Гастроинтестинальные кровотечения (ГИК) часто встречаются у пациентов, которые принимают антикоагулянтные или антиагрегантные препараты для лечения основного заболевания [1]. Эта серьезная проблема, наиболее выраженная у пожилых и/или коморбидных пациентов, ставит перед врачом отделения неотложной помощи сложную дилемму. С одной стороны, любое лечение антикоагулянтами и антиагрегантами следует прекратить, чтобы помочь остановить острое кровотечение. С другой стороны, прекращение этого вида терапии может значительно увеличить риск сердечно-сосудистых или цереброваскулярных осложнений из-за основного заболевания [2].
Число пациентов, которым назначают антикоагулянтную или антиагрегантную терапию для лечения или профилактики сердечно-сосудистых и цереброваскулярных заболеваний, возрастает [3, 4]. Недостатком этих методов лечения, однако, является то, что они увеличивают риск опасных для жизни кровотечений, большинство из которых возникает в виде желудочно-кишечного или внутримозгового кровотечения [5]. В частности, у больных, получающих антикоагулянтную или антиагрегантную терапию, относительный риск кровотечения из верхней части желудочно-кишечного тракта (ЖКТ) увеличивается до 10%, а годовой риск возникновения такого кровотечения составляет от 1,5 до 4,5% [6–8]. Это осложнение ставит пациентов в крайне опасную для жизни ситуацию, поскольку смертность от кровотечений из ЖКТ колеблется от 1 до 13% [9–11]. Растущая доступность различных вариантов антикоагуляции создает как преимущества, так и проблемы для медицинских работников и для пациентов. Несомненно, увеличение арсенала антикоагулянтных средств расширяет возможности лечения, однако в настоящее время мало ясности относительно того, какие пациенты с наибольшей вероятностью выиграют от новых препаратов, а кто может подвергаться наибольшему риску осложнений.
Новые оральные антикоагулянты (НОАК) способствуют лучшей приверженности к лечению и демонстрируют более высокую безопасность в плане неблагоприятных геморрагических событий по сравнению с обычной терапией варфарином [12–15]. Но если говорить о риске именно желудочно-кишечного кровотечения, то некоторые исследователи указывают на увеличение частоты ГИК среди пользователей НОАК [16–20], хотя другие сообщают, что такой риск выше на фоне приема антагонистов витамина K [21–26].
ПАТОФИЗИОЛОГИЯ ГАСТРОИНТЕСТИНАЛЬНЫХ КРОВОТЕЧЕНИЙ НА ФОНЕ НОАК
ЖКТ имеет очень богатое внутри- и подслизистое кровоснабжение, при этом даже у здоровых людей целостность слизистой оболочки ЖКТ регулярно нарушается. Например, эрозии желудка выявляются у 5–10%, а эрозии тонкой кишки – у 10–15% здоровых волонтеров [27, 28]. Уязвимость слизистой оболочки возникает вследствие воздействия кислоты и пищеварительных ферментов, таких как пепсин, трипсин и амилаза, а также внешних воздействий, например, на фоне системной антикоагуляции варфарином [29]. Bнутрипросветные факторы, такие как бактерии, также влияют на микроциркуляторное русло ЖКТ, подвергая хозяина риску кровотечения.
Развитие ГИК при приеме оральных антитромбических средств может быть обусловлено по крайней мере тремя механизмами:
1. системным эффектом антикоагулянта;
2. прямым повреждающим действием;
3. биологическим действием препарата, не связанным с коагуляцией.
Эти механизмы могут реализовываться в комбинации друг с другом: скажем, аспирин может способствовать развитию гастродуоденальной язвы, вызывая местное повреждение и системные антитромбоцитарные эффекты.
Прием дабигатрана этексилата в дозе 150 мг 2 раза/сут и ривароксабана связан с более частыми желудочно-кишечными кровотечениями, чем применение варфарина, но в то же время ассоциирован с более низкими показателями внутричерепного кровоизлияния. Гипотетически это связано с неполным всасыванием НОАК через слизистую оболочку ЖКТ в отличие от варфарина.
