1 Кафедра терапии и ревматологии им. Э.Э. Эйхвальда ФГОУ ВО «Северо-Западный государственный университет
им. И.И. Мечникова», г. Санкт Петербург, Россия;
2 ГБУЗ СПб «Клиническая ревматологическая больница № 25», Санкт Петербург, Россия;
3 Городской клинический центр подагры, Санкт Петербург, Россия
Фебуксостат – недавно появившееся на российском фармацевтическом рынке противоподагрическое средство; оно представляет собой непуриновый, селективный ингибитор изоформ ксантиноксидоредуктазы (КОР), действие которого направлено на снижение уровня мочевой кислоты (МК) в сыворотке крови. Фармакокинетические свойства фебуксостата не зависят от почечного клиренса, что отличает его от аллопуринола и может служить преимуществом при лечении пациентов с хроническими заболеваниями почек. В целом ряде исследований проводится дальнейшая оценка безопасности фебуксостата в отношении сердечно-сосудистой системы, а также позитивного воздействия на сохранение функции почек. Фебуксостат более значимо подавляет КОР, чем аллопуринол, что подтверждается более частым достижением целевого уровня МК, особенно у пациентов с высокой концентрацией уратов в сыворотке крови. Немаловажно и то, что у пожилых пациентов не требуется коррекции дозы фебуксостата.
подагра
гиперурикемия
фебуксостат
аллопуринол
хронические заболевания почек
сердечно-сосудистые заболевания
Подагра – заболевание, которое развивается вследствие отложения кристаллов мочевой кислоты (МК) в суставах и околосуставных тканях. Вариантами клинических проявлений подагры являются приступы острого артрита, рецидивирующие артриты, упорное хроническое воспаление, а также формирование тофусов и отложение кристаллов солей МК в интерстициальной ткани почек. Подагра – одна из наиболее частых причин воспалительных артритов у мужчин [1, 2], но может встречаться и у женщин в постменопаузальном периоде [3].
Распространенность заболевания в мире составляет 0,08% [4], по сравнению с предыдущими десятилетиями ее частота растет [5, 8]. В США распространенность подагры в популяции достигает 3,9% [6], в Великобритании – 2,49% [7,9]. В возрасте 75 лет и старше подагра выявляется у 7% мужчин [10], среди населения Новой Зеландии в той же возрастной группе этот показатель достигает 30% [11]. К причинам увеличения частоты гиперурикемии и подагры среди населения разных стран мира относят увеличение потребления продуктов, богатых пуринами, безалкогольных напитков с фруктозой и, как следствие, ожирение [6, 7, 9], а также увеличение потребления алкоголя [12].
Подагра связана со значительными экономическими затратами [13, 14]. Длительность временной нетрудоспособности для пациентов с подагрой моложе 65 лет составляет 25,1 дня в год, а эпизоды острой подагры приводят к потере в среднем 17,1 дня трудоспособности в год [15].
ПРИНЦИПЫ ЛЕЧЕНИЯ ПОДАГРЫ
Главный принцип опубликованных в 2016 г. рекомендаций EULAR по лечению подагры – снижение уровня МК на фоне длительной противовоспалительной терапии с учетом сопутствующих заболеваний [16]. Впервые предложены принципы и рекомендации лечения подагры, основанные на стратегии Т2Т (treat to target – лечение до достижения цели). Важная роль отводится обучению пациентов, организации доступных консультаций врачей для наиболее эффективного достижения целевых уровней МК. Подчеркивается важность совместного принятия решений врачом и пациентом для оптимизации лечения подагры.
Отдельно обсуждается наиболее серьезное осложнение заболевания – поражение почек. Рекомендовано оценивать их функцию каждые 3–6 мес, особенно при наличии отягощающих факторов (сахарного диабета или артериальной гипертензии).
Согласно рекомендациям европейских экспертов, образование пациентов является неотъемлемой частью комплексного лечения подагры. При этом важное место отводится соблюдению рекомендованной диеты, снижению массы тела и ведению здорового образа жизни.
Стратегия лечения пациента с подагрой включает купирование острой подагрической атаки, а в дальнейшем – коррекцию гиперурикемии (рис. 1).

Для купирования острой подагрической атаки рекомендованы нестероидные противовоспалительные препараты (НПВП) и(или) колхицин, а также глюкокортикостероиды. Основополагающим принципом терапии является как можно более раннее ее начало – концепция pill in the pocket (препарат в кармане).
