формы) и ишемический колит [87]. Например, в литературе описан случай развития диклофенак-индуцированного ишемического колита у 85-летней пациентки, которая обратилась за неотложной помощью по поводу диареи с кровью и упадка сил, слабости, продолжавшихся на протяжении 2 дней. Женщине, получавшей диклофенак в связи с выраженной артралгией, была выполнена колоноскопия, в процессе которой обнаружились множественные петехии и геморрагии, язвы толстого кишечника, псевдомембраны и расположенные подслизисто узлы синюшного цвета. В связи с исключением иных потенциальных причин врачи пришли к заключению, что это поражение было вызвано именно приемом НПВС. Отмена диклофенака привела к улучшению состояния пациентки и полному ее выздоровлению, что может служить дополнительным подтверждением лекарственной этиологии ишемического колита в указанном случае [88].
Анализ федеральной базы данных НР, проведенный Bielefeldt K. [89], показал, что НПВС занимают 6-е место (11,3% случаев) среди причин возникновения лекарственно-индуцированного ишемического колита, уступая лишь антигипертензивным средствам, препаратам для химиотерапии, биологически активным добавкам, иммуносупрессантам и антипсихотикам [89].
Также в литературе имеется описание случая развития коллагенозного колита (КК) (одной из форм микроскопического колита) у 41-летней пациентки с псориатическим артритом на фоне приема диклофенака [90]. Частота развития этого осложнения на фоне использования НПВС точно неизвестна, однако у больных с диагностированным КК частота сопутствующего приема ЛС этой группы составляла 30–70% [91]. Точный патогенез КК в связи с применением НПВС также не установлен, однако, по всей видимости, в нем свою роль играет повышение проницаемости кишечной стенки, которое приводит к развитию воспаления, активации перикриптовых фибробластов и утолщению коллагенового слоя [92].
ПРОФИЛАКТИКА ЛЕКАРСТВЕННОГО ПОРАЖЕНИЯ ЖЕЛУДОЧНО-КИШЕЧНОГО ТРАКТА
В настоящий момент предложено несколько стратегий защиты ЖКТ при приеме различных ЛС, обладающих гастроинтестинальной токсичностью (в том числе антикоагулянтов и НПВС). Самая известная из них – назначение ИПП [66, 67, 93–95].
Однако, как уже неоднократно отмечалось в этом материале, из-за риска развития лекарственно-индуцированных поражений ЖКТ на фоне приема самих ИПП, а также ограниченности их протективного действия верхними отделами ЖКТ актуальной задачей современного здравоохранения является поиск и внедрение в практику новых стратегий гастроинтестинальной протекции. В последние годы все больше данных свидетельствует о том, что новой, эффективной и безопасной стратегией защиты слизистой оболочки ЖКТ как с целью профилактики, так и лечения служит назначение лекарственного препарата ребамипида (Ребагит®, «ПРО.МЕД.ЦС Прага а.о.») [96–98].
Ребамипид (производное аминокислоты 2-хинолинона) обладает цитопротективным действием на протяжение всего ЖКТ, в том числе на фоне негативного воздействия этанола, кислот и щелочей, АСК [99, 100]. Механизм гастропротекторного действия препарата включает:
- уменьшение экспрессии 15-гидроксипростагландин-дегидрогеназы, увеличение содержания простагландина Е2 (PGE2), а также рецептора 4 простагландина E2 (EP4) в слизистой оболочке желудка и желудочном соке;
- активацию ферментов, ускоряющих биосинтез высокомолекулярных гликопротеинов;
- повышение содержания слизи на поверхности желудка;
- улучшение кровоснабжения слизистой желудка;
- активацию барьерной функции, усиление пролиферации и препарации слизистой оболочки желудка [99, 101–105].