Так, более 95% принятого варфарина абсорбируется, а непоглощенная часть препарата в просвете пищеварительного тракта не активна. ГИК у пациентов, получающих варфарин, вероятно, обусловлены системным действием этого антикоагулянта, а не прямым его воздействием на слизистую оболочку ЖКТ.
Напротив, биодоступность НОАК переменна. У пролекарства (неактивного метаболита) дабигатрана этексилата биодоступность составляет лишь 6%; остальная часть препарата обнаруживается в фекалиях. Во время пассажа по ЖКТ по крайней мере две трети пролекарства метаболизируется в активный дабигатран эстеразами пищеварительного тракта. Биодоступность ривароксабана и апиксабана выше (60–80 и 50% соответственно), однако даже при применении этих лекарственных средств существенное количество активного препарата остается в фекалиях. Поэтому в случае со всеми тремя НОАК после их приема активные вещества присутствуют в слизистой оболочке ЖКТ и теоретически могут (в сочетании с системными эффектами) потенцировать кровотечение из уязвимых участков.
Существует разница между различными НОАК в плане вероятности развития ГИК. Результаты, полученные Desai J. et al., показали, что прием дабигатрана этексилат и ривароксабана может быть сопряжен с более высоким риском желудочно-кишечных кровотечений, чем у других НОАК [30]. Недавний метаанализ данных в клинических условиях также подтвердил эту тенденцию [21].
Локальное повреждение богатой сосудами ткани неизбежно вызывает минимальное кровоизлияние в слизистую оболочку. НОАК вследствие как системного, так и местного действия блокируют свертывание крови и образование тромба, а также способны провоцировать продолжение кровотечения [31, 32]. Небольшие повреждения слизистой оболочки могут возникать достаточно часто, ведь она постоянно находится под воздействием агрессивных факторов. В желудке это кислота, пепсин, Helicobacter pylori (который встречается у 30–50% жителей развитых стран), в тонкой кишке – химус, богатый ферментами и желчными кислотами (сильными детергентами), в толстой – активная бактериальная флора, в том числе персистирующие условно-патогенные организмы. Нельзя забывать и о повреждающем действии пищи, содержащей раздражающие специи, органические кислоты, тугоплавкие жирные кислоты, ксенобиотики, крепкого алкоголя и компонентов сигаретного дыма [33]. Широкое использование нестероидных противовоспалительных средств (НПВС) – еще одни ключевой фактор развития пептической язвы и желудочно-кишечных кровотечений в современном мире. Сотни миллионов людей принимают эти препараты в основном в режиме «по требованию», при этом у 20–30% возникают бессимптомные эрозии и язвы верхних отделов ЖКТ, на фоне которых риск развития кровотечений повышается [34, 35].
Исследование ROCKET AF показало, что у пациентов с фибрилляцией предсердий (ФП) пероральный ингибитор фактора Ха ривароксабан не уступает варфарину в профилактике инсульта и системной эмболии [36]. Хотя частота основных и неосновных клинически значимых кровотечений у сравниваемых средств также была одинаковой, ривароксабан приводил к снижению риска внутричерепных кровоизлияний и смертельных кровотечений. В то же время этот препарат вызывал более частые кровотечения из ЖКТ и кровотечения, которые приводили к снижению уровня гемоглобина или требовали переливания крови [18].
Maruyama K. et al. в своем исследовании определили частоту желудочно-кишечных геморрагических событий у пациентов, принимающих НОАК в одном из японских медучреждений, как 4,7% в год, а частота клинически значимых ГИК равнялась 2,0% в год; при этом количество «верхних» ГИК составляло 0,9% в год, а «нижних» – 1,1% в год [37]. Miller C.S. et al. сообщили о ГИК из верхних отделов ЖКТ у 1,5% пациентов и о ГИК из ниже расположенных участков у 1,0% в обзоре 43 клинических испытаний, включавших более 160 000 больных [21]. Отметим, что разница в частоте «верхних» ГИК в исследованиях может быть объяснена различной частотой назначения пациентам ингибиторов протонной помпы (ИПП) и неодинаковым возрастом пациентов.
