ВВЕДЕНИЕ
За последние десятилетия инвазивные микозы в связи с неуклонным ростом заболеваемости стали актуальной медицинской проблемой. Наиболее часто они развиваются у больных гемобластозами, реципиентов трансплантатов кроветворных стволовых клеток. Увеличению числа иммунокомпрометированных пациентов с высоким риском развития глубоких микозов способствует всё большее распространение инвазивных диагностических и лечебных процедур, иммуносупрессивной терапии, возрастание числа ВИЧ-инфицированных, широкое применение антибактериальных препаратов. При этом наиболее высокому риску таких микозов подвержены пациенты в отделениях реанимации и интенсивной терапии (ОРИТ). Еще одну группу пациентов высокого риска составляют больные с тяжелым течением вирусных инфекций. В литературе описаны случаи развития инвазивных микозов у иммунокомпетентных до заболевания пациентов с тяжелым течением гриппа или других острых респираторных вирусных инфекций [1, 2].
Инвазивные микозы у больных новой коронавирусной инфекцией COVID-19 представляют собой тяжелые осложнения с высокой летальностью. По имеющимся данным, у пациентов, инфицированных SARS-CoV-2, наиболее часто развиваются инвазивный аспергиллез, инвазивный кандидоз и мукормикоз [3–5].
В нашей работе представлены опубликованные к настоящему времени данные об эпидемиологии, патогенезе, особенностях диагностики и лечения инвазивного кандидоза, ассоциированного с COVID-19.
ЭПИДЕМИОЛОГИЯ
Согласно эпидемиологическим данным, полученным еще до пандемии новой коронавирусной инфекции, острый диссеминированный кандидоз и кандидемия составляют 75–90% всех случаев инвазивного кандидоза, при этом риск летального исхода в период госпитализации увеличивается в два раза. Candida spp. – одноклеточные дрожжевые микроорганизмы размером 6–10 мкм. В 93–97% случаев возбудителями кандидемии и острого диссеминированного кандидоза выступают C. albicans (15–60%), C. parapsilosis (5–40%), C. glabrata (5–25%), С. tropicalis (5–15%) и C. krusei (3–7%). Источник возбудителя инвазивного кандидоза обычно эндогенный, поскольку Candida spp. – естественные обитатели слизистых оболочек и кожи человека; также возможно внутрибольничное заражение этими возбудителями. В условиях стационара заражение происходит в основном через медицинские изделия, такие как кислородные маски, трубки, катетеры, а также через медицинских работников [6, 7].
Кандидоз является самым распространенным инвазивным микозом у больных с COVID-19 и встречается в этом случае, по результатам ряда исследований, с частотой до 10%. Присоединение вторичной грибковой инфекции происходит обычно через семь и более дней после госпитализации, летальность составляет более 50% [7–12]. Так, в когортном исследовании, проведенном Chowdhary A. et al. (2020), у пациентов с COVID- 19, госпитализированных в ОРИТ, кандидоз или кандидемия обнаруживались в 2,5% случаев, причем 67% случаев были вызваны Candida auris [13].
Патогенез
Наиболее частое проявление инфекции, вызванной грибами рода Candida, – поверхностный кандидоз кожи и слизистых оболочек. Однако у иммунокомпрометированных пациентов возможна грибковая инвазия в системный кровоток и через стенку кишечника [14].
Процесс этот можно представить в виде следующих этапов.
1. Адгезия, опосредующая прикрепление Candida spp. к клеткам организма хозяина путем неспецифических (гидрофобные контакты) и специфических (лиганд-рецепторные взаимодействия) механизмов.
2. Диморфизм – трансформация из дрожжевой формы в гифальную. При этом гифы непосредственно способствуют инвазии, а дрожжевые формы участвуют в диссеминации.
3. Тигмотропизм – направленный рост гиф, который обеспечивается внеклеточным захватом кальция через кальциевые каналы.
4. Инвазия, обеспечивающая проникновение Candida spp. в клетки-хозяина. Она основана на двух взаимодополняющих механизмах: индуцированном эндоцитозе, опосредованном белками-инвазинами, и активном проникновении. Активному проникновению возбудителя в клетки хозяина и повреждению тканей способствует секреция гидролаз – аспарагиновых протеаз, фосфолипаз и липаз.