Помимо гастропротективных свойств, ребамипид обладает энтеропротективным действием за счет восстановления плотных контактов эпителиальных клеток и положительного влияния на клаудин-3 и белки замыкающих контактов (protein zonula occludens-1, ZO-1) [106–107]. Эта особенность препарата крайне важна, поскольку механизмы лекарственного поражения различных отделов ЖКТ универсальны, и нарушение плотных контактов, повышение проницаемости стенки кишечника выступают одними из ведущих патогенетических звеньев таких поражений [107].
Ребамипид улучшает микроциркуляцию в lamina propria слизистой оболочки ЖКТ за счет стимуляции экспрессии проангиогенных факторов, что было показано в работе Tarnawski A.S. et al. [108], а также снижает адгезию бактерий (включая H. pylori) к слизистой ЖКТ [109]
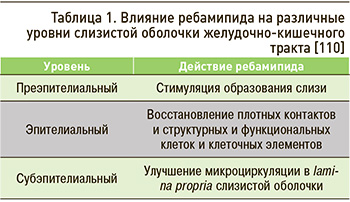 Таким образом, положительные эффекты ребамипида проявляются на всех уровнях слизистой оболочки ЖКТ (табл. 1).
Таким образом, положительные эффекты ребамипида проявляются на всех уровнях слизистой оболочки ЖКТ (табл. 1).
Ребамипид обладает и серьезными фармакокинетическими преимуществами перед другими ЛС с протективными эффектами в отношении ЖКТ (в том числе над многими ИПП). Так, в связи с отсутствием ингибирующего действия на ферменты цитохрома Р450 (CYP) 1A2, 2C9, 2C19, 2D6, 2E1 и 3A4 он характеризуется относительно низким риском развития межлекарственных взаимодействий [111]. Прием ребамида безопасен у пациентов с нефрологическими заболеваниями (что особенно важно для больных пожилого и старческого возраста), так как препарат выводится почками лишь на ≈10%, а основная его элиминация происходит через кишечник с каловыми массами [110].
Эффективность и безопасность ребамипида для профилактики НПВС-индуцированной гастропатии была показана в ряде исследований, в том числе в нескольких РКИ. Так, в плацебо-контролируемом РКИ, выполненном Kim H.K. et al. [112], участники (здоровые добровольцы) были разделены на 2 группы: первая (группа наблюдения) в сочетании с ибупрофеном в дозе 1800 мг/ сут применяла ребамипид 100 мг 3 раза/cут в течение 7 дней; вторая (контроль) группа, наряду с ибупрофеном в той же дозе, получала плацебо. Степень выраженности поражения слизистой оценивалась с использованием модифицированной шкалы Ланца. Было выявлено, что в группе плацебо поражение слизистой оболочки оказалось статистически значимо (р=0,032) более тяжелым по сравнению с группой ребамипида (в среднем 2,9±1,7 vs 1,3±1,0 балла соответственно). Также в группе плацебо в конце периода наблюдения было отмечено статистически значимое снижение кровотока в слизистой оболочке желудка относительно исходного уровня (с 2,8±0,5 до 2,0±0,5 ед. тканевой перфузии; р=0,005), что имело прямую статистически значимую корреляцию с тяжестью ее повреждения (r=–0,677; р=0,001). В группе ребамипида значимых изменений кровотока обнаружено не было. Авторы сделали вывод, что такие результаты являются следствием протективного действия препарата [112].
В другом двойном слепом плацебо-контролируемом РКИ, проведенном Ono S. et al. [113], здоровые добровольцы получали АСК в дозе 81 мг/ сут в комбинации с плацебо или ребамипидом в дозе 300 мг 3 раза/сут в течение 7 дней. В группе ребамипида было выявлено статистически значимо (p=0,039) меньшее количество случав развития поражения слизистой желудка [113].
