Хронический гепатит В (ХГВ) является основной причиной цирроза печени (ЦП) и гепатоцеллюлярной карциномы (ГЦК) в мире [1]. Смертность от ЦП и ГЦК, по последним оценкам, составляет соответственно 310 000 и 340 000 в год [2]. Для лечения ХГВ наиболее широко применяются аналоги нуклеоз(т)идов (АН), обладающие высокой эффективностью и безопасностью [3]. Стойкое подавление репликативной активности вируса в результате длительной терапии АН приводит к уменьшению некровоспаления и фиброза печени.
Уменьшение фиброза печени – один из основных критериев эффективности лечения и условие улучшения прогноза при хронических заболеваниях печени, включая ХГВ. На сегодняшний день наиболее надежным методом оценки фиброза печени признана биопсия, однако инвазивный характер процедуры и потенциальные осложнения ограничивают ее использование в повседневной клинической практике. Транзиентная эластометрия (ТЭ) представляет собой ультразвуковой метод оценки плотности печени, которая измеряется в кПа и позволяет проводить оценку фиброза в динамике [4]. Она продемонстрировала высокую диагностическую ценность у пациентов с хроническим гепатитом С (ХГС) [5–9]. Вместе с тем исследования оценки фиброза печени с помощью ТЭ при длительной терапии пациентов с ХГВ ограниченны.
Цель исследования – оценить динамику фиброза печени у пациентов с ХГВ при длительной терапии нуклеоз(т)идными аналогами.
МАТЕРИАЛ И МЕТОДЫ
В исследование был включен 101 пациент с ХГВ, которые за период с 2008 по 2017 г. получали противовирусную терапию АН (тенофовир дизопроксил фумарат, энтекавир и телбивудин) в течение не менее 3 лет (максимально в течение 9 лет) в клинике ревматологии, нефрологии и профпатологии им. Е.М. Тареева Первого МГМУ им. И.М. Сеченова. В ходе наблюдения у 7 пациентов было отмечено развитие резистентности к терапии, в связи с чем была произведена модификация лечения (замена одного АН на другой); в результате этого суммарное количество случаев составило 108.
Критериями включения в исследование были моноинфекция вирусом гепатита В, наличие показаний к лечению (согласно клиническим рекомендациям EASL 2008 г.), индивидуальное согласие пациента на участие в исследовании, возраст более 18 лет [10].
Из исследования исключались пациенты с ко-инфекцией ХГС, ВИЧ, гепатита дельта, а также с другой патологией печени невирусной этиологии (алкогольная болезнь печени, неалкогольная жировая болезнь печени, аутоиммунные и лекарственные поражения, ГЦК).
Плотность печени измеряли в кПа при помощи транзиентной фиброэластометрии на аппарате «Фиброскан» (Fibroscan®, Echosens, Франция) у 108 пациентов перед началом терапии и в конце наблюдения. Исходные характеристики больных представлены в таблице 1.
Оценку стадии фиброза печени производили при помощи шкалы METAVIR: F0 – отсутствие фиброза (≤ 5,8 кПа), F1 – фиброз портальных трактов (слабый фиброз) (5,9–7,2 кПа), F2 – фиброз с немногочисленными септами (умеренный фиброз) (7,3–9,5 кПа), F3 – фиброз с многочисленными септами (выраженный фиброз) (9,6–12,5 кПа), F4 – цирроз печени (>12,5 кПа). В конце наблюдения в зависимости от результатов пациенты были разделены на две группы: со снижением плотности печени на ≥20% и <20% от исходного уровня.
64 пациента получали препарат энтекавир (Бараклюд, Bristol-Myers Squibb, США) в дозе 0,5 мг/ сут, 25 – тенофовира дизопроксил фумарат (Виреад, Gilead Sciences, США) по 300 мг/сут, 19 – телбивудин (Себиво, Novartis, Швейцария) по 600 мг/ сут.
Эффективность терапии АН оценивали на основании вирусологического ответа (уровень ДНК вируса гепатита В <150 МЕ/мл). Резистентность к проводимой терапии определяли по наличию вирусологического прорыва (повторное появление ДНК вируса гепатита В по данным полимеразной цепной реакции после достижения авиремии) или сохранении виремии ≥36 мес. В ходе наблюдения развитие резистентности к терапии наблюдалось у 7 пациентов.
