К числу патологий, усугубляющих течение ХСН, относится анемия, распространенность которой, по разным данным, колеблется в пределах от 7 до 79% (1–4). Большинством исследователей анемия у больных с ХСН признана независимым фактором риска тяжелого течения и даже увеличения смертности от этого сердечно-сосудистого заболевания [5–6]. Известно, что ХСН сама по себе может вызывать анемию, которая в данном случае по своей сути является анемией хронического заболевания (АХЗ). Karla P.R. et al. показали, что патогенетически связанная, возникшая как следствие ХСН анемия вносит особый негативный вклад в течение сердечной недостаточности [7].
При изучении механизмов возникновения анемии у больных ХСН большое внимание уделялось роли почек, влиянию дефицита железа на фоне синдрома мальабсорбции и желудочно-кишечных кровотечений, вызванных приемом ацетилсалициловой кислоты, гемодилюции, снижению продукции эритропоэтина, непосредственной ишемии костного мозга из-за снижения насосной функции сердца и ряду ятрогенных факторов (воздействию ингибиторов АПФ, бета-адреноблокаторов) [4, 8–18]. Вместе с тем некоторые патогенетические аспекты развития анемии у этой группы пациентов остаются до конца не ясными.
В последние годы активно обсуждается роль системного воспаления у больных ХСН, что основано на «цитокиновой» теории ее патогенеза [19]. Эти данные даже побудили исследователей к изучению эффективности применения антицитокиновых препаратов в дополнение к базисной терапии ХСН [20]. Например, в 2018 г. были опубликованы результаты исследования CANTOS, в котором приняли участие пациенты с ХСН после перенесенного инфаркта миокарда, имеющие повышенные уровни С-реактивного белка (СРБ). На фоне дополнительной терапии канакинумабом (моноклональным антителом против интерлейкина-1β) они имели более низкую частоту повторных сердечно-сосудистых событий [21].
В ряде экспериментальных работ показано, что у больных ХСН отмечаются высокие показатели провоспалительных цитокинов (интерлейкина- 1β, интерлейкина-6, фактора некроза опухоли-α и СРБ), участвующих в формировании АХЗ [22–23].
При этом в генезе анемии при ХСН до конца не ясна роль гепсидина – гормона со свойствами отрицательного регулятора обмена железа в организме. Вызывая деградацию ферропортина, он тем самым блокирует транспорт железа из ретикулоэндотелиальной системы в кровоток и всасывание его в энтероцитах («гепсидиновый барьер») [24–25]. Этот механизм способствует формированию функционального дефицита железа и резистентности пациентов к стандартной терапии анемии препаратами железа. В доступной литературе встречаются немногочисленные и противоречивые данные о роли провоспалительных цитокинов и гепсидина в генезе анемии у больных ХСН. В одних исследованиях замечена более низкая концентрация гепсидина у больных ХСН при наличии анемии и отсутствие взаимосвязи концентрации гепсидина и гемоглобина [26–27]. В других обсуждается увеличение уровня циркулирующего гепсидина при утяжелении ХСН и снижении гемоглобина [2]. При этом корреляции между содержанием интерлейкина-6 (ИЛ-6) и гепсидином не замечено [28].
Открытие белка гепсидина и клиническая значимость взаимодействия между ним и ферропортином стала причиной для выделения исследователями нового патофизиологического феномена «ось гепсидин – ферропортин» [29]. Это дало толчок к исследованию целого ряда препаратов для лечения АХЗ, нацеленных непосредственно на эту ось, которые служат дополнением к стандартной терапии анемии и способствуют снижению резистентности к препаратам железа. В настоящее время проводятся клинические и доклинические исследования антагонистов гепсидина: прямых ингибиторов гепсидина, веществ, препятствующих связыванию ферропортина с гепсидином, а также ингибиторов его экспрессии [25, 30]. Однако, несмотря на значительное внимание к молекуле гепсидина, ни одно из доклинических и клинических исследований на данный момент не затрагивает использование таргетных препаратов в лечении анемии у больных с ХСН.
В связи с изложенным можно констатировать, что роль цитокиновой активации и гепсидина при анемии у пациентов с ХСН до настоящего времени остается мало изученной. Это определило цель и задачи настоящей работы – изучить показатели провоспалительных цитокинов и гепсидина при анемии, патогенетически связанной с ХСН. Это может способствовать детализации механизма развития такой анемии с целью создания новых, безопасных и патогенетически обоснованных подходов к лечению.
