Диагностика
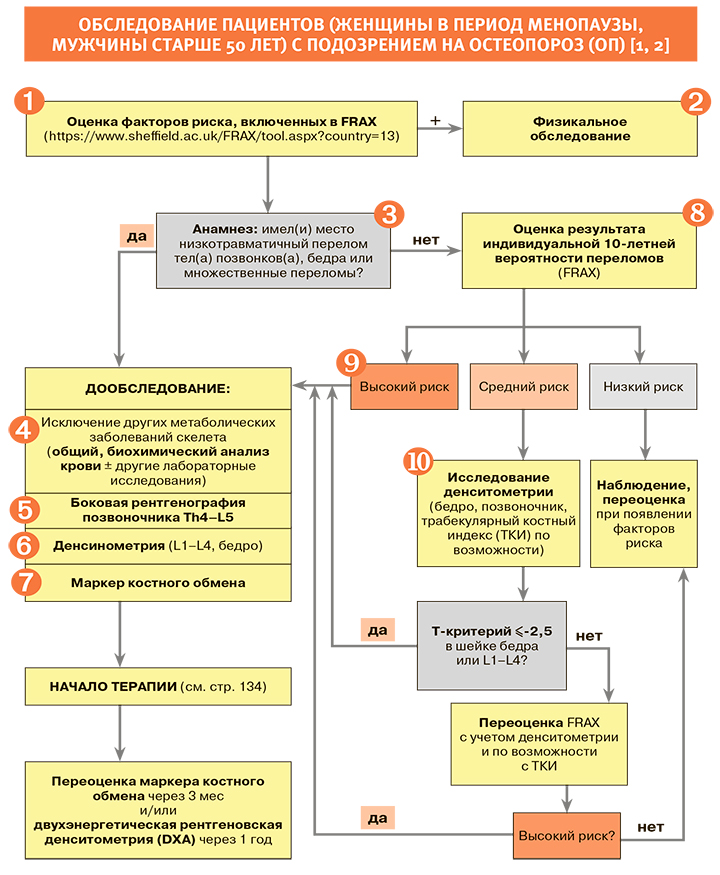
1.
• До развития низкотравматического перелома остеопороз (ОП) не имеет клинических проявлений [1, 2].
• Все клинические факторы риска, включенные в модель расчета риска переломов ВОЗ (FRAX), и нюансы их оценки приведены на русскоязычной странице официального сайта FRAX. Практическому врачу удобно выяснить наиболее важные детали анамнеза, сразу включив их в анализ вероятности перелома FRAX, в модель, разработанную для России [3].
• Скрининг для выявления групп с высокой вероятностью переломов рекомендован с использованием алгоритма FRAX среди всех женщин в постменопаузе и мужчин старше 50 лет [1, 2].
2.
• При физикальном обследовании заподозрить компрессионный(ые) перелом(ы) деформации тела позвонка(ов) позволяют:
- снижение роста пациента на ≥2 см за 1–3 года регулярного наблюдения или ≥на 4 см за период, прошедший после 25-летнего возраста. При измерении роста в пользу возможных компрессионных переломов позвонков говорит также невозможность пациента полностью распрямиться, наличие расстояния от стены до затылка;
- складки кожи на спине и боках (симптом «лишней кожи»), уменьшение расстояния между реберными дугами и гребнями подвздошных костей меньше ширины 2 пальцев;
- характерная кифотическая деформация грудной клетки, относительное увеличение живота в объеме («выпячивание» передней брюшной стенки), относительное удлинение конечностей и укорочение грудной клетки;
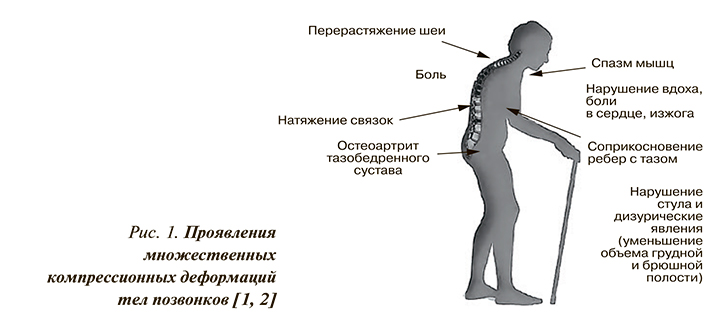
- перерастяжение шеи, боль, натяжение связок, нарушения стула и дизурические явления (уменьшение объема грудной и брюшной полости), спазм мышц, нарушение вдоха, боли в сердце, изжога, остеоартрит тазобедренного сустава.