Биохимический анализ крови, количественное определение ДНК вируса гепатита B в сыворотке крови (методом полимеразной цепной реакции), серологические маркеры (HBeAg и anti-HBe у HBeAg-позитивных пациентов) проводили до начала исследования, далее – каждые 6 мес. Количественные значения ДНК вируса гепатита B оценивали в log10 МЕ/мл.
Статистический анализ выполнялся с использованием SPSS версии 22.0, а также MedCalc 11.2. Критерий Колмогорова–Смирнова использовался для определения нормальности распределения количественных показателей. Количественные переменные представлены в виде медианы (Ме) и интерквартильного размаха (25-й и 75-й процентили), качественные признаки – в виде абсолютного числа и доли, выраженной в процентах. В связи с ненормальным распределением для анализа данных применялись непараметрические методы статистики: при сравнении количественных величин – критерии Манна–Уитни и Вилкоксона, для качественных переменных – критерий Фишера. К методу бинарной логистической регрессии прибегали для выявления предикторов отсутствия выраженного снижения плотности печени. Критическое значение уровня статистической значимости при проверке нулевых гипотез принималось равным 0,05.
РЕЗУЛЬТАТЫ
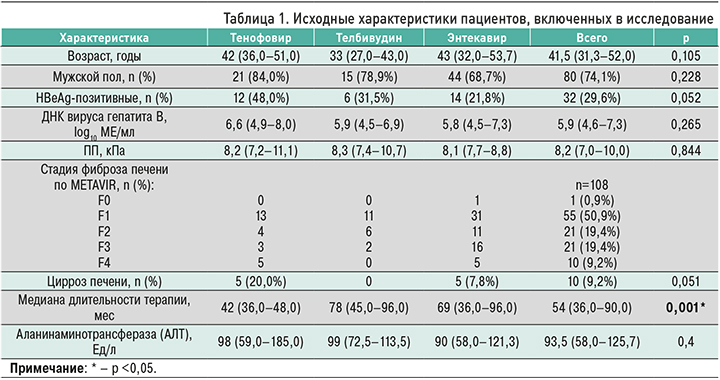
Среднее время между первым (исходным) и последним исследованием составило 49±10 мес. При лечении АН наблюдалось значительное снижение плотности печени – с 8,2 (7,4–10,1) до 6,7 (6,0–7,8) кПа (р <0,0001; рис. 1). Медиана снижения плотности печени составила 1,4 (1,1–2,2) кПа, или 17,5% (12,3–24,3). В целом снижение этого показателя наблюдалось у 98% (106/108) пациентов после лечения АН. Регресс фиброза на ≥1 балл по шкале METAVIR был отмечен в 57% (61/108) случаев.
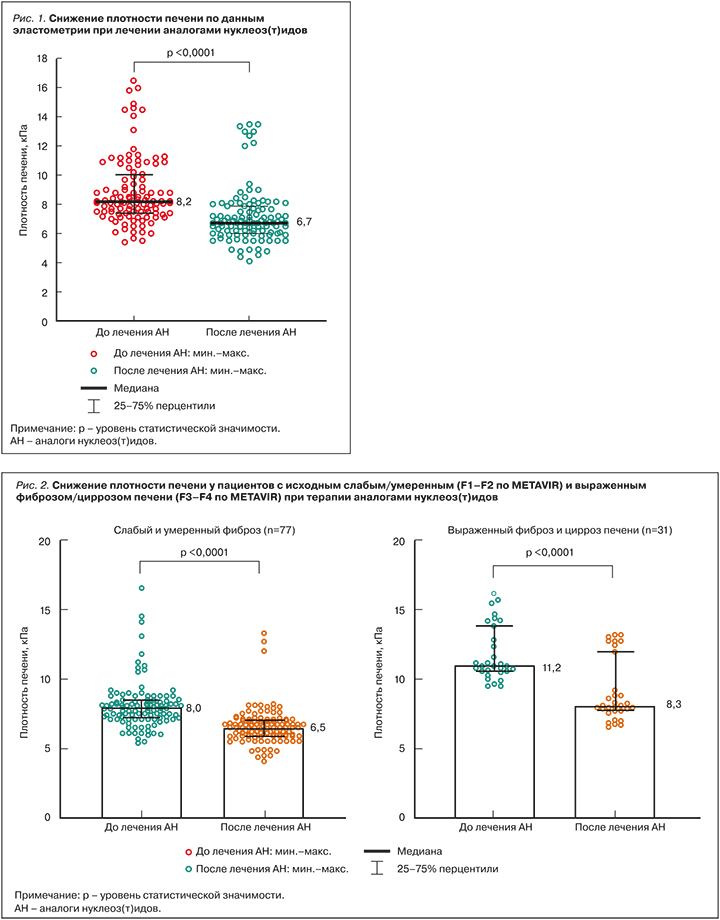
У пациентов с исходно тяжелым фиброзом (F3–F4 по METAVIR) отмечалось большее снижение плотности печени относительно больных с минимальным и умеренным фиброзом (F1–F2 по METAVIR): в первом случае это снижение составило с 11,2 (10,8–14,1) до 8,3 (7,9–12,2) кПа, во втором – с 8,0 (7,2–8,5) до 6,5 (5,9–7,0) кПа (рис. 2). Медиана снижения плотности печени составила соответственно -2,9 (-3,6–(-1,9)) и -1,3 (-1,7–(-1)) кПа (р <0,0001).