Коррекция гиперурикемии до достижения целевого уровня МК во многом определяет эффективность лечения подагры. В настоящее время рекомендовано начинать уратснижающую терапию (УСТ) уже после первого приступа подагрического артрита. Терапия должна проводиться уратснижающими препаратами в эффективной дозе для достижения целевого уровня МК в крови, составляющего менее 6 мг/дл (360 мкмоль/л). Уровень МК ниже 5 мг/дл (300 мкмоль/л) рекомендован для пациентов с тяжелым течением подагры (тофусы, хроническая артропатия, частые обострения). Следует отметить, что показатели содержания в крови МК у пациентов с подагрой ниже 3 мг/дл (180 мкмоль/л) не рекомендованы для длительного поддержания [17, 18].
В настоящее время в лечении гиперурикемии используются урикодепрессоры, урикозуретики, а также препараты, включающие фермент уриказу [19–21].
Урикодепрессоры тормозят продукцию МК посредством воздействия на ксантиноксидазу (КО) – фермент, участвующий в расщеплении пуринов (оксидация гипоксантина до ксантина, с последующим метаболизмом ксантина до МК) (рис. 2). В свою очередь, ингибирование КО способствует уменьшению количества свободных радикалов, образующихся на всех этапах формирования МК [17].
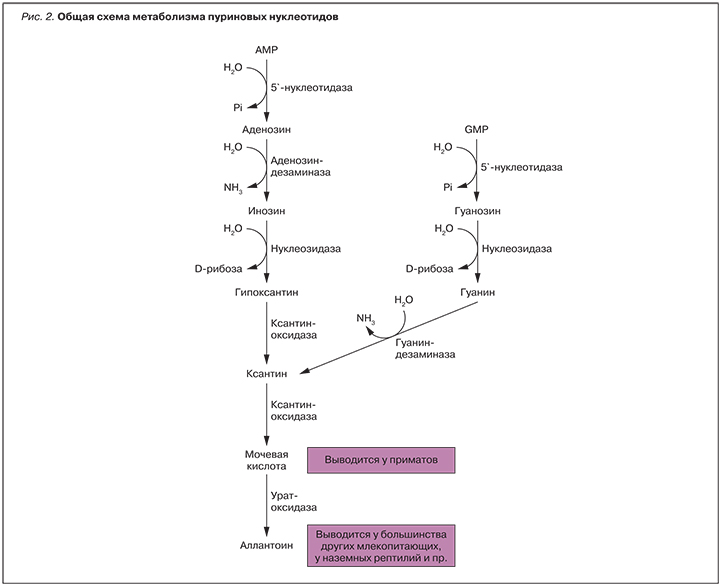
У большинства пациентов с подагрой препаратом выбора при инициации УСТ служит аллопуринол, который назначается в дозе 50–100 мг/сут, а затем доза препарата постепенно увеличивается на 100 мг через каждые 2–4 нед до достижения целевых значений уровня МК в крови. У 30–50% пациентов с нормальной функцией почек целевых значений МК достигнуть не удается. У ряда пациентов аллопуринол не эффективен даже в максимальной дозе 900 мг/сут [23]. Следует отметить, что при наличии у больных подагрой значимых нарушений функции печени и почек, гемохроматоза и беременности применение препарата противопоказано [22].
МЕСТО ФЕБУКСОСТАТА В ТЕРАПИИ ПОДАГРЫ
В случаях, когда целевое значение МК не может быть достигнуто при приеме максимально переносимой дозы аллопуринола, а также при наличии противопоказаний к аллопуринолу или его непереносимости, препаратом выбора является фебуксостат-2-(3-циано-4-изобутоксифенил)-4-метилтиазол-5-карбоксильная кислота, являющийся непуриновым селективным ингибитором КО, разрешенным для лечения подагры в 2008 г. [30]. Он проявляет мощное ингибирование окисленных и восстановленных форм КО [27–29] в отличие от активного метаболита – аллопуринола (оксипуринол), который ингибирует лишь восстановленную форму КО [31–33]. Кроме того, препарат не оказывает негативных воздействий на другие ферментные пути, которые являются частью пуринового и пиримидинового метаболизма, в том числе при высоких концентрациях [30].
ЕМА рекомендует фебуксостат «для лечения хронической гиперурикемии, когда имеют место отложения кристаллов, выявленные методами визуализации на доклинической стадии подагрического артрита» [34, 35]. В Австралии показанием к назначению фебуксостата является подагра [36], в то время как в Японии фебуксостат показан для лечения не только подагры, но и для коррекции бессимптомной гиперурикемии, которая является доказанным фактором риска сердечно-сосудистых заболеваний, а также одной из причин хронической почечной недостаточности [37, 38].