5. Формирование биопленок, способствующее большей устойчивости возбудителя к антимикробным агентам и иммунным факторам хозяина. Связано это со сложной архитектурой матрикса, повышенной экспрессией эффлюксного насосного аппарата и метаболической пластичностью [15–19].
Candida spp. обладают способностью обходить иммунную систему организма хозяина путем снижения слияния фагосом с лизосомами, уменьшения опсонизации, разрушения факторов комплемента, высвобождения каталазы и супероксиддисмутазы, снижения концентрации антимикробных пептидов, нарушения баланса между Th1- и Th2- лимфоцитами [20]. Кроме того, они вырабатывают кандидализин – пептидный цитолитический токсин, который является критическим фактором в процессе перехода кандид из комменсальной в патологическую форму. Кандидализин обладает двойной функцией – лизиса мембран макрофагальных фагосом и активации иммунокомпетентных клеток. При этом зачастую происходит иммунопатологический ответ с чрезмерной активацией клеточного звена [21].
Candida и COVID-19
Возможные факторы риска развития инвазивного кандидоза у пациентов с COVID-19 продолжают изучаться. Согласно имеющимся к настоящему времени сведениям, таким фактором риска прежде всего выступает сама инфекция, вызванная вирусом SARS-CoV-2, особенно у госпитализированных пациентов с тяжелым поражением легких. Есть исследования, в которых показано, что частота инвазивного кандидоза у пациентов с COVID-19 выше, даже если они не имеют дополнительных факторов риска [12, 22]. SARS-CoV-2 может повреждать энтероциты, снижать барьерную функцию кишечника, приводить к микробной транслокации и изменению микробиоты пациентов с COVID-19, что влечет за собой увеличение содержания Candida spp. Таким образом, данная категория пациентов входит в группу повышенного риска транслокации грибов рода Candida с последующей кандидемией [23–25].
Вероятность развития инвазивного кандидоза повышает наличие дополнительных факторов риска, таких как пожилой возраст, сахарный диабет, применение иммуносупрессантов и ряд других. В значительном числе случаев кандидоз развивался у пациентов с центральным венозным катетером, госпитализированных в ОРИТ. Отрицательную роль играла также необоснованно высокая частота назначения антибактериальных препаратов пациентам с COVID-19. По данным литературы, антибиотики получали около 70% таких больных, тогда как частота подтвержденной вторичной бактериальной инфекции составляла лишь 1–10%. Очевидно, что нерациональное применение антибактериальных препаратов широкого спектра действия способствует селекции и размножению условно-патогенных микроорганизмов, повышая риски вторичной грибковой инфекции [11, 26–33].
Наконец, известно, что для лечения тяжелой инфекции COVID-19 применяются иммуносупрессоры. В ряде работ высказано предположение о возможной связи между использованием блокатора рецепторов интерлейкина 6 (ИЛ-6) тоцилизумаба и развитием кандидоза [34, 35]. Также опубликованы результаты исследований, в которых на небольшой выборке получено подтверждение повышенного риска развития кандидемии и диссеминированного кандидоза при применении системных глюкокортикостероидов (ГКС) [36].
Роль системных ГКС в развитии инвазивного кандидоза у пациентов с COVID-19 объясняется их фармакологическими свойствами и подтверждается значительным числом исследователей. Препараты этого класса оказывают выраженное угнетающее влияние на клетки иммунной системы (Т-лимфоциты, макрофаги, лейкоциты и др.) и подавляют синтез провоспалительных цитокинов, таких как ИЛ-1b, ИЛ-2, ИЛ-6 и фактор некроза опухоли-альфа (ФНО-α). Под действием ГКС снижается фагоцитарная активность и нарушается баланс Th1/Th2 с преобладанием Th2-опосредованного иммунного ответа и выработкой специфических цитокинов [37]. Вследствие ингибирования ГКС секреции ФНО-α подавляется способность моноцитов ингибировать рост Candida [38]. Также показана способность ГКС способствовать адгезии грибковых клеток к клеткам хозяина и размножению их в желудочно-кишечном тракте (ЖКТ) и системном кровотоке [36, 38].
