Сердечная недостаточность (СН) относится к числу самых распространенных заболеваний в России и Европе [1, 2]. СН представляет собой сложный клинический синдром, возникающий в результате структурно-функциональной перестройки миокарда желудочков и приводящий к характерным клиническим проявлениям в покое или при физической нагрузке. По сравнению с СН с низкой фракцией выброса левого желудочка вопросы диагностики СН с сохраненной фракцией выброса (СНсФВ) являются на сегодняшний день одними из наиболее дискуссионных, а различные аспекты рациональных диагностических алгоритмов у пациентов с этой формой заболевания, в частности, перед внесердечными хирургическими вмешательствами, остаются малоизученными и во многом нерешенными. Эта проблема тем более актуальна, что у больных хирургического профиля наличие сопутствующей СН служит отягощающим соматическим фактором, своевременная диагностика которого позволяет оптимизировать периоперационные стратегии [3]. Своевременное выявление и учет СНсФВ для хирургических пациентов имеет принципиальное значение, поскольку в условиях хирургического стресса (кровопотеря, инфузионная терапия) неадекватный ответ при измененной геометрии левого желудочка (ЛЖ) может влиять на послеоперационные исходы.
 Цель предлагаемого исследования – оценить частоту СНсФВ у пациентов с хроническими неинфекционными заболеваниями перед плановым внесердечным хирургическим вмешательством.
Цель предлагаемого исследования – оценить частоту СНсФВ у пациентов с хроническими неинфекционными заболеваниями перед плановым внесердечным хирургическим вмешательством.
МАТЕРИАЛ И МЕТОДЫ
Протокол исследования, формы медицинской документации и информированного согласия были одобрены Этическим комитетом ФГАОУ ВО «Российский национальный инновационный медицинский университет им. Н.И. Пирогова» (протокол от 17.12.2018 № 180). Исследование проводилось на базе ГБУЗ «Городская клиническая больница № 24» Департамента здравоохранения г. Москвы.
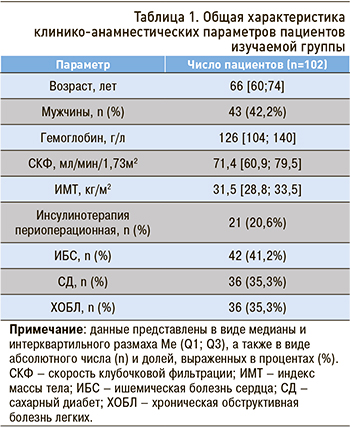
В исследование были включены 102 пациента (в том числе 43 мужчины), направленных на плановое эхокардиографическое обследование (ЭхоКГ) перед внесердечным хирургическим вмешательством. Медиана возраста составила 66 (60; 74) лет. Общая характеристика группы представлена в таблице 1.
Пациенты имели анамнез хронического неинфекционного заболевания (ишемическая болезнь сердца, хроническая обструктивная болезнь легких, сахарный диабет) без активных жалоб в покое, с субъективной оценкой своего функционального статуса как удовлетворительного. По данным клинического осмотра и электрокардиографии (ЭКГ), у исследуемых отмечался синусовый ритм.
До проведения трансторакальной ЭхоКГ выполнялся забор крови для оценки концентрации мозгового натрийуретического пептида (BNP) в покое методом иммуноферментного анализа (Вектор-бест).
Трансторакальное ЭхоКГ проводилось на ультразвуковом сканере Vivid7 PRO EXPERT (GE, США). Оценивались индекс массы миокарда ЛЖ, относительная толщина стенки ЛЖ, тип геометрии ЛЖ, параметры трансмитрального импульсно-волнового допплеровского исследования, тканевой допплерографии фиброзного митрального кольца, индексированный к площади поверхности тела объем левого предсердия и скорость трикуспидальной регургитации, на основании которой рассчитывалось систолическое давление в легочной артерии (СДЛА).