Сводный портрет пациента с клиническими проявлениями множественных компрессионных деформаций тел позвонков приведен на рисунке 1.
• Также при физикальном обследовании следует обращать внимание на симптомы заболеваний, приводящих к вторичному ОП (генетические, гипогонадные, эндокринные, гематологические и другие нарушения), которые достаточно специфичны для каждой патологии [1, 2].
3.
• При переломах в анамнезе необходимо уточнить их локализацию и характер травмы. При низкотравматических переломах крупных костей скелета (бедра, тел(а) позвонков(а), множественных переломов), выявленных в анамнезе или при обследовании, рекомендовано устанавливать диагноз ОП и назначать лечение НЕЗАВИСИМО ОТ РЕЗУЛЬТАТОВ ДЕНСИТОМЕТРИИ ИЛИ FRAX (при условии исключения других заболеваний скелета). Наличие единственного перелома тела позвонка повышает риск последующих переломов позвонков в 3–5 раз, а риск переломов бедра и другой локализации – в 2–3 раза [1, 2].
• В отличие от ОП, низкотравматический перелом в остром периоде, как правило, имеет яркую клиническую картину. Он сопряжен с болью, нарушением функции и диагностируется рентгенологически врачом-травматологом, который в зависимости от характера предшествующей травмы может заподозрить ОП [1, 2].
4.
• Лабораторные методы исследования – клинический, биохимический анализ крови (обязательно) и другие методы (по показаниям) – рекомендуется проводить всем пациентам с впервые установленным диагнозом ОП, а также при неэффективности ранее назначенной терапии для дифференциальной диагностики с другими причинами повышенной хрупкости скелета (табл. 1).
• Результаты клинического и биохимического анализа крови при первичном ОП не имеют специфических изменений. Выявление анемии, повышенного СОЭ, нарушений фосфорно-кальциевого обмена позволяет заподозрить другие заболевания, приводящие к снижению прочности скелета, или какую-либо причину вторичного ОП. Кроме того, лабораторные исследования позволяют уточнить ограничения к назначению той или иной терапии (например, гипокальциемия – противопоказание к назначению антирезорбтивных препаратов, гиперкальциемия и повышение ЩФ – к назначению терипаратида и т.д.). Лабораторные исследования следует повторять при неэффективности терапии ввиду возможности развития сочетанных заболеваний или дефицита витамина D как причины недостаточного ответа на лечение [1, 2].
5.
• Стандартное рентгенологическое исследование грудного и поясничного отделов позвоночника (Th4–L5) в боковой проекции для выявления компрессионных переломов тел позвонков рекомендовано пациентам с болью в спине, снижением роста на 4 см за жизнь или на 2 см при регулярном медицинском контроле, больным, принимающим глюкокортикоиды (ГКС), имеющим длительно некомпенсированный сахарный диабет (СД) 2 типа или находящимся на инсулинотерапии, а также пациентам c диагностированными переломами другой локализации. Наиболее значимые факторы риска развития компрессионных переломов тел позвонков – применение ГКС и СД 2 типа [1, 2].
• Компрессионный перелом тела позвонка – снижение высоты тела позвонка (компрессионная деформация) в переднем, среднем или заднем отделах на ≥20% по сравнению с другими отделами этого же позвонка (рис. 2).

• Основной рентгенологический симптом ОП – повышение рентгенопрозрачности костной ткани – неспецифичен и в значительной степени зависит от технических условий съемки и качества проявления рентгенограмм. В отсутствие характерных компрессионных деформаций тел позвонков (компрессионных переломов) установление диагноза ОП на основании данных рентгенографии неправомочно [1, 2].