В двойном слепом плацебо-контролируемом исследовании Naito Y. et al. [114] по изучению эффективности ребамипида при профилактике поражения слизистой оболочки желудка, вызываемого индометацином, здоровые добровольцы (20 мужчин в возрасте 20–26 лет) были рандомизированы на 2 группы: первая группа в течение 7 дней наряду с указанным НПВС в дозе 25 мг 3 раза/сут получала плацебо (3 раза/сут), вторая – ребамипид (по 100 мг 3 раза/сут). Всем участникам на этапе включения и после окончании курса приема препаратов выполнялось эндоскопическое исследование с целью контроля состояния слизистой верхних отделов ЖКТ. В итоге было обнаружено, что распространенность поражения слизистой оболочки желудка составила 70% в группе плацебо и только 14% – в группе ребамипида. Уровень липидного пероксида и активность миелопероксидазы в слизистой желудка были значительно повышены в группе плацебо. На основании полученных данных авторами было сделано заключение о наличии у ребамипида гастропротективных эффектов в отношении развития индометацин-индуцированного поражения желудка.
Еще одно двойное слепое рандомизированное перекрестное исследование, которое было посвящено сравнению эффективности ребамипида и фамотидина в профилактике повреждения слизистой желудка, вызванного приемом индометацина у здоровых добровольцев без H. pylori [115], позволило установить: ребамипид в дозе 100 мг 3 раза/ сут более эффективен, чем фамотидин 10 мг 2 раза/сут, способствовал предупреждению развития острого НПВС-индуцированного поражения слизистой желудка, по данным эндоскопического исследования [115]. Так, субъективная симптоматика была отмечена у 58% лиц, принимавших ребамипид, и у 75% добровольцев, получавших фамотидин. Распространенность гастропатии (2 и более балла по модифицированной шкале Ланца) составила 17% в группе ребамипида и 25% – в группе фамотидина. Случаев развития пептических язв, а также значительной разницы в содержании липидного пероксида, активности миелопероксидазы в тканях и концентрации индометацина в сыворотке крови у всех пациентов выявлено не было [115].
В проспективном рандомизированном открытом пилотном исследовании с ослепленными конечными точками GLORIA [116] изучалась эффективность совместного применения ребамипида и целекоксиба в аспекте профилактики НР со стороны верхних отделов ЖКТ у пациентов с ревматоидным артритом и болью в нижних отделах спины (n=75, средний возраст 67±13 лет). С этой целью участники были рандомизированы на 2 группы: первая получала монотерапию целекоксибом в дозе 100 мг 2 раза/сут, вторая – комбинированную терапию целекоксиб + ребамипид по 100 мг 3 раза/ сут. Оценку состояния слизистой оболочки желудка проводили методом эндоскопического исследования на этапе включения в исследование и спустя 3 мес лечения. Результаты были следующие: у 17,6% пациентов в группе монотерапии целекоксибом развились НР со стороны верхних отделов ЖКТ (5 случаев язвенного поражения и 1 случай непереносимости лечения вследствие диспептических явлений), тогда как в группе комбинированной терапии с ребамипидом подобные явления не наблюдались (p=0,0252). Авторами был сделан вывод о том, что терапия ребамипидом позволяет профилактировать осложнения со стороны верхних отделов ЖКТ, вызванные длительным применением селективных ингибиторов ЦОГ-2 [116].
В рандомизированном многоцентровом контролируемом открытом исследовании STORM сравнивалась частота развития осложнений со стороны ЖКТ в группах пациентов, получавших ребамипид или мизопростол в комбинации с НПВС в течение 12 нед [117]. Наличие осложнений оценивалось с использованием эндоскопии исходно и в конце периода наблюдения. Распространенность пептических язв составила 4,5% в группе ребамипида и 4,4% в группе мизопростола, а в группах высокого риска этот показатель был равен 4,0 и 3,9% соответственно. Это позволило авторам заключить, что ребамипид не менее эффективен, чем мизопростол, для профилактики гастропатии, вызванной длительным приемом НПВС [117].