При подавлении КО фебуксостат формирует комплекс с обеими формами — редуцированной и оксидированной (D-форма и О-форма) [39, 40]. Аллопуринол связывается только с D-формой энзима, при этом две формы фермента могут обмениваться ионами молибдена (пинг-понг-механизм), превращаясь из одной формы в другую и снижая эффективность аллопуринола. Фебуксостат позволяет получить клинический эффект при значительно меньшей концентрации препарата в плазме по сравнению с аллопуринолом [39–44]. В отличие от аллопуринола фебуксостат практически не влияет на другие ферменты пуринового и пиримидинового метаболизма, что позволяет называть его селективным ингибитором КО [42]. КО, ингибированная аллопуринолом, может быстро реактивироваться под действием спонтанной реоксидации молибденового центра. Фебуксостат же не разрушается под действием окислительного статуса кофакторов, вследствие чего формируются стабильные связи и реактивации энзима не происходит [39]. Аллопуринол является ингибитором действия энзима, лишь временно снижая его активность. В то время как фебуксостат практически полностью заполняет узкие каналы, ведущие к молибденовому центру, обеспечивая стойкое подавление активности фермента [41].
После перорального приема фебуксостат быстро и почти полностью (84%) абсорбируется. У здоровых добровольцев пик плазменной концентрации наступает через 1 ч. Период полувыведения является дозозависимым и составляет 4–18 ч при дозах 10–120 мг [42–44]. При повторных приемах этот период может удлиняться [45]. Доказано, что фебуксостат можно принимать совместно с пищей и антацидами без существенного изменения фармакодинамики [42].
Метаболизм препарата происходит преимущественно в печени за счет связи с глюкуронозилтрансферазой [47], 25–45% препарата экскретируется с мочой в конъюгированном виде и только 1–6% выводится в неизмененном виде [39, 42, 43, 45]. Именно поэтому на фармакокинетические свойства фебуксостата нарушение функции почек оказывает минимальное влияние [46, 48]. У большинства пациентов наличие умеренной и даже тяжелой почечной недостаточности не отразилось на свойствах фебуксостата по снижению уровня уратов [50]. У пациентов с патологией печени также не требуется коррекции дозы фебуксостата [51].
ФЕБУКСОСТАТ ПРИ ХРОНИЧЕСКОЙ БОЛЕЗНИ ПОЧЕК
Гиперурикемия и подагра ассоциированы с развитием хронической болезни почек (ХБП). В Великобритании популяционное исследование показало, что распространенность ХБП (>2 ст.) среди пациентов с уровнем МК >10 мг/дл (584,9 мкмоль/л) и страдающих подагрой составляла соответственно 86 и 53%. ХБП представляется одним из главных факторов риска развития подагры, тогда как подагра может вызывать ХБП. Повышение сывороточного уровня МК ассоциировано с нарушением функции почек и развитием артериальной гипертензии, что является независимыми факторами риска заболеваний сердечно-сосудистой системы. О значении повышенного уровня МК свидетельствуют данные, полученные на животных моделях с индуцированной гиперурикемией [71], о снижении системного артериального давления и гломерулярной гипертензии после лечения фебуксостатом. Исследование FOCUS [72] показало, что стабилизация или увеличение расчетной скорости клубочковой фильтрации (СКФ) находится в обратно пропорциональной зависимости от сывороточного уровня МК. Уменьшение уровня МК на 1 мг/дл вызывало увеличение расчетной СКФ на 1 мл/мин. У пациентов с наиболее выраженным снижением уровня МК возможно ожидать уменьшения проявлений почечной недостаточности или даже стабилизации функции почек. Также было подтверждено, что фебуксостат эффективен и хорошо переносится у пациентов с подагрой и умеренным или выраженным снижением функции почек [74].
Эффективность и безопасность фебуксостата у реципиентов трансплантации почки и посттрансплантационной гиперурикемией были оценены у 51 пациента, показывая, что лечение фебуксостатом ведет к понижению уровня МК без серьезных побочных эффектов, что является одним из факторов длительного функционирования трансплантата [73, 75].
ПРОФИЛЬ БЕЗОПАСНОСТИ ФЕБУКСОСТАТА
По результатам проведенных клинических исследований, фебуксостат в целом хорошо переносился [56, 57] и проявил себя как более эффективный, чем аллопуринол, препарат для снижения уровня МК в крови до целевого показателя [66, 67]. Наиболее частыми негативными последствиями являются инфекционные заболевания верхних дыхательных путей (в исследованиях APEX, CONFIRMS, FOCYS и EXCEL), по результатам исследования FACT наиболее распространенными побочными эффектами были нарушения функции печени, являвшиеся наиболее частыми причинами отмены препарата [62, 63]. Примерно у 1% пациентов, получавших фебуксостат в представленных исследованиях, отмечалась тошнота, артралгии и кожная аллергическая реакция [63, 64].
При длительном применении фебуксостата у 5,5% пациентов отмечалось повышение концентрации тиреотропного гормона (>5,5 мкМЕ/мл), что явилось основанием для осторожного подхода к назначению препарата лицам, имеющим нарушения функции щитовидной железы [68].