• После первого рентгенологического исследования динамический контроль необходимо проводить лишь в случаях документально подтвержденного проспективного снижения роста или при возникновении/возобновлении боли в спине или изменения осанки. Повторное рентгенологическое исследование позвоночника также рекомендовано у пациентов при принятии решения об отмене медикаментозного лечения ОП, так как у пациентов со свежими переломами тел позвонков прекращение приема препаратов нежелательно [1, 2].
• В качестве дополнительных инструментальных методов дифференциальной диагностики ОП и других заболевания могут рассматриваться различные виды мультиспиральной компьютерной томографии (МСКТ), магнитно-резонансной томографии (МРТ) и сцинтиграфия скелета [1, 2].
6.
• Денситометрия бедра и позвоночника может быть использована для:
- установки или подтверждения диагноза ОП согласно рекомендациям ВОЗ от 1994 г. (табл. 2);
- прогнозирования/расчета риска переломов в зависимости от степени снижения МПК;
- наблюдения за динамикой состояния пациентов на фоне терапии или без лечения.
Рекомендовано устанавливать диагноз ОП и назначать терапию при снижении МПК на ≥2,5 стандартных отклонений (SD) по Т-критерию в шейке бедра и/или в целом в бедре и/или в поясничных позвонках (L1–L4, L2–L4), измеренной методом двухэнергетической рентгеновской денситометрии (DXA) [1, 2, 9].

7.
• Основные биохимические маркеры костного ремоделирования, которые наиболее оправдано определять в клинической практике, сведены в таблице 3.
• Маркеры костного ремоделирования (резорбции при назначении антирезорбтивной терапии и костеобразования при назначении анаболической терапии) рекомендовано определять исходно и через 3 мес от начала терапии с целью ранней оценки эффективности лечения (ожидая как минимум 30%-ное изменение) и приверженности к терапии. Достаточно оценивать один маркер, но одним и тем же лабораторным набором [1, 2].
• Уровень маркеров костного ремоделирования в верхней четверти референтного интервала рекомендуется оценивать как дополнительный фактор риска для инициации терапии ОП у женщин в постменопаузе с остеопенией [1, 2].

• Не рекомендуется использовать маркеры костного ремоделирования для прогнозирования риска переломов у индивидуального пациента, а также выбора типа терапии (антирезорбтивной или анаболической) [1, 2].
• Практическим специалистам не рекомендуется устанавливать первичный диагноз ОП на основании любых лабораторных исследований. Диагноз ставится только на основании низкотравматического перелома, снижения МПК или совокупности факторов риска [1, 2, 4, 5].
8.
• Если в ходе оценки жалоб, анамнеза, физикального обследования и рентгенографии позвоночника низкотравматические переломы не выявлены, решение о необходимости назначения терапии ОП принимается на основании 10-летней вероятности развития низкотравматического перелома. В результате расчета FRAX врач получает индивидуальную 10-летнюю вероятность (в процентах) перелома бедра и основных низкотравматических переломов – клинически значимых переломов тел позвонков (т.е. переломов, которые сопровождаются болью), переломов бедра, плеча и лучевой кости [1, 2, 3].
• Устанавливать диагноз ОП и назначать лечение независимо от показателя денситометрии рекомендовано пациентам c высокой индивидуальной 10-летней вероятностью основных низкотравматических переломов, т.е. в случаях, когда результат оценки FRAX соответствует Российскому порогу вмешательства (рис. 3) и/или превышает «высокую вероятность переломов» (табл. 4) по усредненным Европейским данным [1, 2].


• FRAX не был валидизирован у пациентов, ранее получавших или получающих на момент обращения фармакотерапию по поводу ОП. Однако пациенты, прекратившие прием препаратов 2 и более года назад, могут считаться нелечеными [1, 2].
• FRAX может быть рассчитан с включением МПК/Т-критерия в шейке бедра, и не учитывает МПК поясничного отдела позвоночника. ВОЗ определила, что для многих вторичных причин ОП риск переломов был обусловлен прежде всего влиянием основного заболевания на МПК. По этой причине при включении МПК в шейке бедра в онлайн-расчет FRAX отмеченные значения «вторичные причины ОП» автоматически инактивируются [1, 2].