После оценки структурно-функциональных параметров в покое всем пациентам выполняли диастолический стресс-тест (ДСТ) на велоэргометре ножного педалирования (LODE CORIVAL, Нидерланды) или велоэргометре ручного педалирования (BELBERG BE-02). Исследование начинали с педалирования со скоростью не менее 60 оборотов в минуту при начальной нагрузке 50 Ватт (для лиц моложе 75 лет) и 25 Ватт (для лиц старше 75 лет). Каждая ступень нагрузки длилась 3 мин (180 с), по истечении которых нагрузку увеличивали на 25 Ватт. При достижении частоты сердечных сокращений (ЧСС) 100 ударов в минуту эргометрию продолжали в течение 3 мин без увеличения нагрузки. По достижении целевых критериев (субмаксимальная ЧСС, одышка, ангинозный приступ, падение артериального давления от исходного более чем на 20 мм рт.ст., отказ пациента от продолжения исследования) пробу прекращали. Далее в течение 5 мин после остановки теста оценивали следующие изменения:
- показатель давления наполнения ЛЖ (Е/е’) >14;
- СДЛА, mmHg (>50);
- индекс конечного систолического объема левого предсердия (ИКСО ЛП), мл/м2 (>34);
- ФВ ЛЖ (%).
Во время пробы контролировали параметры артериального давления (АД), ЧСС, сердечный ритм и сатурацию. Критерием прекращения пробы является полностью выполненная нагрузка в течение 3 мин после достижения ЧСС 100 ударов в минуту при стабильных условиях выполнения эргометрии. Отказ от продолжения тестирования, снижение систолического АД менее чем на 20 мм рт.ст. от исходного или повышение выше 220 мм рт.ст., появление симптомной аритмии, одышки, которая не позволяет продолжить исследование, боли в нижних конечностях служат критериями досрочной остановки пробы.
 Все участники подписали добровольное информированное согласие перед участием в исследовании. В качестве системы для ультразвуковой кардиовизуализации использовалась экспертный сканер VIVID7.
Все участники подписали добровольное информированное согласие перед участием в исследовании. В качестве системы для ультразвуковой кардиовизуализации использовалась экспертный сканер VIVID7.
Для статистического анализа применялись программные пакеты Microsoft Office Excel, STATISTICA 10.0 (Statsoft, USA), SPSS 23.0 (IBM, USA). При анализе данных использовались тест Шапиро–Уилкса, непараметрический критерий Манна–Уитни, непараметрический критерий Крускала–Уоллиса, критерий χ2 Пирсона, регрессионный анализ, индекс Юдена. Различия считались статистически значимыми при значении двухстороннего p <0,05.
РЕЗУЛЬТАТЫ
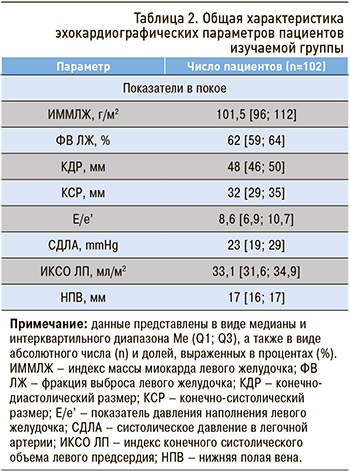
Общая характеристика эхокардиографических параметров пациентов изучаемой группы приведена в таблице 2. В нашей работе все пациенты имели ФВ ЛЖ ≥50%. Исходя из этого показателя, в соответствии с критериями анестезиологического риска параметры сократительной функции при трансторакальной ЭхоКГ не расценивались как нарушенные [4]. В то же время мы решили оценить наличие критериев СН среди пациентов с сохраненной ФВ на основании показателей ремоделирования ЛЖ и концентрации BNP в плазме крови.