МАТЕРИАЛ И МЕТОДЫ
Исследование проводилось на базе кардиологического отделения Нижегородской областной клинической больницы им. Н.А. Семашко в течение 12 мес (с 2016 по 2017 г.). Научная работа была одобрена локальным этическим комитетом Нижегородской государственной медицинской академии (протокол от 21.11.2016 № 15).
С целью определения частоты анемии и ее причин проспективно обследовано 873 больных с ХСН. Среди них анемией страдал 191 (22%) человек. У 95 (49,7%) таких пациентов причина снижения гемоглобина была установлена (постгеморрагические, дефицитные, анемии, связанные с воспалительными и онкологическими заболеваниями), и соответственно они были исключены из исследования. Другие 96 (50,3%) человек с ХСН и анемией имели снижение уровня гемоглобина, не связанное с каким-то известным этиологическим фактором. Это состояние было расценено как анемия при ХСН, и настоящая группа участников рассматривалась как основная в исследовании.
В группу контроля были включены пациенты с ХСН без анемии (n=35), сопоставимые по гендерному составу (р=0,45), возрасту (р=0,68) и показателю фракции выброса левого желудочка (р=0,08).
Этиологическими факторам ХСН в основной (n=96) и контрольной (n=35) группах были гипертоническая болезнь (ГБ) в сочетании с ишемической болезнью сердца (ИБС) – у 71 (74%) и 19 (54,3%), ГБ – у 13 (13,5%) и 9 (25,7%), ИБС – у 10 (10,5%) и 6 (17,1%), дилатационная кардиомиопатия – у 2 (2%) и 1 (2,9%) человек соответственно.
Пациенты получали базисную патогенетическую и симптоматическую терапию ХСН в соответствии с актуальными на момент лечения рекомендациями, за исключением случаев непереносимости или противопоказаний к применению препаратов.
Всем больным проводилось стандартное клиническое, лабораторное и инструментальное обследование. ХСН оценивали по классификации В.Х. Василенко – Н.Д. Стражеско с дополнениями, функциональный класс (ФК) определяли по шкале оценки клинического состояния (ШОКС), согласно рекомендациям Общества специалистов по сердечной недостаточности (ОССН, 2018). С целью верификации ХСН и оценки ее тяжести измеряли также концентрацию N-концевого фрагмента мозгового натрий-уретического пептида (NT-proBNP) [31–32].
Показатель гемоглобина оценивали по общему анализу крови с помощью анализаторного метода. Анемией считали уровни гемоглобина ниже 130 г/л у мужчин и 120 г/л у женщин (WHO, 2011). Также определяли гематокрит, количество эритроцитов, эритроцитарные индексы: среднее содержание гемоглобина в эритроците (MCH), средний объем эритроцита (MCV) [33].
Наличие и стадию ХБП устанавливали согласно рекомендациям KDIGO (2012).
Кроме этого, у пациентов исследовались концентрация эритропоэтина (ЭПО) сыворотки и показатели феррокинетики – сывороточное железо (СЖ) и сывороточный ферритин (СФ). Дефицит железа определяли при СФ <100 мкг/л; при СФ в интервале 100–300 мкг/л и СЖ ниже референсных значений железодефицит считали функциональным [34].
У 74 пациентов (54 в основной группе и 20 в группе контроля) количественно методом иммуноферментного анализа оценивали показатели цитокинового профиля (ИЛ-1β, ИЛ-6, фактор некроза опухоли-α; тест-система «Вектор БЕСТ», Россия) и гепсидина (тест-система Cloud-Clone Corporation, США).
Всем больным проводилось инструментальное обследование: электрокардиография (ЭКГ) в 12 отведениях и эхокардиография (ЭхоКГ) на аппарате PHILIPS iE33 (Нидерланды). Оценивались следующие структурно-функциональные характеристики миокарда: фракция выброса левого желудочка (ФВ ЛЖ); размеры ЛЖ – конечный систолический размер (КСР), конечный диастолический размер (КДР), толщина межжелудочковой перегородки, толщина задней стенки ЛЖ, масса миокарда левого желудочка (ММЛЖ), индекс массы миокарда левого желудочка (ИММЛЖ). Исследуемых пациентов с ХСН разделяли в зависимости от состояния ФВ ЛЖ сердца: с сохранной ФВ (≥50% – ХСНсФВ), промежуточными значениями ФВ (40–49% – ХСНпФВ), низкой ФВ (<40% – ХСНнФВ) [31–32].