Гастропротективные эффекты ребамипида и мизопростола у пациентов, получающих длительную терапию НПВС, также сравнивались в работе Kim J.H. et al. [118]. В общей сложности в исследование вошли 479 пациентов, которые случайным образом были распределены на 2 группы: одна в дополнение к НПВС получала ребамипид в дозе 100 мг 3 раза/сут (n=242), другая – мизопростол по 200 мг 3 раза/сут (n=237). Период наблюдения составил 12 нед, первичной конечной точкой служило выявление язв желудка при эндоскопическом исследовании. Распространенность язвенного поражения в конце периода наблюдения была сопоставимой в обеих группах (20,3% в группе ребамипида и 21,9% в группе мизопростола; p=0,6497). При этом в ходе оценке тяжести диспептической симптоматики отмечены статистически значимые различия: их частота была ниже в группе ребамипида (p=0,0002). Также у пациентов на ребамипиде была статистически значимо меньше потребность в дополнительном приеме антацидов для купирования симптоматики (p=0,0258). Меньшим в этой группе оказалось и количество пациентов, выбывших из исследования (10,3 vs 18,6% в группе мизопростола; p=0,0103). Таким образом, ребамипид показал себя эффективным препаратом для лечения и профилактики осложнений, ассоциированных с длительным использованием НПВС, который может рассматриваться как альтернатива мизопростолу [118].
В связи с увеличивающейся частотой назначения двойной антиагрегантной терапии особый интерес представляет двойное слепое плацебо-контролируемое РКИ, проведенное Tozawa K. et al. [119]. Целью его было определение гастропротективных свойств ребамипида на фоне приема АСК в низких дозах (АСКнд) в монотерапии или комбинации с клопидогрелом у здоровых добровольцев (n=32). Срок наблюдения составлял 14 дней. Пациенты случайным образом были распределены на 4 группы:
- А: плацебо 3 раза/сут + АСКнд (100 мг) 1 раз/сут;
- В: ребамипид по 100 мг 3 раза/сут + АСКнд 1 раз/сут;
- С: плацебо + АСКнд + клопидогрел по 75 мг 1 раз/сут;
- D: ребамипид + АСКнд + клопидогрел.
Тяжесть поражения слизистой желудка оценивалась путем проведения эзофагогастродуоденоскопии (ЭГДС) в начале и конце периода наблюдения с использованием модифицированной шкалы Ланца (МШЛ). Выраженность субъективной симптоматики определялась с помощью шкалы оценки выраженности симптомов со стороны ЖКТ. Было выявлено, что ребамипид значительно снижал выраженность повреждения слизистой желудка. Так, в группе А было отмечено статистически значимое снижение количества баллов по МШЛ в конце периода наблюдения по сравнению с исходными данными (p <0,05). Аналогичная тенденция отмечалась и в группе двойной антиагрегантной терапии с плацебо (С): 0–3 балла в конце периода наблюдения против 3–4 в начале (p <0,01). В то же время в группах приема ребамипида (В и D) в конце периода наблюдения значимого снижения МШЛ не наблюдалось. Полученные данные позволили констатировать, что ребамипид может быть использован для первичной профилактики поражения ЖКТ, вызванного как монотерапией АСКнд, так и двойной антиагрегантной терапией [119].