Фебуксостат не рекомендуется применять у пациентов с ишемической болезнью сердца или застойной сердечной недостаточностью. В настоящее время проводится оценка сердечно-сосудистой безопасности препарата в рамках ускоренного исследования, которое началось в 2011 г. [69, 70].
ЗАКЛЮЧЕНИЕ
Фебуксостат – недавно вошедший в клиническую практику в России уратснижающий препарат. Механизм его действия, особенности его фармакокинетики и фармакодинамики позволяют ожидать значимых результатов в достижении целевых значений уровня МК крови у пациентов с подагрой. Особое место препарат может занять у пациентов с ХБП [52–55].
В настоящее время продолжаются клинические исследования, направленные на оценку эффективности терапии фебуксостатом в сочетании с ингибиторами реабсорбции уратов в почках у пациентов с тяжелой подагрой, которые не достигают целевых уровней МК при использовании монотерапии.
В качестве УСТ возможно применение урикозурических препаратов (бензбромарон, пробеницид) в варианте монотерапии при непереносимости ингибиторов КО, а также в комбинации с аллопуринолом или фебуксостатом. Однако использование урикозуретиков ограничено в условиях развившейся подагрической нефропатии, а также из-за риска гепатотоксичности [24, 25].
Следует отметить, что в клиническую практику вошли новые урикозуретики – лезинурад и веринурад, блокирующие почечный URAT1-транспортер [26, 19].
Лезинурад является селективным ингибитором реабсорбации МК. Препарат блокирует URAT1 – транспортер органических анионов, тем самым способствуя выведению МК из организма. Безопасность и эффективность применения оценивалась в трех рандомизированных плацебо-контролируемых исследованиях при участии 1537 пациентов. Это ингибитор обратного захвата МК, подавляющий активность транспортной системы URAT1, отвечающий за основной объем реабсорбции МК в почках. Ингибируя URAT1, лезинурад усиливает выведение МК из организма, тем самым снижая ее концентрацию в сыворотке крови. Также лезинурад ингибирует систему транспорта органических анионов-4 (OAT-4). OAT-4 отвечает за транспорт МК и является причиной развития гиперурикемии при применении диуретиков. Кроме того, у человека лезинурад не ингибирует OAT1 и OAT3 – транспортные системы, расположенные в почках и отвечающие за межлекарственное взаимодействие.
Веринурад – самый новый из селективных ингибиторов реабсорбции МК (группа урадов), созданных как для лечения подагры, так и для контроля бессимптомной гиперурикемии [65].
Пациентам с тяжелым течением подагрического артрита, у которых общепринятые методы лечения остаются неэффективными, показана терапия пеглотиказой, являющейся рекомбинантной уриказой, способной метаболизировать МК в растворимый аллантоин. В 2011 г. в издании Journal of the American Medical Association опубликованы результаты американского исследования, целью которого было оценить эффективность и переносимость пеглотиказы при хронической подагре, не поддающейся обычной терапии.
Авторы делают вывод, что лечение больных тяжелой подагрой с высоким уровнем уратов, не переносящих или невосприимчивых к действию аллопуринола, пеглотиказой в дозе 8 мг 1 или 2 раза в месяц в течение полугода приводит к значимому снижению концентрации кислоты.
Комплексная терапия пациентов, страдающих подагрой, с применением как традиционных, так и новейших препаратов до достижения целевого уровня МК – путь к достижению ремиссии заболевания и улучшению качества жизни больных.
Марианна Семеновна Петрова, к.м.н., руководитель Городского клинического центра подагры, доцент кафедры терапии и ревматологии им. Э.Э. Эйхвальда ФГОУ ВО «Северо-Западный государственный медицинский университет им. И.И. Мечникова». Адрес: 190068, Санкт Петербург, Большая Подъяческая, д. 30.
Мария Меджидовна Мусийчук, врач-ревматолог ГБУЗ СПб «Клиническая ревматологическая больница № 25». Адрес: 190068, Санкт Петербург, Большая Подъяческая, д. 30.
Оксана Владимировна Инамова, к.м.н., главный врач ГБУЗ СПб «Клиническая ревматологическая больница
№ 25». Адрес: 190068, Санкт Петербург, Большая Подъяческая, д. 30.
Вадим Иванович Мазуров, д.м.н., профессор, академик РАН, завкафедрой терапии и ревматологии им. Э.Э. Эйхвальда ФГОУ ВО «Северо-Западный государственный медицинский университет им. И.И. Мечникова», заслуженный деятель науки. Адрес: 191015, Санкт Петербург, ул. Кирочная, д. 41.