Для оценки размеров почек, состояния паренхимы проводили ультразвуковое исследование почек на сканере PHILIPS – EPIQ 5 (Нидерланды). Функциональное состояние почек оценивали с помощью определения сывороточного креатинина и расчета скорости клубочковой фильтрации (СКФ) по формуле CKD-EPI (2011). Для исключения эрозивно-язвенных процессов желудка и двенадцатиперстной кишки проводили фиброгастродуоденоскопию на гастроинтестинальном видеоскопе OlympusGIF-Q 165 (Япония).
Критериями включения в исследование служили наличие симптомов ХСН в покое и/или при нагрузке; имеющиеся признаки систолической и/или диастолической дисфункции сердца по результатам эходопплеркардиографии; получение информированного согласия пациентов на участие в исследовании; возраст от 18 лет.
Из исследования исключали лиц, отказавшихся от участия в исследовании; пациентов с установленными причинами анемии; больных, получающих препараты железа на амбулаторном этапе в течение предыдущих 6 мес до рандомизации; больных с первичной патологией почек, тяжелой почечной недостаточностью, сахарным диабетом, алкогольной зависимостью и психическими заболеваниями, тяжелой степенью ожирения и кахексией; беременных женщин.
Статистическая обработка полученных данных выполнялась с использованием пакета программ (StatSoft; Statistica 12.0). Распределение величин оценивалось посредством критериев Колмогорова–Смирнова и Шапиро–Уилка. Количественные признаки, имеющие нормальное распределение, представляли в виде М±σ, где М – среднее арифметическое значение, σ – среднеквадратичное отклонение. Количественные признаки, имеющие ненормальное распределение, выражали в виде Ме [Q25; Q75], где Ме – медиана, Q25 и Q75 – первый (25%) и третий (75%) квартили соответственно. При нормальном распределении анализировали данные при помощи t-критерия Стьюдента (t). При непараметрическом распределении количественных признаков применялся U-тест Манна–Уитни (U), при мультигрупповом – критерий Краселла–Уоллиса (Н) с последующим использованием теста множественных сравнений. Взаимосвязь определяли посредством корреляционного анализа с помощью коэффициента корреляции Спирмена (ρ). Различие в независимых группах по качественным бинарным признакам проводилось с использованием таблиц сопряженности c использованием критерия χ², вычисленного по методу Пирсона. Различия считали статистически значимыми при р <0,05.
Клинико-демографическая характеристика пациентов основной группы и группы контроля представлена в таблице 1.
РЕЗУЛЬТАТЫ
Больные основной группы имели легкую (63 человека – 65,6%) или среднюю (33 человека – 34,4%) степень тяжести анемии. Она имела нормоцитарный (MCV=87,71±6,07 фл), нормохромный (MCH=27,84±2,9 пг) характер. При этом гемоглобин у пациентов с анемией достоверно снижался при прогрессировании стадии (р=0,001) и ФК (р=0,0002) ХСН.
Больные с анемией при ХСН оказались достоверно более «тяжелыми», чем пациенты без анемии, за счет меньшего числа пациентов с ХСН I стадии и II ФК. Они имели более длительный сердечно-сосудистый анамнез, большее количество баллов по ШОКС и более высокую концентрацию NT-proBNP (см. табл. 1).