Bang C.S. et al. провели систематический обзор исследований, проводившихся с даты создания PubMed, Кокрановской библиотеки, Embase и KoreaMed по апрель 2018 г., в которых оценивался защитный эффект антисекреторных препаратов в отношении ГИК, ассоциированных с антикоагулянтами. Всего было найдено и проанализировано 6 когортных исследований или исследований типа «случай–контроль». В этих испытаниях ИПП оказывали защитное действие против верхнего ГИК у пациентов, получавших дикумариновые антикоагулянты (отношение риска [RR] 0,56; 95% доверительный интервал [ДИ] от 0,38 до 0,83; I 2, 0%), а вот H2-блокаторы не проявляли схожего эффекта (RR 0,97; 95% ДИ от 0,52 до 1,81; I 2, 0%). В то же время у пациентов, принимавших дабигатрана этексилат, кислотосупрессоры не показали протекторного действия в отношении желудочно-кишечных кровотечений, связанных с этим НОАК (RR 0,78; 95% ДИ от 0,44 до 1,37; I 2, 81,8%) [38].
ОЦЕНКА РИСКА КРОВОТЕЧЕНИЙ НА ФОНЕ ПРИЕМА НОАК В КЛИНИЧЕСКОЙ ПРАКТИКЕ
Достаточно сильное кровотечение, требующее серьезного медицинского вмешательства (такого, как переливание крови, хирургическое пособие) либо приводящее к критическим осложнениям или смерти, классифицируется как тяжелое (большое) [39, 40]. Такое кровотечение обычно требует прекращения антикоагулянтной терапии, по крайней мере временного [41]. В клинической практике в режиме реального времени в качестве тяжелого обычно рассматривается кровотечение, связанное со значительным риском смерти (например, внутримозговое или желудочно-кишечное), серьезными осложнениями (например, внутриглазное или внутримозговое) или требующее переливания крови [42].
При проведении пероральной антикоагуляции необходим осознанный подход к оценке риска кровотечений. Для этого у пациентов с неклапанной ФП было разработано и апробировано несколько оценочных шкал (табл. 1): HEMORR 2 HAGES, HAS-BLED, ATRIA, RIETE и AMPLIFY [43–47]. Однако ни один из этих инструментов не является специфическим для НОАК, так как эти системы оценки создавались в основном для пациентов, получающих антагонисты витамина К. Итоговые значения, которые указывают на высокий риск кровотечения с помощью HEMORR 2 HAGES, HAS-BLED и ATRIA, составляют ≥4, ≥3 и ≥5 баллов соответственно.
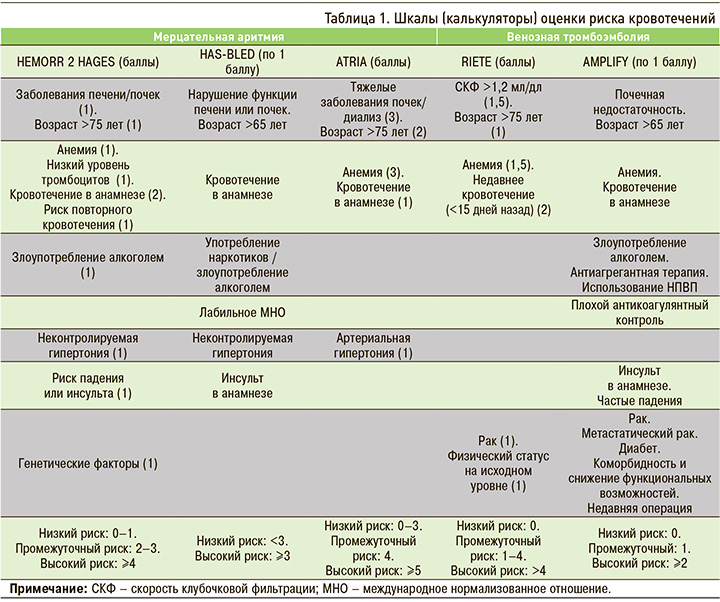
Результаты HAS-BLED показали лучшую прогностическую эффективность в отношении тяжелых и клинически значимых нетяжелых кровотечений по сравнению со шкалами HEMORR 2 HAGES и ATRIA. Кроме того, показатель HAS-BLED >2 коррелировал с 85%-ным повышением риска клинически значимого нетяжелого кровотечения, 2,4-кратным риском тяжелого кровотечения и 2,9-кратным риском смерти. При этом оценка по ATRIA >3 не была связана с клинически значимым нетяжелым кровотечением, но ассоциировалась с высокой смертностью от всех причин и тяжелым кровотечением [48].