Клиническая картина
Клинические проявления кандидемии и острого диссеминированного кандидоза, вызванных Candida spp., существенно не различаются. Клинические признаки кандидемии неспецифичны и практически не отличаются от симптомов бактериального сепсиса: у 95–97% больных отмечается повышение температуры тела выше 38 °C, рефрактерное к антибактериальной терапии, у 20–25% – острая дыхательная недостаточность, у 15–25% – инфекционно-токсический шок, у 30–40% – признаки поражения различных органов. Острый диссеминированный кандидоз возникает в результате гематогенного распространения Candida spp. в организме. При этом возможно поражение практически всех органов и тканей организма, но наиболее часто в патологический процесс вовлекаются легкие, почки, органы зрения, головной мозг, сердце, кости, а также кожа и подкожная клетчатка [6].
У пациентов с инвазивным кандидозом, осложнившим течение новой коронавирусной инфекции COVID-19, описаны такие клинические проявления, как лихорадка с ознобом, гипотензия, дезориентация, абдоминальные боли, инфекции мочевых путей, безболезненные пустулезные элементы на коже на фоне эритемы [39–41].
ДИАГНОСТИКА
У больных с факторами риска и соответствующими клиническими признаками кандидемии или острого диссеминированного кандидоза диагностические мероприятия следует проводить незамедлительно. Диагностика основана на выявлении грибов рода Candida в крови и других стерильных в норме субстратах. Рекомендованные лабораторные и инструментальные исследования приведены в таблице.
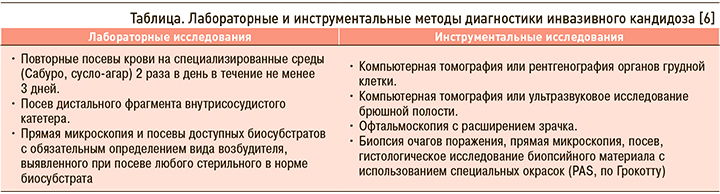
Критерии диагностики кандидемии – однократное выделение Candida spp. при посеве крови, полученной у больного с лихорадкой или другими признаками генерализованной воспалительной реакции. Критерии диагностики острого диссеминированного кандидоза – кандидемия в сочетании с выявлением Candida spp. при гистологическом исследовании и/или посеве материала из глубоких тканей или выявление Candida spp. при гистологическом исследовании и/или посеве материала из глубоких тканей двух и более локализаций [6].
Положительная гемокультура служит «золотым стандартом» диагностики инвазивного кандидоза [42]. Однако нужно помнить, что иногда при кандидемии или диссеминированном кандидозе в инфицированных тканях или в крови могут быть лишь единичные грибковые клетки, что делает их обнаружение в культуре крови затруднительным и требует дополнительных диагностических процедур [43]. Исследование гемокультуры также занимает достаточно длительное время [44]. Быстрым и точным современным методом идентификации Candida spp. является времяпролетная масс-спектрометрия с матрично-активированной лазерной десорбцией/ионизацией (MALDI-TOF-масс-спектрометрия) [45, 46].
РЕЗИСТЕНТНОСТЬ Candida к противогрибковым препаратам
Резистентность Candida spp. к азолам формируется через два механизма. Гиперэкспрессия или мутация гена ERG11, кодирующего фермент 14-α-деметилазу, приводит к гиперпродукции эргостерола или снижению аффинитета к азолам. Второй механизм связан с формированием мембранных эффлюксных помп. Известно несколько транспортных систем, осуществляющих активное выведение азолов. Активация систем выведения, часто ассоциирующаяся с изменениями в структуре мембраны, связана с мутациями генов Cg CDR1, Cd CDR1, Cd MDR1 [47, 48]. Относительно редко резистентность к азолам наблюдается у C. albicans, в то время как C. glabrata, C. krusei, C. auris и C. tropicalis обычно резистентны к этим препаратам [48].