При выполнении трасторакального ЭхоКГ в покое у 82,8% (n=84) пациентов были выявлены признаки ремоделирования ЛЖ. Были обнаружены следующие изменения структурных показателей и отклонения от нормальных значений параметров внутрисердечной гемодинамики: увеличение индекса массы миокарда ЛЖ в соответствии с полом – у 57,8%; дилатация ЛП, определяемая как увеличение ИКСО ЛП, – у 34,3%; повышение СДЛА в покое – у 16% пациентов; снижение средней скорости движения фиброзного митрального кольца е’ – у 62,7% пациентов. При этом параметр, отражающий давление наполнения ЛЖ в покое (Е/е’), был повышен только у 5,9% исследуемых.
С учетом вышеуказанных результатов, а также при повышении у пациентов уровня BNP >80 пг/л, мы, согласно алгоритмам HFA-PEFF, диагностировали наличие критериев СНсФВ [5]. Повышение BNP было отмечено в 40,2% (n=41) всех наблюдений. Оценив выборку в соответствии с вышеуказанными критериями, мы установили, что 32,3% (n=33) пациентов имеют инструментальные признаки СНсФВ. В покое у 67,7% (n=69) пациентов критериев СНсФВ выявлено не было.
Далее пациентам, у которых в покое не были выявлены признаки СН, был проведен эргометрический ДСТ. После нагрузочного тестирования было установлено, что 19,7% (n=20) исследуемых соответствуют критериям СНсФВ на основании повышения концентрации BNP при эргометрии. При сравнении ЭхоКГ-параметров было обнаружено статистически значимое увеличение ФВ ЛЖ, E/e’, ИКСО ЛП и уровня BNP (табл. 3). Повышение BNP мы оценивали после проведения ДСТ, и оно было выявлено в 63,7% всех наблюдений.

При сопоставлении клинических, инструментальных и лабораторных критериев СНсФВ в покое критериям СН соответствовало 32,3% пациентов, тогда как ДСТ позволил диагностировать заболевание у 52% всех исследуемых.
ОБСУЖДЕНИЕ
СНсФВ представляет собой вариант сердечной недостаточности, который долгое время протекает бессимптомно и трудно диагностируется. К сожалению, информация об этом фенотипе заболевания широко не распространена среди специалистов хирургического и анестезиологического профиля.
Внутрибольничная периоперационная смертность является достоверно более высокой у больных с любым диагнозом СН (вне зависимости от показателя ФВ ЛЖ) по сравнению с пациентами без СН [3]. По мнению авторов, пациенты со стабильным течением хронических неинфекционных заболеваний, таких как ишемическая болезнь сердца, сахарный диабет, хроническая обструктивная болезнь легких, относятся к основным кандидатам для углубленного диагностического обследования на предмет наличия СНсФВ. Именно у таких коморбидных больных перед внесердечными хирургическими вмешательствами требуется углубленный алгоритм диагностического периоперационного обследования.
В повседневной клинической практике вопрос диагностики СНсФВ у хирургических пациентов остается клинически значимой и нерешенной проблемой [3, 5]. И если для пациентов с умеренно сниженной и низкой ФВ ЛЖ риск потенциальных осложнений априори расценивается анестезиологами как высокий (поскольку под термином «сердечная недостаточность» подразумевается снижение ФВ ЛЖ), то в отношении пациентов с фенотипом СНсФВ отсутствуют подходы к стратификации периоперационного кардиального риска при внесердечных оперативных вмешательствах [4, 6–9]. Вопрос своевременной диагностики СНсФВ в группе хирургических пациентов остается важной проблемой реальной клинической практики [6].