Европейское общество кардиологов в алгоритме назначения оральных антикоагулянтных средств пациентам с ФП рекомендует опираться на шкалу HAS-BLED для определения модифицируемого риска кровотечений [49]. Американский колледж кардиологии рекомендует, исходя из данных шкалы RIETE, стратифицировать пациентов на группы с низким (0 факторов риска), средним (1 фактор риска) и высоким (≥2 факторов риска) риском: каждая категория имеет соответственно 1,6, 3,2 и 12,8% вероятность тяжелых кровотечений в первые 3 мес базовой антикогулянтной терапии [50]. Важно учитывать, что высокий риск кровотечения не должен рассматриваться как противопоказание или причина для прекращения антикоагуляции: в большинстве случаев польза от антикоагуляции превышает опасность кровотечений. Скорее, высокие риски должны быть идентифицированы и скорректированы в зависимости от ситуации, чтобы минимизировать риск кровотечения [49, 50].
Препараты группы НОАК могут назначаться как в виде монотерапии, так и в составе двойной терапии в комбинации с низкими дозами аспирина и тройной терапии в комбинации с аспирином, клопидогрелем или прасугрелем. В комбинации с аспирином риск гастроинтестиальных кровотечений увеличивается; особенно страдает в такой ситуации тонкая кишка. Несмотря на свою явно положительную роль в профилактике сердечно-сосудистых заболеваний, аспирин удваивает риск развития желудочно-кишечного кровотечения, причем риск ГИК сохраняется у людей, которые принимают его более 1 года [51–53].
ЛЕЧЕНИЕ И ПРОФИЛАКТИКА КРОВОТЕЧЕНИЙ НА ФОНЕ ПРИЕМА НОАК
Как уже упоминалось выше, в исследованиях по применению НОАК наибольшую проблему представляли желудочно-кишечные кровотечения, тогда как риск кровотечений в других органах был низким. Во время ГИК, связанного с НОАК, лабораторное измерение уровня коагуляции не рекомендуется, поскольку достоверность или ценность любого простого теста коагуляции при определении антикоагулянтного статуса не была доказана. Исходя из периодов полураспада НОАК и их быстрого клиренса, у пациентов с нормальным почечным клиренсом для нивелирования эффекта антикоагулянта, как правило, достаточно поддерживающих мер, обычно в течение 18–24 ч. В ситуациях, угрожающих жизни, требуется экстренное хирургическое вмешательство.
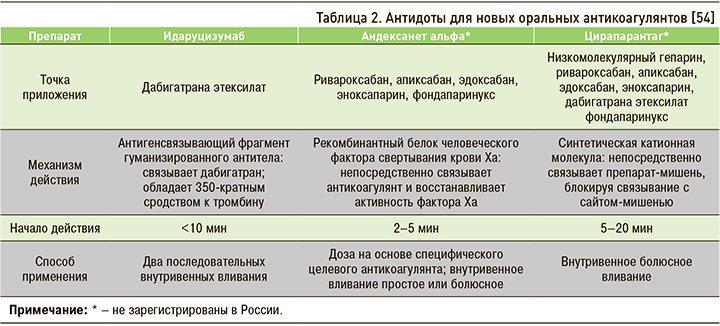
На сегодняшний день изучены и находятся на различных этапах процесса утверждения три «антидота» против НОАК [54], данные о которых обобщены в таблице 2.
