От хронической боли страдает не менее 20% взрослого населения Земли, что составляет более 1,5 млрд человек. В большинстве случаев причиной появления хронической боли становятся скелетно-мышечные заболевания [1]. Хроническую боль рассматривают в последнее время не просто как симптом какого-либо заболевания, а самостоятельную болезнь, представляющую серьезную угрозу здоровью и жизни пациентов и требующую особого внимания и адекватного лечения. В формировании хронической боли принимает участие множество генетических, психосоциальных и нейробиологических факторов, изучение которых имеет серьезное теоретическое и практическое значение [2].
Ведущую роль среди факторов, способствующих развитию хронической боли, наряду с воспалением, дегенеративными процессами, периферической сенситизацией, нейропластическими изменениями в нервной системе, играет феномен центральной сенситизации (ЦС). ЦС – нейробиологический процесс, связанный с изменениями нейронов и нейронального окружения, сопровождающийся повышением возбудимости нервной системы и значительным снижением болевого порога. Установлено, что ЦС играет ключевую роль в патогенезе ряда болевых синдромов: хронической тазовой боли, боли в нижней части спины, хронической головной боли напряжения, мигрени, хронической боли в плече и области локтя и другого, а также при таких ревматологических заболеваниях, как фибромиалгия, остеоартрит (ОА), ревматоидный артрит (РА) [3–6].
Диагностика ЦС основывается на клинической оценке характеристик болевого ощущения, психофизиологическом тестировании, оценке реакций на различные болевые и неболевые стимулы (количественное сенсорное тестирование, wind-up, определение порогов боли на давление – альгометрия). Современные методы нейровизуализации, такие как функциональная магнитно-резонансная томография (ф-МРТ) и позитронно-эмиссионная томография (ПЭТ), также используются для диагностики ЦС; они демонстрируют активацию не только областей мозга, ответственных за обработку болевых сигналов, но и многих других, связанных с эмоционально-когнитивной оценкой внешнего стимула и формированием поведенческой реакции [8–10].
Следует отметить, что ЦС отражает повышенный ответ не только на болевые, но и многие другие стимулы: проприоцепцию, стресс, механическое давление, холод, тепло, электрический стимул и др. [11]. Тем не менее роль ЦС наиболее изучена именно при хронических болевых синдромах. Она проявляется нарушением и недостаточным функционированием нисходящих антиноцицептивных систем, повышенной чувствительностью к боли, а также увеличенной временной суммацией боли [7, 12, 13]. Симптомы ЦС при хронической боли включают вторичную гипералгезию, аллодинию, расширение зоны восприятия за пределами иннервации периферического нерва, а также длительное ощущение боли после прекращения действия болевого или неболевого стимула.
В 2015 г. Yunus М. описал значение ЦС для целого ряда заболеваний, при которых зачастую нет травмы, воспаления или другого известного источника ноцицептивной стимуляции [14]. Классическим примером заболевания, при котором ЦС является основной причиной боли, служит фибромиалгия. Боль при этом заболевании носит интенсивный генерализованный характер, а повреждение тканей человеческого организма и признаки воспаления отсутствуют. [15]
В клинической практике нередко отмечаются overlap-синдромы, когда признаки ноцицептивной боли, связанной с повреждением и/или воспалением, сочетаются с болью, обусловленной ЦС. Признаки ЦС обнаруживают при синдроме раздраженного кишечника, дисменорее, мигрени, головной боли напряжения, миофасциальном болевом синдроме (МФБС), дисфункции височно-нижнечелюстного сустава, рассеянном склерозе и послеоперационной боли.
Заболевания и синдромы, при которых выявляются признаки ЦС, как правило, высоко коморбидны друг другу. Так, например, при мигрени часто отмечаются дисфункции височно-нижнечелюстного сустава, МФБС и болевые синдромы другой локализации [16]. Аналогично от 40 до 60% пациентов с синдромом раздраженного кишечника страдают фибромиалгией и мигренью [14].
Важное место в клинической картине синдромов ЦС занимают психические нарушения, в частности депрессия, тревожность и различные расстройства сна, такие как бессонница и неудовлетворенность сном. У пациентов с ЦС в анамнезе часто выявляются психические и физические травмы, а также более частые хронические болевые синдромы у ближайших членов семьи, что способствует формированию у пациентов особого «болевого поведения» [6].
Накопленные на сегодняшний день данные изменили взгляд на хроническую боль при таких ревматических заболеваниях (РЗ), как ОА и РА. Для клинициста важно, что центральные механизмы не только оказывают влияние на интенсивность болевого синдрома, его клинические характеристики и распространенность, но также могут определять недостаточную эффективность противоболевой терапии [17, 18].