В двойном слепом РКИ, выполненном Pittayanon R. et al., изучалось протективное действие ребамипида на слизистую оболочку верхних отделов ЖКТ у пациентов (n=95), длительно (≥1 года) получающих антиагрегантную терапию, без кровотечения из язв или перфорации язвы в анамнезе [120]. Участники были случайным образом распределены в группу ребамипида (300 мг/сут) или плацебо; при этом пациенты, принимающие ИПП, в исследование не включались. Первичной конечной точкой было появление дефекта слизистой оболочки верхних отделов ЖКТ по данным ЭГДС через 3 и 12 мес. Вторичными конечными точками служили изменения гематокрита, ЖКК и боль в грудной клетке. Были получены следующие результаты: в течение 12-месячного периода наблюдения дефекты слизистой оболочки верхних отделов ЖКТ развились у 43,3% пациентов в группе ребамипида против 65,5% в группе плацебо (р=0,07); частота пептических язв ≥5 мм в группах исследования составила 6,7 против 27,6% соответственно (р=0,03). Показатели гематокрита статистически значимо между группами не различались. Кровотечений и болей в грудной клетке в процессе проведения исследования выявлено не было. Полученные результаты свидетельствовали о том, что у пациентов, длительно получающих двойную антиагрегантную терапию, прием ребамипида позволяет предотвратить появление пептических язв желудка [120].
Имеются данные и об эффективности ребапимида в профилактике лекарственно-индуцированных поражений ЖКТ на фоне приема новых оральных антикоагулянтов. Так, в многоцентровом проспективном открытом наблюдательном сравнительном РКИ в параллельных группах у 309 пациентов с неклапанной фибрилляцией предсердий, которым впервые был назначен дабигатрана этексилат, с помощью глобальной совокупной шкалы тяжести диспепсии (GOS) оценивалась выраженность и частота возникновения симптоматики [121]. После этого пациенты с количеством баллов ≥3 были рандомизированы в 3 группы, которые получали соответственно терапию ИПП, Н2-блокатором или ребамипидом. Частота развития диспепсии на фоне терапии дабигатрана этексилатом составила 17,2%, при этом в 77% случаев манифестация симптоматики наблюдалась в первые 10 дней приема препарата, 5 пациентов ввиду диспепсии выбыли из исследования. В конце периода наблюдения (4 нед) средний балл по GOS у пациентов с диспепсией составил 3,5±7,7, при этом количество больных с ≥3 баллами уменьшилось со 100 до 11,3%. В подавляющем большинстве случаев (83–100%) на фоне проводимой терапии состояние пациентов улучшалось (уменьшение количества баллов по шкале GOS ≤2), при этом статистически значимой разницы между 3 группами выявлено не было [121].
Ребамипид, в отличие от ИПП, способен не только защищать желудок, но и, возможно, снижать риск развития рака желудка, что было показано в популяционном национальном когортном исследовании материалов базы данных страховых компаний Кореи (Health Insurance Review and Assessment Service database) [122]. В анализ включались данные пациентов, перенесших эндоскопическую подслизиcтую диссекцию в 2011–2014 гг. За период наблюдения (73,416 человеко-лет) у 711 больных был впервые диагностирован рак желудка, в том числе у 377 пациентов, получавших низкие дозы (средняя кумулятивная доза <9,000 мг) ребамипида, и у 334 пациентов, находившихся на терапии высокими дозами (средняя кумулятивная доза ≥9,000 мг) этого препарата (37 157,4 и 36 258,3 на 100 000 человеко-лет соответственно; log-rank test, p=0,052). Были выявлены значительные различия в заболеваемости раком желудка в зависимости от возраста, пола и первоначального диагноза во время оценки индекса эндоскопической подслизиcтой диссекции. После введения поправки на эти аспекты было обнаружено, что применение ребамипида в высоких дозах ассоциировано с уменьшением риска развития рака желудка (отношение пределов функций риска 0,858; 95% ДИ 0,739–0,995; р=0,043) [122, 123].
Ребамипид обладает не только гастро- но и энтеропротективными эффектами. Так, систематический обзор и метаанализ, сделанные Zhang S. et al. (15 контролируемых РКИ; n=965) [123], позволили установить, что ребамипид был более эффективен, чем плацебо, в плане профилактики и лечения НПВС-индуцированных энтеропатий (отношение рисков 2,70; 95% ДИ 1,02–7,16; р=0,045). Серьезных НР выявлено не было [124].