Предыдущие рандомизированные исследования показали, что профилактическое использование ИПП и Н2-блокаторов снижает риск эндоскопически установленных язв у пациентов, получающих аспирин [55–57]. Эти исследования, однако, не были в состоянии оценить клинические желудочно-кишечные события и, более того, не определили потенциальную выгоду для пациентов, получающих комбинированную антитромбоцитарную терапию. Испытания, показывающие роль ИПП в предотвращении рецидивов желудочно-кишечного кровотечения, были проведены в популяциях с высоким риском ГИК.
Согласно различным международным и отечественным рекомендациям (например, NICE), ИПП показаны пациентам с тяжелой гастроэзофагеальной рефлюксной болезнью или другой доказанной патологией пищевода (язва пищевода, пищевод Барретта), зарегистрированными язвами двенадцатиперстной кишки или желудка, а также больным с неисследованной диспепсией, неязвенной диспепсией или легкими симптомами диспепсии коротким курсом в низкой дозе для оценки ответа на лечение [58]. Любое другое использование ИПП считается неоправданным. Применение ИПП на фоне антикоагулянтной терапии (кроме терапии аспирином в низких дозах) сегодня не оговорено ни в одной инструкции к препаратам этого класса, зарегистрированным в России [59].
С момента появления ИПП увеличивается осведомленность о потенциальных неблагоприятных воздействиях, связанных с ними. Управление по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA) выпустило предупреждение о возможном повышенном риске переломов, связанных с остеопорозом, и инфекции Clostridium difficile (CDI), на фоне терапии ИПП [60, 61]. Из-за этих потенциальных угроз Американское общество гериатрии (AGS) в 2015 г. обновило критерии Бирса с целью минимизировать использование ИПП в течение >8 нед у пожилых людей, за исключением пациентов с высоким риском [62]. Аналогичным образом критерии START/STOPP рекомендуют прекратить прием или снизить дозу ИПП у пожилых людей с неосложненной язвенной болезнью или эрозивным пептическим эзофагитом, которые находились на лечении >8 нед [65].
Существует еще ряд неблагоприятных эффектов ИПП, которые были выявлены в течение последних двух десятилетий, включая повышенный риск внебольничной пневмонии, дефицита витамина B12, а в последнее время деменции и болезни почек. Хотя и было опубликовано несколько обзоров о нежелательных реакциях при приеме ИПП, ни один из них не был специально посвящен пожилому населению. Более того, недавно обнаруженные побочные эффекты, такие как заболевание почек и деменция, ранее не рассматривались в опубликованных обзорах. Эти потенциальные риски вызывают особую обеспокоенность при ведении пожилых пациентов, поскольку эта группа населения априори подвергается повышенному риску возникновения этих проблем, которые приводят к значительному росту заболеваемости и смертности [66].
Совместное назначение гастропротективных агентов (ИПП или Н2-блокаторов), как установлено, связано с 50% сокращением риска ГИК [37]. Однако этот защитный эффект ограничен верхним отделом ЖКТ у лиц, имеющих анамнез язвенной болезни или ГИК. ИПП продемонстрировали несколько лучший защитный эффект, чем Н2-блокаторы, а оптимальный эффект отмечался у пациентов, которые совместно применяли и ИПП и H2-блокаторы (85%-ное сокращение риска ГИК) [67]. При этом следует помнить, что кислотосупрессия может уменьшить абсорбцию дабигатрана этексила [68, 69].
Помимо антисекреторных средств, другие превентивные меры по снижению риска кровотечений на фоне приема НОАК включают модификацию факторов риска (например, эрадикацию Helicobacter pylori, воздержание от алкоголя, исключение совместного назначения НПВП). Пациентам с высоким риском ГИК (например, ≥3 по шкале HAS-BLED, с желудочно-кишечными кровотечениями в анамнезе) показаны гастропротективные средства [17, 30, 70], также рекомендуется использование апиксабана или дабигатрана этексилата в низкой дозе [71, 72]. В то же время важно отметить, что более низкая доза НОАК менее эффективна в профилактике инсульта [73]. Интересно, что прием НОАК может привести к раннему диагнозу опухоли ЖКТ, поскольку они могут вызвать более раннее кровотечение [74, 75].