ЭхоКГ позволяет определить нарушение релаксации с использованием продольной митральной кольцевой скорости ранней диастолической ткани (e’) и повышенного давления наполнения ЛЖ через соотношение раннего митрального притока (E) к e’, т.е. E/e’. Пациенты с СНсФВ могут иметь неэффективное опорожнение ЛП, увеличение его размера и нарушение функции [10]. В работах по изучению патофизиологии диастолической дисфункции было показано, что нарушение диастолического наполнения ЛЖ выступает важным фактором индуцированного повышения давления в ЛП [10]. Эти исследования продемонстрировали, что нормальное сердце способно увеличить трансмитральный поток во время упражнений с небольшим увеличением или вовсе без увеличения давления в ЛП за счет снижения минимального диастолического давления ЛЖ [10]. Однако при СН снижения диастолического давления в ЛЖ при физической нагрузке не происходит, а увеличение трансмитрального градиента и митрального кровотока достигается за счет повышения давления в ЛП [11– 13]. Подобный механизм объясняет повышенное давление наполнения во время упражнений у пациентов с СН, и именно он лежит в основе ДСТ [11]. ДСТ включает измерение скорости E/e’ и скорости трикуспидальной регургитации до и во время тренировки [12]. К сожалению, мы не встречали данных о широком использовании этого теста в России в рутинной клинической практике. Между тем неинвазивный ДСТ является крайне необходимым исследованием у пациентов с СНсФВ, поскольку у пациентов с сердечной недостаточностью и нормальным значением ФВ ЛЖ давление наполнения в состоянии покоя может быть нормальным, и тогда могут потребоваться эргометрические критерии для постановки диагноза. Дополнительная оценка E/e’ во время ЭхоКГс эргометрией улучшает чувствительность диагностики СНсФВ по сравнению только с оценкой в покое [14]. Оборудование, на котором выполнялось исследование, не является экспертным и используется в рутинной клинической практике. Операторами исследований у нас были врачи-исследователи, владеющие методикой трансторакальной ЭхоКГ.
Многие пациенты с СН имеют симптомы заболевания только при физической нагрузке, что обычно объясняется увеличением давления наполнения ЛЖ, необходимого для поддержания адекватного наполнения и ударного объема [5, 15, 16]. Получение данных ЭхоКГ во время физической нагрузки может выявить диастолическую и систолическую дисфункцию ЛЖ и улучшить диагностические подходы к выявлению СНсФВ [17–20]. Пациенты, истории болезней которых мы проанализировали, на основании рутинных ЭхоКГ-критериев не имели в покое клинически значимых нарушений внутрисердечной гемодинамики. У всех пациентов была сохраненная ФВ ЛЖ, отсутствовали регионарные нарушения сократимости миокарда, не было клапанных болезней сердца, левый желудочек не был дилатирован. При подробном анализе показателей трансторакальной ЭхоКГ нами были выявлены признаки ремоделирования левого желудочка у 82,8% пациентов.
Наличие ремоделирования ЛЖ само по себе служит фактором риска развития СНсФВ, поэтому нами были детально изучены показатели диастолического наполнения ЛЖ. Среди изменений структурных показателей и отклонений от нормальных значений параметров внутрисердечной гемодинамики в покое у 34,3% пациентов была выявлена дилатация ЛП, у 16% пациентов отмечалось повышение расчетного СДЛА, и только у 5,9% исследуемых наблюдалось повышение Е/е’ в покое.
Таким образом, при сопоставлении клинических, инструментальных и лабораторных критериев СНсФВ на основании данных трансторакальной ЭхоКГ покоя мы могли диагностировать СН у 32,3% пациентов. После выполнения ДСТ были выявлены следующие изменения: дилатация левого предсердия, по данным ИКСО ЛП, – у 39,2%, повышение СДЛА – у 34,3%, увеличение основного критерия наполнения ЛЖ (Е/е’) при эргометрии – у 26,5% пациентов.
ЗАКЛЮЧЕНИЕ
СНсФВ относится к трудно диагностируемым состояниям. В изученной выборке пациентов с хроническими неинфекционными заболеваниями перед плановым внесердечным хирургическим вмешательством признаки структурного ремоделирования были выявлены у 82,2% человек. На основании инструментальных и лабораторных данных 32,3% пациентов в покое имели критерии СНсФВ. При этом полезным диагностическим инструментом послужил ДСТ, который позволил обнаружить почти 1/3 случаев ранее не выявленной СН.