За время исследования доля пациентов с выраженным фиброзом (≥F2), уменьшилась с 48 (52/108) до 14% (15/108; р <0,0001; рис. 3). Уменьшение стадии фиброза печени (≥1 балла по METAVIR) наблюдалось у всех участников с исходной стадией фиброза F2 и F3. К концу наблюдения у всех больных с исходным ЦП был отмечен регресс фиброза (медиана снижения ПП составила 1,85 кПа, р <0,05). При этом у 3 пациентов с исходным F4 был отмечен регресс фиброза до стадий F3 (n=2) и F2 (n=1).
Ни у одного больного с ЦП не было выявлено увеличения плотности ткани печени (рис. 4).

Значительное снижение плотности печени (на ≥30%) наблюдалось у 12/108 (11,1%) пациентов. У абсолютного большинства больных (74,1%) имело место уменьшение этого показателя на 10–29,9%, у 13 (12%) – менее чем на 10%, у 2 изменения отсутствовали, и еще в 1 случае отмечалось повышение плотности печени (рис. 5).
Пациенты со значительным снижением плотности печени (≥20%) и без значительного ее снижения (<20%) не различались по возрасту, полу, исходной виремии, наличию HBeAg и ЦП до начала терапии (табл. 2). При этом у пациентов со значительным снижением этого показателя реже наблюдалось развитие резистентности к терапии и вирусологического прорыва, а также исходно были более высокие значения аланинаминотрансферазы (АЛТ) и плотности ткани печени.
Многофакторный анализ, выполненный на основе данных однофакторного анализа, показал, что более высокие исходные значения плотности печени (относительный риск 1,96; 95% доверительный интервал: 1,38–2,78; р <0,0001) ассоциированы со значимым ее снижением при терапии АН.
При лечении энтекавиром значительное снижение плотности печени отмечалось в 51,6% (33/64), тенофовиром – в 20% (5/25), телбивудином – в 36,8% (7/19) случаев. При этом пациенты во всех трех группах значимо не отличались по показателям исходной плотности ткани печени. Стоит отметить, что длительность терапии тенофовиром была статистически значимо ниже, чем телбивудином и энтекавиром (медиана 42, 66 и 54 мес соответственно; р=0,001).
В ходе лечения вирусологический ответ был получен у 96/108 (88,9%) участников. У пациентов с вирусологическим ответом в ходе терапии было отмечено более значительное снижение плотности печени, чем у пациентов, не достигших такого ответа (медиана снижения -1,4 и 1,0 соответственно; р=0,023). Добавим, что у пациентов без вирусологического ответа наблюдалось снижение вирусной нагрузки относительно исходного уровня (медиана снижения -5,9 log10 МЕ/мл).
ОБСУЖДЕНИЕ
В настоящее время показано, что обратное развитие фиброза печени возможно при условии успешного лечения хронического заболевания печени [11, 12]. Возможность обратного развития фиброза печени при длительной (более 3 лет) терапии АН у больных ХГВ на сегодняшний день является предметом активного изучения.
В нашем исследовании мы отметили снижение плотности печени в среднем на 1,5 кПа (с 8,2 до 6,7 кПа), или на 17,5% от исходного у абсолютного большинства (98%) пациентов, получавших АН в течение ≥3 лет. Наши данные согласуются с результатами других исследований: так, в работах Kim M.N. et al. и Zeng J. et al. плотность печени в среднем снизилась с 11,8 до 5,9 кПа и с 8,7 до 5,9 кПа через 3 года и 8 лет терапии АН соответственно [13, 14]. Метаанализ 24 исследований с участием 2228 пациентов с ХГВ продемонстрировал уменьшение этого показателя в среднем на 5,19 кПа через 5 лет терапии различными АН [15].