ИПП произвели революцию в лечении многочисленных заболеваний верхних отделов ЖКТ. Общие преимущества такой терапии и улучшение качества жизни значительно перевешивают потенциальные риски у большинства пациентов, хотя пациенты, у которых нет клинических показаний к применению ИПП, подвержены риску осложнений этого лечения. Для оценки баланса пользы и вреда от применения ИПП важна стратификация риска у пожилых, ослабленных, госпитализированных больных, пациентов с хроническими заболеваниями. Клиницистам крайне важно проводить переоценку необходимости продолжительной терапии ИПП в долгосрочной перспективе, принимая во внимание экономически эффективную практику назначения лекарств [76].
Пациентам, имеющим атрофический гастрит, особенно с метаплазией и дисплазией, гипо- и ахлоргидрией ИПП не показаны, так как у этих пациентов уже не остается «точек приложения» этих препаратов – протонных помп. При этом эрозивно-язвенные поражения у них бывают не реже, чем у пациентов с сохраненной секрецией, по нашим данным. Эти пациенты чаще относятся к старшей возрастной группе, а причины поражения слизистой ЖКТ у них следующие: дуоденогастральный рефлюкс (ДГР), нарушение микроциркуляции вследствие хронической сердечной недостаточности или психологических стрессов, прием большого количества лекарственных препаратов, в том числе НПВС, кортикостероидов и многих других. Уровень кислотопродукции у них можно определить с помощью суточной pH-метрии, экспресс pH-метрии или при исследовании пепсиногена-1 (<30 мкг/л) и гастрина-17 (≥10 пмоль/л). Учитывая, что антисекреторные препараты у таких пациентов малоэффективны или вовсе не эффективны, возникает потребность в поиске других средств гастро- и энтеропротекции [77].
Несколько лет назад в России появился препарат ребамипид, выпускаемый под коммерческим названием Ребагит® чешской фармацевтической компанией PRO.MED.CS Praha a.s.. Ребамипид продемонстрировал профилактическое действие на различных моделях острой язвы желудка, включая язвенную болезнь, вызванную лекарственными препаратами [78]. В 1990 г. ребамипид был одобрен в Японии для лечения язвенной болезни желудка. Впоследствии он был также апробирован для лечения поражений слизистой оболочки желудка (эрозия, кровотечение, покраснение и отек) при остром гастрите и обострении хронического гастрита. Доклинические исследования показывают, что ребамипид усиливает стимуляцию биосинтеза простагландинов в слизистой оболочке желудка [79], увеличивает синтез гликопротеинов слизи в поверхностных слоях слизистой оболочки желудка [80], активирует эпидермальный фактор роста и его рецепторную экспрессию [81], восстанавливает эпителий желудка и тонкой кишки [82]. Сообщается также, что он удаляет свободные радикалы [83], подавляет активацию нейтрофилов [84] и выработку воспалительных цитокинов (например, интерлейкина-8) [85]. Эти данные подтверждают противовоспалительную активность ребамипида на слизистой оболочке желудка и позволяют предположить, что препарат оказывает влияние на физиологические функции слизистой оболочки желудка; это отличает его от других гастропротекторных средств [86–88].
Ребамипид был многократно описан как средство защиты слизистой оболочки ЖКТ с хорошими статистически значимыми результатами в профилактике и лечении язвенных поражений, вызываемых НПВП, H. pylori или эндоскопической диссекцией подслизистой оболочки [88–94].
Именно Ребагит® может рассматриваться как тот препарат, который эффективно справляется с профилактикой и лечением желудочно-кишечных кровотечений при приеме НОАК как на фоне монотерапии (особенно дабигатрана этексилатом), так и комбинированной двойной или тройной терапии. Препарат наиболее эффективен в ситуациях с добавлением низких доз аспирина [95]. При этом у пациентов, получающих НОАК, Ребагит® может оказывать реальное защитное действие не только на уровне желудка, но и тонкой кишки, в которой антисекреторные средства не проявляют энтеропротективной эффективности.
Появление в арсенале врачей ребапимида имеет большое значение в связи с тем, что ГИК, связанные с приемом НОАК, серьезная медицинская проблема, значение которой в связи с постоянным расширением использования этого класса антикоагулянтов будет только возрастать.