ЦЕНТРАЛЬНАЯ СЕНСИТИЗАЦИЯ И ХРОНИЧЕСКАЯ БОЛЬ ПРИ СКЕЛЕТНО-МЫШЕЧНЫХ ЗАБОЛЕВАНИЯХ
Хроническую боль при РЗ длительное время связывали исключительно с ноцицептивными и структурными изменениями в суставах и окружающих тканях, рассматривая ее как ноцицептивную боль. При воспалении сустава выделяется ряд медиаторов: простагландины, брадикинин и провоспалительные цитокины (фактор некроза опухоли-α, интерлейкин-1, интерлейкин-6, фактор роста нервов-β), которые сенсибилизируют периферические болевые рецепторы и вносят значительный вклад в возникновение и поддержание боли [19–21]. Источником боли при РА и ОА выступает не только синовиальная оболочка; активация сенсорных волокон происходит также в суставных капсулах, связках, менисках, субхондральной кости, сухожилиях и мышцах [22]. В месте повреждения возникает периферическая сенситизация: повышается чувствительность ноцицепторов, снижается порог их возбуждения, они становятся чувствительными к подпороговым болевым раздражениям. Происходит активация так называемых молчащих ноцицепторов, которые в обычных условиях не отвечают на первичные болевые стимулы [7].
Хроническое воспаление перестраивает работу противоболевых систем, вовлекая нейрогенные механизмы в патогенез болевого синдрома. Повторяющаяся стимуляция периферических рецепторов в результате хронического воспаления приводит к гиперактивности спинальных и супраспинальных ноцицептивных нейронов, которая является пусковым моментом для развития ЦС. Биохимические механизмы ЦС достаточно хорошо изучены. Повышенная возбудимость болевых нейронов связана с усиленным выделением на уровне спинного мозга ряда нейротрансмиттеров, таких как глутамат и субстанция Р. Глутамат – один из важнейших возбуждающих нейротрансмиттеров, действующий на три подгруппы рецепторов: рецептор α-амино-3-гидрокси-5-метил-4-изоксазелопропионовой кислоты, рецептор NMDA и семейство метаботропов, связанных с G-белком. В то время как α-амино-3-гидрокси-5-метил-4-изоксазелопропионовая кислота отвечает за базовый ответ на патологические раздражители, NMDA-рецептор усиливает и расширяет болевой ответ. Данные изменения модулируют пластичность центральной нервной системы, что приводит ко вторичной гипералгезии и аллодинии, которые характеризуют ЦС [23].
Постоянный ноцицептивный поток с периферии имеет ключевое значение в возникновении и поддержании ЦС. Это установлено даже при таком синдроме, как фибромиалгия, когда отсутствуют четкие, локализованные, вызывающие боль поражения [24, 25]. В исследовании Affaitati G. et al. (2011) [26] было показано, что инъекции в периферические триггерные точки и гидроэлектрофорез у 68 пациентов с фибромиалгией, имеющих миофасциальные болевые синдромы, и 56 больных с фибромиалгией, страдающих региональной болью в суставах, уменьшают боль при фибромиалгии и увеличивают пороги боли в местах, отдаленных от инъекции. Это служит дополнительным доказательством того, что болезненные периферические раздражители способствуют активации центральных механизмов боли.
Наиболее распространенным заболеванием суставов является ОА, развивающийся в результате субклинического воспаления в хряще и окружающих тканях (low grade inflammation). ОА страдает более 10% населения земного шара [27]. Пациенты с этой патологией испытывают хроническую боль, которая приводит к значительной инвалидизации и снижению качества жизни. При ОА выявлен ряд диссоциаций: в некоторых случаях отсутствуют корреляции между степенью структурных изменений в области пораженного сустава и интенсивностью боли; у 40% пациентов, имеющих изменения в суставах и окружающих тканях, боль отсутствует вовсе; до 44% больных после адекватно выполненного эндопротезирования коленных суставов продолжают испытывать боль [28–30]. Эти данные позволяют предполагать, что в развитии хронической боли у пациентов с ОА участвует не только ноцицептивный механизм.
С помощью специальных опросников (PainDETECT, DN4) у 30–66% пациентов при ОА обнаруживаются специфические сенсорные феномены, характерные для нейропатической боли [31–34]. Однако объективные признаки органического поражения соматосенсорной нервной системы при ОА, как правило, не выявляются, в связи с чем нейропатические сенсорные феномены (ощущение онемения, покалывания, жжения и др.) объясняют функциональными расстройствами центральной нервной системы, а именно развитием ЦС.
Исследование порогов боли с помощью количественного сенсорного тестирования продемонстрировало, что пациенты с ОА более чувствительны к экспериментальным болевым стимулам. При изучении чувствительности к болевому стимулу в областях тела, близких к пораженным суставам, было показано, что пациенты с ОА имеют более низкие пороги ноцицептивного ответа на механические и термические стимулы, чем здоровые лица из группы контроля. Это подтверждает наличие у первых периферической сенситизации и ЦС [4, 35–38].