Лекарственные средства из класса эхинокандинов воздействуют на мембрано-ассоциированный фермент β-13-D-глюкан-синтетазу, блокирующий синтез β-1,3-глюкана – основного структурного компонента клеточной стенки большинства грибов. Ферментный комплекс состоит из структурной/каталитической субъединицы, которая кодируется генами FKS [49]. В случае формирования клинической резистентности происходит модификация субъединиц генов FKS [50]. У большинства грибов рода Candida мутации возникают в двух высококонсервативных участках генов FKS1, а у C. glabrata – и FKS2. Возникающие аминокислотные замены вызывают повышение значений минимальных подавляющих концентраций (МПК), при этом некоторые из них могут снижать чувствительность глюкансинтетазы к препарату более чем в 3000 раз [51]. На текущий момент мутации в генах FKS остаются единственным механизмом устойчивости, четко ассоциированным с клинической неэффективностью терапии [52]. Наиболее частыми типами мутаций, ответственными за снижение чувствительности или резистентность к эхинокандинам, являются замены S663P или F659L [52–54], однако появляются данные и о новых типах мутаций, сопровождающихся высокими показателями устойчивости (потеря функции гена FKS1 в сочетании с точечной мутацией E655K в FKS2) [55]. Резистентность возбудителей к эхинокандинам обычно возникает на фоне терапии и связана с повторным или длительным их применением, при этом есть сведения о достаточно быстром формировании устойчивости и после коротких курсов лечения [56, 57].
Устойчивость к полиенам (амфотерицин В) развивается редко, и среди грибов рода Candida наблюдается у Candida auris [58]. Встречаются также штаммы, резистентные к большинству противогрибковых средств, и в этом случае контролировать возможные внутрибольничные вспышки особенно сложно [59].
ЛЕЧЕНИЕ
Риск развития инвазивного кандидоза, ассоциированного с COVID-19 (COVID-ИК), у больных в ОРИТ без специфических факторов риска невысок, поэтому рутинная первичная антифунгальная профилактика не рекомендуется.
Раннее эмпирическое назначение эхинокандинов повышает выживаемость больных инвазивным кандидозом. Показанием для эмпирической терапии COVID-ИК у больных в ОРИТ служит резистентная к адекватной антибиотикотерапии лихорадка продолжительностью более 4 сут в сочетании с наличием ≥2 факторов риска (длительное применение центрального венозного катетера, полное парентеральное питание, использование ГКС или иммуносупрессоров). Всем пациентам с инвазивным кандидозом показано раннее удаление/замена всех внутрисосудистых катетеров и других возможных источников возбудителя (мочевых катетеров, шунтов, протезов). Важные компоненты лечения – устранение или уменьшение выраженности факторов риска (отмена или снижение дозы ГКС, компенсация сахарного диабета и др.). При наличии факторов риска и клинических признаков COVID-ИК эмпирическую терапию следует начинать немедленно.
К препаратам выбора для эмпирической терапии всех вариантов COVID-ИК, кроме менингита и эндофтальмита, относятся эхинокандины – анидулафунгин, каспофунгин и микафунгин. Триазолы (вориконазол, флуконазол) можно назначать только в случае выделения чувствительного к препарату возбудителя COVID-ИК при стабильном состоянии пациента, а также для лечения кандидозного менингита и эндофтальмита. Помимо этого, вориконазол и флуконазол используют для деэскалационной терапии после стабилизации больного на фоне применения эхинокандина. Липосомальный амфотерицин В или липидный комплекс амфотерицина B применяют в случае неэффективности, токсичности или недоступности эхинокандинов. Амфотерицин В, позаконазол и итраконазол не рекомендованы для лечения COVID-ИК.
Продолжительность лечения составляет не менее 14 сут после исчезновения клинических признаков COVID-ИК и отрицательного посева крови [60].
ЗАКЛЮЧЕНИЕ
Грибковые инфекции, в частности ассоциированный с COVID-19 инвазивный кандидоз, относятся к частым осложнениям новой коронавирусной инфекции COVID-19. Особенно это касается госпитализированных пациентов с тяжелым течением инфекции и дополнительными факторами риска, среди которых наиболее значимыми являются сопутствующий сахарный диабет, онкогематологические заболевания, применение системных ГКС, антагонистов ИЛ-6 и других иммуносупрессоров, антибактериальных препаратов. Учитывая отсутствие специфических клинических проявлений COVID-ИК, позднюю диагностику, сложности терапии, связанные с ростом резистентности грибов рода Candida к антимикотическим препаратам, высокую летальность, необходима повышенная настороженность врачей в отношении этого осложнения новой коронавирусной инфекции для его своевременной диагностики и лечения.