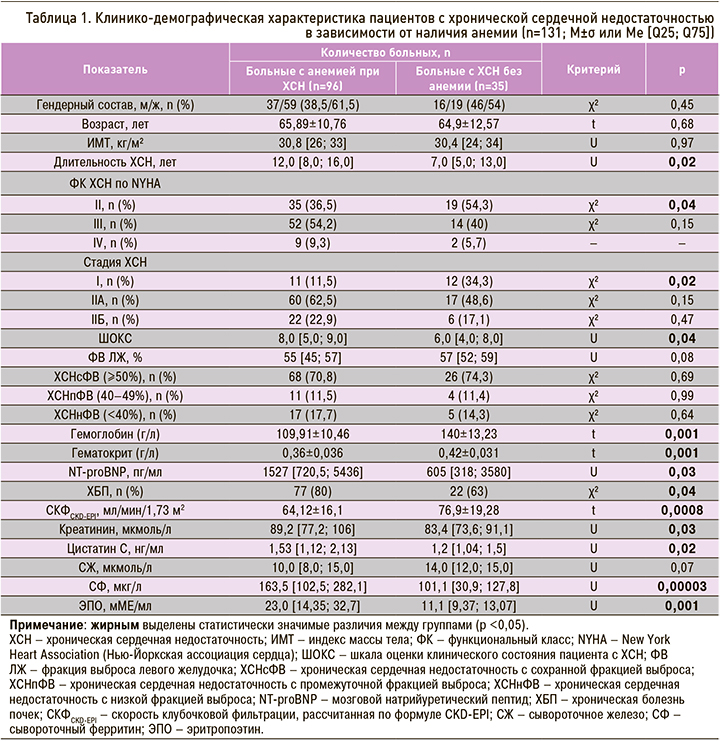
Выявлено, что среди пациентов с анемией при ХСН чаще встречалась ХБП: 80 против 63% в группе контроля (χ2=4,18; p=0,04). Об этом свидетельствовали показатели функционального состояния почек (см. табл. 1), которые были достоверно хуже у пациентов основной группы. Уровень гемоглобина у пациентов без ХБП был в среднем на 8,25 мл/ мин/1,73 м2 выше, чем при наличии признаков нарушения функции почек (p=0,01), и снижался по мере нарастания стадии ХБП (p=0,0007). Также обнаружено, что в основной группе уровень гемоглобина снижался при ухудшении функционального состояния почек по креатинину (ρ=-0,37; p <0,05), СКФ (ρ=0,58; p <0,05) и цистатину С (ρ=-0,50; p <0,05). В группе контроля подобных взаимосвязей обнаружено не было.
Уровень СЖ у пациентов с анемией при ХСН был умеренно снижен или нормальный. Он уменьшался по мере нарастания тяжести ХСН (р=0,005) и был ниже у лиц с ХБП (р=0,021). СФ в этой группе больных оказался нормальным или даже умеренно повышенным, причем был достоверно выше, чем у пациентов без анемии (см. табл. 1).
Было установлено, что 40 человек (42%) с ХСН имели дефицит железа. У 17 участников (18%) он был функциональным, а 56 человек (58%) имели анемию без дефицита железа. СФ у больных основной группы нарастал по мере прогрессирования ХСН по ШОКC (ρ=0,21; p <0,05), утяжеления анемии (ρ=-0,27; p <0,01) и снижения почечной функции по креатинину (ρ=0,28; p <0,01), цистатину С (ρ=0,34; p <0,05), СКФ (ρ=-0,28; p <0,01).
Пациенты с анемией при ХСН имели нормальные (71,4%) или умеренно повышенные (28,6%) показатели ЭПО – достоверно большие, чем среди больных без анемии. При этом повышенные значения ЭПО в основной группе ассоциировались с большей концентрацией фактора некроза опухоли-α (ФНО-α, р=0,01) и гепсидина (р=0,036).
Показатели цитокинового профиля (ИЛ-1β, ИЛ-6, ФНО-α) и гепсидин у пациентов с анемией при ХСН были значительно выше, чем у пациентов без анемии (табл. 2).
При этом ФНО-α и гепсидин достоверно нарастали по мере утяжеления ХСН по ФК (табл. 3).
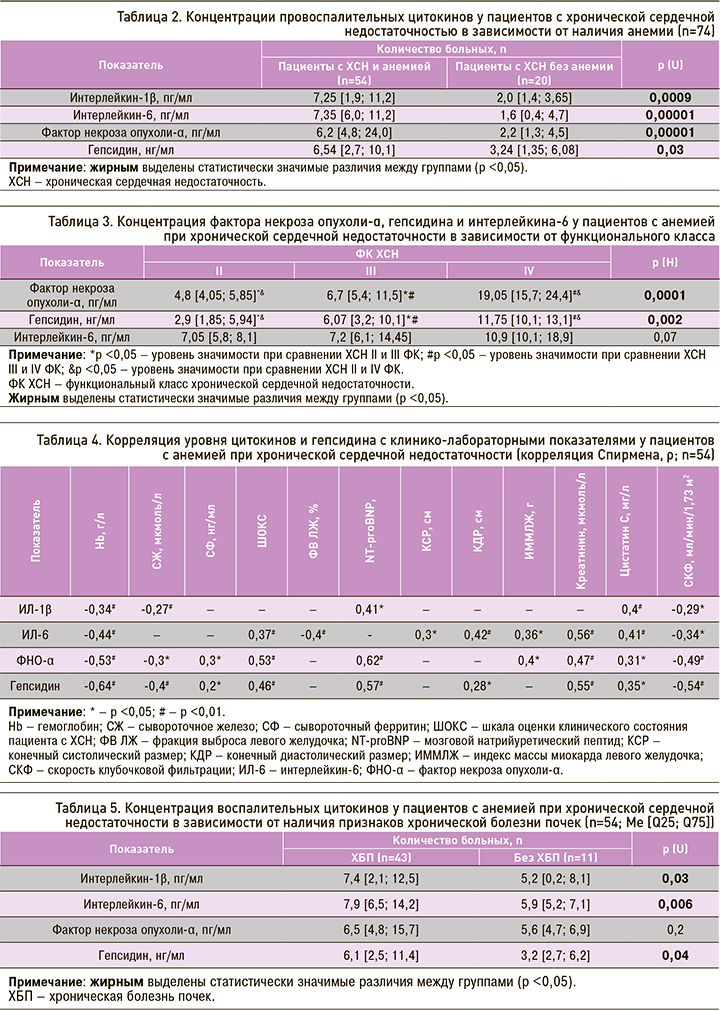
Среди пациентов с анемией при ХСН обнаружена умеренная корреляция по показателям ИЛ-6 и гепсидина и значительная – по уровню ФНО-α с тяжестью ХСН (ШОКС). В основной группе наблюдалась также прямая взаимосвязь концентрации ФНО-α, гепсидина и ИЛ-1β с уровнем NT-proBNP. Обнаружено, что уровень ИЛ-6 достоверно нарастал по мере снижения ФВ ЛЖ. Интерес представляет и положительная корреляция ИЛ-6 с показателями размеров ЛЖ у больных с анемией (КДР, КСР, ИММЛЖ), ФНО-α – с ИММЛЖ, гепсидина – с КДР (табл. 4).
Обращает на себя внимание то, что у больных с анемией при ХСН и признаками ХБП в целом были выявлены достоверно более высокие показатели ИЛ и гепсидина, чем среди пациентов без ХБП (табл. 5).