В проспективном двойном слепом плацебо-контролируемом перекрестном РКИ по изучению эффективности ребамипида у пациентов с диклофенак-индуцированным повреждением слизистой тонкого кишечника, выполненном Niwa Y. et al., была продемонстрирована способность этого препарата защищать слизистую оболочку тонкого отдела кишечника от негативного воздействия НПВС [125]. В данном исследовании 10 здоровых добровольцев были разделены на 2 равные группы: первая в течение 1 нед получала ребамипид по 300 мг + диклофенак по 75 мг + омепразол по 20 мг/сут, вторая – плацебо + диклофенак по 75 мг + омепразол по 20 мг/сут. После этого терапия в группах менялась еще на 7 дней. Для контроля состояния слизистой оболочки пациентам в обеих группах выполнялась капсульная видеоэндоскопия как перед началом, так и в конце терапии. В результате было показано, что количество пациентов с повреждением слизистой оболочки тонкого отдела кишечника было статистически значимо (р=0,023) больше в группе плацебо, даже несмотря на то, что они получали профилактическую дозу омепразола. При этом в группе плацебо было также выявлено 2 случая развития язвенного поражения и 1 случай кровотечения, в то время как в группе ребамипида подобных осложнений не наблюдалось [125].
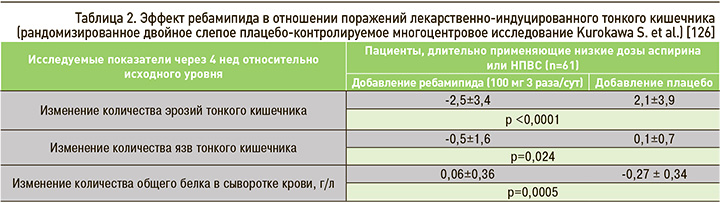
Ребамипид является эффективным препаратом в лечении поражения тонкого кишечника, ассоциированного с приемом НПВС, что, в частности, было показано в двойном слепом плацебо-контролируемом многоцентровом РКИ, осуществленном Kurokawa S. et al. [126]. В данную работу вошел 61 пациент, который длительно (>3 мес) принимал АСКнд или НПВС. Пациенты с признаками энтеропатии, по данным капсульной видеоэндоскопии, были рандомизированы в группу плацебо или ребамипида в дозе 100 мг 3 раза/сут в течение 4 нед. Для контроля эффективности терапии в конце периода наблюдения выполнялась повторная капсульная эндоскопия, а также оценивалось количество общего белка сыворотки крови. На основании полученных данных (табл. 2) авторами был сделан вывод о том, что применение ребамипида не только способствует заживлению НПВС-индуцированных дефектов слизистой оболочки кишечника, но также улучшает нутритивный статус пациентов [126].
В мультицентровом двойном слепом плацебо-контролируемом РКИ по оценке эффективности применения высоких доз ребампида у пациентов со средней и тяжелой энтеропатией (>3 дефектов слизистой оболочки тонкого отдела кишечника в виде эрозий и язв, по данным капсульной видео эндоскопии), вызванной применением АСК, покрытой кишечнорастворимой оболочкой, в дозе 100 мг/сут на протяжении 3 мес, пациентам назначали ребампид (по 300 мг 3 раза/cут) или плацебо в течение 8 нед (соотношение пациентов 2:1) [127]. Первичной конечной точкой было изменение количества дефектов слизистой кишечника после 8 нед терапии. Вторичными конечными точками выступали полное заживление дефектов слизистой оболочки и изменение баллов по шкале Льюиса. В группе ребамипида (но не в группе плацебо) статистически значимо (p=0,046) уменьшилось количество дефектов слизистой оболочки; а также наблюдалось большее (32%) количество случаев полного заживления дефектов по сравнению с группой плацебо (7,7%; р=0,13). Также на фоне 8-недельной терапии ребамипидом, в отличие от плацебо, статистически значимо (p=0,02) уменьшалась тяжесть повреждения слизистой, оцененной по шкале Льюиса. Авторы заключили, что прием ребамипида является эффективной стратегией лечения энтеропатии средней и тяжелой степени, вызванной приемом АСКнд в кишечнорастворимой оболочке [127].