В одной экспериментальной работе оценивался болевой ответ на введение гипертонического раствора в переднюю большеберцовую мышцу у 14 пациентов с ОА и 14 здоровых добровольцев, сопоставимых по возрасту и полу. Больные ОА отмечали увеличение интенсивности болевых ощущений и расширение болевых зон, распространяющихся на пальцы ног, тогда как у здоровых лиц отмечалась более низкая интенсивность боли и ее меньшее распространение [39].
В исследовании Imamura М. et al. у пациентов с ОА, в отличие от здоровых добровольцев, отмечалась гипералгезия под воздействием давления в семи различных дерматомах и гипералгезия в девяти группах мышц нижней конечности. Авторы предположили, что как периферические, так и центральные механизмы играют большую роль в патогенезе хронического болевого синдрома при ОА. Причем периферические механизмы более значимы на ранних стадиях, а центральные механизмы доминируют на поздних стадиях заболевания [40].
Другим распространенным РЗ является РА, который характеризуется системным аутоиммунным воспалением с преимущественным поражением периферических суставов и формированием симметричного прогрессирующего эрозивного полиартрита, а также внесуставными проявлениями и поражением внутренних органов [41–43]. Болевой синдром при РА возникает вследствие хронического воспаления, однако интенсивность болевых ощущений далеко не всегда коррелирует с активностью заболевания и может оставаться высокой, несмотря на успешно проводимую противовоспалительную терапию.
В частности, анализ результатов лечения 12 тыс. пациентов с достоверным РА установил его недостаточный противоболевой эффект. Динамика интенсивности болевого синдрома оставалась схожей как при монотерапии базисными противовоспалительными препаратами, так и при комплексной терапии с добавлением генно-инженерных биологических препаратов [44].
Исследование Rupp I. et al., в котором приняли участие более 800 пациентов с РА, показало, что в течение первых 3 лет после постановки диагноза и начала терапии динамика интенсивности болевого синдрома была положительной, однако в дальнейшем болевой синдром вновь стал нарастать, несмотря на проводимое лечение. Это можно объяснить тем, что первоначальное уменьшение боли было связано с контролем воспаления, а последующее ее усиление – с другими патогенетическими механизмами хронического болевого синдрома [45].
Свидетельством важной роли ЦС в патогенезе боли при РА служит коморбидность с фибромиалгией. Так, Haliloglu S. et al. обследовали 835 пациентов с РА, системной красной волчанкой, анкилозирующим спондилитом, ОА, семейной средиземноморской лихорадкой, болезнью Бехчета, подагрой, синдромом Шегрена, васкулитом, ревматической полимиалгией или полимиозитом. Распространенность фибромиалгии среди пациентов с этими заболеваниями колебалась от 1,4 до 25% (самая низкая была при подагре, самая высокая – при васкулите). Из 197 пациентов с РА 6,6% был выставлен диагноз фибромиалгия. Ее наличие затрудняло диагностику и терапию пациентов. При наличии коморбидной фибромиалгии пациенты испытывали более интенсивную боль, имели более выраженные функциональные нарушения и утомляемость, что могло быть ошибочно интерпретировано как сохранение высокой активности заболевания и приводить к изменению базисной терапии. Исследователи пришли к выводу, что ЦС является основным механизмом коморбидности фибромиалгии и РЗ. Они еще раз продемонстрировали важность диагностики фибромиалгии при РЗ с целью избежать диагностических и терапевтических ошибок [46].
Участие ЦС в патогенезе хронического болевого синдрома у пациентов с РА было подтверждено рядом экспериментальных исследований. С помощью электроэнцефалографии у больных РА в сравнении с контрольной группой (сопоставимой по полу и возрасту) было выявлено усиление корковых реакций на повторную стимуляцию, свидетельствующее об изменениях в модуляции боли в центральной нервной системе [47]. Morris V.H. и его коллеги показали, что капсаицин вызывает большую площадь гипералгезии у пациентов с РА по сравнению со здоровыми лицами. Эта область усиленной гипералгезии может соответствовать расширению рецепторных полей нейронов спинного мозга, что характерно для ЦС [48].
Накоплены данные о взаимосвязи интенсивности болевого синдрома с эмоциональным состоянием у пациентов с РА. В 2008 г. с помощью ф-МРТ Schwienhardt Р. et al. продемонстрировали взаимосвязь активации медиальной префронтальной коры с тяжестью депрессии у 20 больных РА [49]. В исследовании Robinson М. et al. также было установлено, что у пациентов с РА и депрессией выше активность медиальной префронтальной коры при пальпации пораженного сустава [50]. ф-МРТ способна определить области мозга, участвующие в формировании болевых реакций у пациентов с суставной патологией. Болевые сигналы, исходящие из пораженных суставов кисти, активируют таламус, вторичную сенсорную кору, а также лимбическую систему (цингулярную и инсулярную кору) – области мозга, связанные с эмоциональными реакциями. Это свидетельствует о гипервозбудимости центральной нервной системы, которая определяет повышенную чувствительность к боли при ЦС [51, 52].