Установлено, что уровень провоспалительных цитокинов и гепсидина сыворотки крови у пациентов основной группы увеличивался пропорционально снижению почечной функции: обнаружены прямые корреляции ИЛ-1β, ИЛ-6, ФНО-α и гепсидина с цистатином С и отрицательная корреляция со СКФ, а также умеренная взаимосвязь уровней ИЛ-6, ФНО-α и гепсидина с концентрацией креатинина сыворотки (см. табл. 4). При этом у пациентов без анемии не было обнаружено подобных корреляций с маркерами почечной функции.
При сравнении показателей цитокинового профиля у пациентов с анемией при ХСН в зависимости от функционального характера дефицита железа определено, что функциональный железодефицит у них ассоциировался с достоверно большими концентрациями цитокинов (ИЛ-6 – р=0,04; ФНО-α – р=0,02) и гепсидина (р=0,004). Концентрации ФНО-α и гепсидина повышались пропорционально уровню СФ и обратно пропорционально уровню СЖ у пациентов с анемией при ХСН.
Также выявлена умеренная отрицательная корреляция между концентрацией гемоглобина с ИЛ-6 и значительная – между ФНО-α и гепсидином с гемоглобином. При увеличении уровня ИЛ-1β концентрация гемоглобина также хоть и не выраженно, но достоверно уменьшалась (см. табл. 4).
ОБСУЖДЕНИЕ
Анемия, патогенетически связанная с ХСН, регистрировалась в половине случаев всех анемических состояний у этих больных. Для нее была характерна легкая и умеренная степень тяжести и нарастание тяжести с прогрессированием ХСН. Развитие анемии при ХСН было взаимосвязано с нарушением функционального состояния почек: в подавляющем большинстве случаев (80%) она наблюдалась при наличии ХБП. Уровень гемоглобина прогрессивно снижался при увеличении креатинина и цистатина С в сыворотке крови, а также уменьшении СКФ. Результаты исследования наглядно проиллюстрировали прочные взаимопотенциирующие связи между течением и прогрессированием ХСН, анемией и снижением почечной функции. Это дополняет и соотносится с другими литературными данными [11, 35, 36].
Надо отметить, что в настоящем исследовании анемия при ХСН характеризовалась как наличием, так и отсутствием дефицита железа. При этом железодефицит у таких больных в половине случаев был функциональным. Согласно нашим данным, 58% пациентов вовсе не имели дефицита железа, что соотносится с данными Н.И. Соломахиной с соавт. [1]. Дополнительно нами было выявлено, что увеличение степени тяжести ХСН в сочетании с нарастанием почечной дисфункции негативно влияло на феррокинетические процессы у больных с анемией при ХСН и ассоциировалось с развитием функционального дефицита железа, что, в свою очередь, способствовало формированию АХЗ и сочетанию АХЗ с железодефицитом у таких больных. Это имеет важное прикладное значение в плане дифференциального подхода к лечению и избегания необоснованного назначения препаратов железа в отсутствие его дефицита.