Механизм терапевтического действия ребамипида при поражении кишечника изучался в экспериментальной работе Lai Y. et al. [128]. Лабораторным мышам назначалась АСК в дозе 200 мг/кг/сут в течение 5 дней с целью индукции повреждения слизистой тонкого кишечника, после чего животные получали терапию ребамипидом в дозе 320 мг/ кг/ сут на протяжении 5 дней. У мышей, получавших ребамипид, воспаление было выражено в значительно меньшей степени, при этом также было обнаружено усиление плотных контактов между эпителиоцитами. После лечения ребамипидом повышался пролиферативный индекс; экспрессия ЦОГ-2, β-катенина и концентрация PGE2 в тканях тонкого кишечника были статистически значимо выше у мышей, получавших терапию ребамипидом (р <0,05). Таким образом, терапевтические эффекты ребамипида при НПВС-индуцированной энтеропатии (в том числе аспирин-индуцированной) обусловлены восстановлением барьерной функции клеток, стимуляцией экспрессии ЦОГ-2 и накоплением β-катенина [128].
Еще один патофизиологический механизм терапевтического действия ребамипида заключается во влиянии на кровоснабжение слизистой оболочки ЖКТ. Этот эффект был наглядно продемонстрирован в плацебо-контролируемом двойном слепом перекрестном РКИ [129], где наряду с прочим оценивалось влияние АСКнд и ребамипида на кровоток в тонком кишечнике и повреждение его слизистой оболочки у здоровых добровольцев. В группе контроля испытуемые принимали АСК 100 мг/сут и плацебо, в основной группе – АСК в той же дозе и ребамипид по 300 мг/сут. До и после каждого периода лечения всем пациентам выполняли капсульную эндоскопию и УЗИ тонкого кишечника с контрастным усилением. Применение АСКнд приводило к снижению кровоснабжения слизистой оболочки тонкого кишечника в группе плацебо, тогда как в группе ребамипида существенной динамики показателей, характеризующих кровоток в слизистой оболочке тонкого кишечника, отмечено не было. Иными словами, ребамипид оказывает протективное действие, препятствуя аспирин-индуцированному ухудшению кровоснабжения слизистой оболочки тонкого кишечника [129].
Применение ребамипида оправдано и у пациентов с колонопатиями, что также выгодно отличает его от ИПП. Так, в исследовании Takagi Т. et al. [130], проведенном на крысах линии Wistar путем введения тринитробензолсульфоновой кислоты (ТНБС), был смоделирован колит, схожий с ВЗК и проявляющийся гиперемией, отеком, утолщением, изъязвлением и некрозом стенки толстой кишки. Подопытным животным с 7-го дня после моделирования колита с помощью клизм вводили раствор ребамипида или плацебо в течение недели. Было обнаружено, что через 2 нед с момента введения ТНБС у грызунов, получавших плацебо, сохранялась симптоматика выраженного язвенного колита, в то время как у крыс, получавших ребамипид, выраженность поражения была значительно меньше. Положительное действие ребамипида было подтверждено в том числе гистологически: менее выраженное утолщение кишечной стенки и агрегация клеток воспаления. Таким образом, введение ребамипида способствовало восстановлению поврежденного эпителия толстого отдела кишечника [130].