ЛЕЧЕНИЕ ХРОНИЧЕСКОЙ СКЕЛЕТНО-МЫШЕЧНОЙ БОЛИ, АССОЦИИРОВАННОЙ С ЦЕНТРАЛЬНОЙ СЕНСИТИЗАЦИЕЙ
Наличие смешанного хронического болевого синдрома у пациентов с РА и ОА в виде сочетания воспалительной боли и боли, обусловленной ЦС, имеет не только теоретическое, но и важное практическое значение для разработки программ комплексной патогенетической терапии.
Для уменьшения боли, связанной с ЦС, используют стратегии, направленные как на устранение источника боли (уменьшение ноцицептивной афферентации с периферии), так и на мозговые механизмы сверху вниз. На сегодня комбинированная терапия, сочетающая различные стратегии, является наиболее перспективной. Однако исследования эффективности комбинированной фармакотерапии с применением препаратов центрального действия у пациентов с РА и ОА пока малочисленны.
В двойном слепом плацебо-контролируемом 13-недельном рандомизированном исследовании, посвященном оценке применения антидепрессанта дулоксетина (60–120 мг/сут) в лечении боли у пациентов с ОА (n=231), авторы доказали эффективность этого препарата как в отношении боли, так и функционального состояния пациентов [53].
В другом рандомизированном проспектовом исследовании у пациентов с ОА коленных суставов проводилось сравнение эффективности монотерапии НПВП и комбинированной терапии НПВП + антиконвульсант. Было установлено, что комбинированная терапия в течение 4 нед достоверно уменьшила интенсивность боли и улучшила функциональное состояние больных (рис. 1) [54].
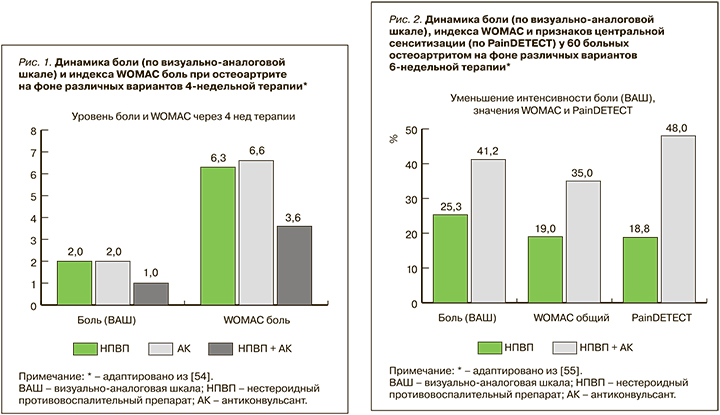
В 6-недельном исследовании, проведенном в НИИР им. В.А. Насоновой, был изучен эффект комбинированной терапии у пациенток с ОА коленных суставов, имеющих признаки ЦС. Лечение, наряду с НПВП и хондропротекторами, включало препарат центрального действия – антиконвульсант прегабалин. Сравнительный анализ двух схем лечения – монотерапии (ацеклофенак) и комплексной терапии (ацеклофенак + прегабалин) – показал достоверно большую эффективность второй схемы в отношении выраженности боли, эмоционального и функционального состояния пациенток, а также более быстрое наступление клинического действия при ее использовании (рис. 2) [55].
Эффективность препаратов из группы антиконвульсантов также была изучена у пациентов, перенесших тотальное эндопротезирование коленного сустава. В исследовании Buvanendran А. et al. было показано, что у больных, принимавших антиконвульсант в течение 32 ч после операции, потребность в дополнительной обезболивающей терапии была меньше по сравнению с группой пациентов, получавшей плацебо. Кроме того, в группе терапии с использованием антиконвульсанта был больше объем движений в коленном суставе после операции: 84 против 76° [56].
ЗАКЛЮЧЕНИЕ
Серия клинических и экспериментальных исследований демонстрирует важную роль ЦС в развитии хронического болевого синдрома у значительной доли пациентов с ОА и РА. Этот нейробиологический феномен оказывает влияние не только на характер и интенсивность болевых ощущений, но и функциональное и эмоциональное состояние пациентов. Выявление признаков ЦС у больных с хронической суставной болью имеет большое значение для разработки методов персонифицированной терапии, основанной на определении фенотипа болевого синдрома, с целью оказания более эффективной противоболевой помощи.