Как и в других исследованиях, мы наблюдали уменьшение доли пациентов с выраженным фиброзом (≥F2) с 48 до 14% (р <0,0001). Marcellin P. et al. также выявили уменьшение (с 38 до 12%) доли пациентов с тяжелым фиброзом (Ishak >4 баллов) при лечении тенофовиром в течение 5 лет [16].
Многими авторами было показано, что снижение плотности печени, как правило, отмечается у пациентов, достигших вирусологического ответа [13, 17–21]. В нашей работе у пациентов с вирусологическим ответом также имело место более значительное снижение плотности печени, чем у пациентов, не достигших такого ответа (р=0,023). В предыдущих исследованиях также было обнаружено уменьшение плотности печени не только у больных с вирусологическим ответом, но и при снижении виремии [13, 22].
В более ранних исследованиях описано обратное развитие фиброза и у больных ЦП [23, 29]. Нами было выявлено снижение плотности печени в среднем на 1,85 кПа у пациентов с исходным ЦП, в том числе регресс фиброза на ≥1 балл по шкале METAVIR у 3 (33%) пациентов. Buti M. et al. показали, что при лечении тенофовира дизопроксила фумаратом в течение 6 лет улучшение гистологической картины (уменьшение стадии фиброза на ≥1 единицу по шкале Ishak) наблюдалось у 93,8% пациентов с ЦП [24]. В метаанализе Facciorusso A. et al. также было установлено, что через 1 год терапии АН у 30,4% пациентов с ЦП плотность печени составила менее 11 кПа [15]. Однако необходимы дальнейшие исследования для оценки точного влияния снижения плотности печени на риск дальнейшего прогрессирования портальной гипертензии и развития ГЦК.
У больных с исходными стадиями фиброза F3– F4 по METAVIR наблюдалось более значительное уменьшение плотности печени в сравнении с пациентами с исходными стадиями F1–F2 по METAVIR: -2,9 кПа и -1,5 кПа соответственно. Chon Y.E. et al. были получены аналогичные результаты: плотность печени снизилась на 6,9 кПа у пациентов с исходным ЦП и на 3,3 кПа у пациентов с исходной F3 стадией через 5 лет терапии (р=0,001) [25]. В работе Rinaldi L. et al. также было продемонстрировано снижение плотности печени в среднем на 1,5 кПа и на 6 кПа у больных с исходным фиброзом F1–F2 и F3–F4 соответственно, получавших энтекавир или тенофовир в продолжение 2 лет [26]. При этом необходимо подчеркнуть, что в нашей работе у больных со значительным снижением плотности печени (≥20%) исходная активность АЛТ была выше, чем у больных с менее значительным снижением плотности печени (107 против 87 ЕД/л соответственно; р=0,041). Многими авторами, в частности, в исследованиях Fung J. et al., было показано, что даже небольшое повышение АЛТ (более 1N) увеличивает значения плотности печени независимо от гистологической стадии фиброза [27, 28]. Таким образом, можно предполагать, что уменьшение некровоспалительных изменений в результате терапии АН приводит к более выраженному снижению плотности ткани печени. Вопрос о том, насколько при этом уменьшается количество фиброзной ткани в печени, остается открытым.
Таким образом, ТЭ является неинвазивным, точным и удобным для клинической практики методом оценки плотности ткани печени, однако требуются дальнейшие исследования для выяснения ограничений к его использованию и интерпретации результатов.
ЗАКЛЮЧЕНИЕ
У больных ХГВ при длительном лечении АН плотность печени снизилась в среднем на 1,5 кПа, у 57% больных было отмечено уменьшение стадии фиброза на ≥1 баллов по шкале METAVIR. У всех пациентов с исходным ЦП наблюдалось снижение плотности печени, у 33% из них было достигнуто уменьшение стадии фиброза по шкале METAVIR. Доля пациентов с выраженным фиброзом (≥F2) в результате терапии уменьшилась с 48 до 14%. У участников исследования с исходно тяжелым фиброзом печени (F3–F4) наблюдалось большее снижение плотности печени, чем у пациентов с исходным фиброзом (F1–F2). Выраженное снижение плотности печени было отмечено в 42% случаев и было ассоциировано с более высокими ее значениями до начала терапии.