В проспективном двойном слепом плацебо-контролируемом перекрестном пилотном РКИ по изучению эффективности комбинированной терапии ИПП и ребамипидом пациенты, получавшие АСК в дозе 100 мг/сут и омепразол в дозе 20 мг/ сут, были разделены на 2 группы: первая течение 4 нед в дополнение к указанным средствам получала ребамипид по 300 мг, вторая – плацебо в [131]. Участники в обязательном порядке протоколировали выраженность симптомов нарушения функции ЖКТ до и спустя 1 и 4 нед после включения в исследование, на основании чего высчитывался итоговый балл. Как показали результаты, у пациентов, принимавших ребамипид, общая распространенность и выраженность симптомов нарушения функции нижних отделов ЖКТ достоверно отличалась и была ниже по сравнению с группой плацебо после 4 нед лечения (p=0,0093). Это позволило предположить, что ребамипид является препаратом, который может эффективно применяться в комбинации с ИПП для профилактики поражения ЖКТ (особенно нижних отделов и тонкого кишечника) у пациентов длительно принимающих АСКнд [131].
Применение ребамипида с целью лечения и профилактики осложнений со стороны ЖКТ, в том числе на фоне приема НПВС, нашло отражение в ряде российских национальных клинических рекомендаций. В работе А.Е. Каратеева с соавт. «Рациональное использование нестероидных противовоспалительных препаратов. Клинические рекомендации» [97] отмечается, что назначение ребамипида служит эффективным методом профилактики осложнений со стороны как верхних, так и нижних отделов ЖКТ, тонкого и толстого отдела кишечника (градация рекомендации В). При этом назначение ИПП не снижает, а, наоборот, может повышать риск развития НПВС-индуцированной энтеропатии [97]. Для предупреждения этого потенциального осложнения фармакотерапии целесообразно использование ребамипида, который не только эффективно профилактирует, но и способствует заживлению эрозий и язв у пациентов с НПВС-индуцированной энтеропатией по сравнению с плацебо (ОШ 2,70; 95% ДИ 1,02–7,16; р=0,045) [97,131]. Для этих целей рекомендуется назначение ребамипида в дозе 100 мг 3 раза/сут курсами по 2 мес [97].
В клинических рекомендациях «Коморбидная патология в клинической практике. Алгоритмы диагностики и лечения» авторы отмечают, что прием ребамипида – эффективный способ профилактики эрозивно-язвенного поражения слизистой оболочки верхних отделов ЖКТ, тонкой и толстой кишки [98]. У пациентов с факторами риска возникновения эрозивно-язвенных поражений ЖКТ назначение АСК может проводиться в комбинации с ИПП и/ или ребамипидом, который является универсальным гастроэнтеропротектором [132]. Для профилактики развития НПВС-индуцированных гастро- и энтеропатий ребамипид назначается в дозе 100 мг 3 раза/сут курсом длительностью 2 мес [132].
Важно отметить, что ребамипид не только защищает слизистую оболочку ЖКТ на всем его протяжении при применении НПВП, антиагрегантов (включая моно- или двойную антиагрегантную терапию) и НОАК, но еще и устраняет повышенную проницаемость кишечной стенки. А ведь исследования последних лет показывают, что повышенная проницаемость слизистой кишечника – один из значимых механизмов развития ряда системных заболеваний, в частности сердечно-сосудистых. Очевидно, что ребамипид, как единственный корректор повышенной проницаемости слизистой оболочки ЖКТ, обладает потенциалом для широкого использования не только в гастроэнтерологической, но и кардиологической практике у пациентов с различными кардиоваскулярными патологиями, а также риском возникновения [96].
ЗАКЛЮЧЕНИЕ
Таким образом, лекарственно-индуцированное поражение ЖКТ является актуальной проблемой современного здравоохранения. Накоплено значительное количество научных данных о типах поражения и ЛС, которые потенциально могут приводить к его развитию. Меры профилактики и лечения при этом напрямую зависят от типа поражения и органа-мишени. Применение препарата ребамипид (Ребагит®, «ПРО.МЕД.ЦС Прага а.о.») может рассматриваться как научно обоснованная и подтвержденная данными хорошо контролируемых клинических исследований стратегия профилактики и лечения лекарственно-индуцированного поражения ЖКТ независимо от его локализации.