Нами определено, что пациенты с анемией при ХСН имели нормальные или несколько повышенные значения ЭПО. Кроме того, оказалось, что для пациентов с уровнем ЭПО, превышающим референсные значения, были характерны более высокие концентрации ФНО-α и гепсидина. Эти данные не противоречат результатам некоторых работ, которые продемонстрировали увеличение уровня эндогенного ЭПО при утяжелении ХСН и рассматривали анемию как результат относительного дефицита ЭПО, неадекватного степени анемии, и относительной ЭПО-резистентности, возникающих, вероятно, на фоне цитокиновой активации [18, 37]. Подобные закономерности до настоящего времени полностью не изучены.
Согласно полученным нами результатам, у пациентов с анемией при ХСН отмечались достоверно более высокие уровни провоспалительных цитокинов и гепсидина, чем у пациентов с нормальным уровнем гемоглобина. Их концентрация нарастала по мере утяжеления ХСН и снижения насосной функции сердца. Показатели цитокинового профиля были взаимосвязаны с показателями структурных характеристик миокарда (КДР, КСР, ММЛЖ), что свидетельствовало о взаимосвязи уровня провоспалительных цитокинов сыворотки крови и ремоделирования миокарда при анемии. Это дополняет данные литературы о значимой роли воспалительного компонента в патогенезе ХСН [38].
Также нами установлено наличие более высокой концентрации показателей цитокинового профиля гепсидина среди пациентов с анемией при ХСН и признаками ХБП и наличие отрицательной корреляции этих показателей с маркерами почечной функции. Это позволяет предположить наличие роли почек в формировании цитокиновой активации и возникающей на его фоне АХЗ у больных с ХСН.
Немаловажным в понимании механизма развития анемии, патогенетически связанной с ХСН, оказалось то, что функциональный дефицит железа у больных сердечной недостаточностью ассоциировался с более высокими концентрациями ИЛ-6, ФНО-α и гепсидина. При этом показатели ФНО-α и гепсидина нарастали параллельно формированию функционального железодефицита (снижению СЖ и повышению СФ). Это, в свою очередь, закономерно отражалось на уровне гемоглобина у больных с ХСН и анемией. Таким образом, получалось, что по мере утяжеления ХСН и снижения функции почек у этих больных цитокиновая активация вызывала увеличение образования гепсидина, который способствовал формированию функционального дефицита железа и развитию АХЗ. Эти данные могут свидетельствовать о непосредственной его роли в секвестрации железа и формировании АХЗ у пациентов с ХСН, что дополняет сведения, полученные в исследовании на мышиных моделях [39].
ЗАКЛЮЧЕНИЕ
Настоящее исследование показало нарастание концентрации провоспалительных цитокинов и гепсидина у пациентов с анемией при ХСН по мере утяжеления ХСН и ухудшения почечной функции, а также их корреляцию с уровнем гемоглобина. Это свидетельствует о роли иммунного воспаления и гепсидина при формировании анемии, патогенетически ассоциированной с ХСН. Взаимосвязь показателей цитокинового профиля и гепсидина с маркерами работы почек указывают на вклад почек в эти механизмы.
Понимание внутренних патогенетических аспектов развития анемии у больных с ХСН имеет высокую ценность. Анемия при ХСН является потенциально устранимым состоянием, которое влияет на течение ХСН, прогноз и качество жизни таких пациентов. Вместе с тем часто такая анемия является резистентной к лечению препаратами железа, что обусловлено влиянием белка гепсидина на фоне нарастания цитокиновой активации.
Уточнение роли цитокиновой активации и гепсидина в генезе анемии у больных с ХСН будет способствовать детализации механизма ее развития и пониманию воздействия этого белка на организм в целом; это может стать предпосылкой к созданию новых, безопасных и патогенетически обоснованных подходов к лечению. Возможно, в обозримом будущем именно подключение антицитокиновой терапии, антагонистов гепсидина, ингибиторов его экспрессии и ингибиторов связывания гепсидина с ферропортином к стандартной терапии станет ключевым аспектом в лечении АХЗ у больных ХСН и улучшит реакцию пациентов не только на парентеральные, но и пероральные препараты железа.