• Алгоритм оценки 10-летней вероятности переломов имеет ограничения, требующие клинического суждения врача. Так, при наличии у пациента множественных переломов риск последующих переломов будет занижен инструментом FRAX. Таким пациентам лечение ОП назначается независимо от показателя FRAX. FRAX не учитывает дозу ГКС, количество сигарет и алкоголя, не включает некоторых вторичных причин ОП, например, СД 2 типа, а также риск падений. Перерасчет индивидуальной 10-летней вероятности переломов в зависимости от дозы ГКС представлен в таблице 5.

9.
При высокой вероятности переломов по FRAX пациенту соответствующего возраста на основании совокупности факторов риска не показано проведение рентгеновской денситометрии; такой пациент однозначно нуждается в лечении остеопороза [1, 2].
10.
• Проведение двухэнергетической рентгеновской денситометрии (DXA) рекомендовано лицам с индивидуальной 10-летней вероятностью переломов (FRAX) в интервале между низкой и высокой вероятностью переломов (см. табл. 4, оранжевую зону рис. 3), т.е. когда назначение терапии ОП сомнительно; для оценки эффективности проводимой терапии, а также динамики состояния МПК у лиц без терапии с интервалом не менее 12 мес от первого исследования.
• Согласно всем проведенным исследованиям, рентгеновская денситометрия служит наиболее точным методом мониторинга терапии, доказавшим связь изменений на фоне терапии со снижением риска переломов при применении зарегистрированных препаратов для лечения ОП, поэтому этот метод используется для динамического контроля, но не чаще 1 раза в 12 мес [1, 2].
• Результат трабекулярного костного индекса (ТКИ), полученный в ходе стандартной рентгеновской денситометрии поясничного отдела позвоночника, рекомендуется использовать для одномоментного включения в алгоритм FRAX с целью повышения чувствительности метода [1, 2].
• По результатам метаанализа, ТКИ показал независимый вклад в предсказание риска переломов и на сегодняшний день этот показатель введен в алгоритм FRAX для повышения чувствительности метода. ТКИ – тканевой показатель, оценивающий пиксельные отклонения по шкале градаций серого на денситометрических изображениях поясничного отдела позвоночника, другими словами, непрямой показатель трабекулярной микроархитектоники. Отдельной точки вмешательства в отличие от DXA для этого показателя не существует. Однако введение параметра позволяет улучшить чувствительность FRAX и учитывает недостающие данные МПК поясничного отдела позвоночника. Для женщин в постменопаузе была также разработана градатация ТКИ по степени нарушения микроархитектоники: деградированная микроархитектоника ТКИ ≤1,2; частично деградированная микроархитектоника >1,2, но <1,35 и нормальная микроархитектоника ≥1,35. Однако данная классификация используется в исследовательских целях и пока не может служить основанием для лечения [1, 2].
• Практическим специалистам не рекомендуется устанавливать диагноз ОП на основании данных ультразвуковой денситометрии, измерения МПК не аксиального скелета (например, МПК пяточной кости, лучевой кости и т.д.), а также использовать нестандартные, неодобренные производителем денситометров способы укладки пациентов (например, денситометрия лежа на боку) и другие локализации исследования МПК, измеренные при рентгеновской денситометрии (например, треугольник Варда) [1, 2].
Лечение

1.
• Все средства терапии остеопороза (ОП) должны назначаться в сочетании с препаратами кальция (500–1000 мг/сут) и витамина D (минимум 800 МЕ/сут), поскольку доказанная эффективность по результатам рандомизированных клинических исследований (РКИ) была продемонстрирована именно в такой комбинации [1, 2].
• Обладая слабым антирезорбтивным действием, кальций потенцирует эффект основных препаратов для лечения ОП, предотвращает гипокальциемию и рекомендуется при любых терапевтических режимах и схемах. Витамин D активирует абсорбцию кальция в кишечнике и минерализацию скелета, но только при наличии адекватного поступления самого кальция. Назначение антирезорбтивной терапии может резко повысить потребность в кальции, необходимом для восстановления костной ткани, поэтому для профилактики/лечения патологии скелета наряду с предварительной коррекцией уровня витамина D очень важно обеспечить адекватное поступление кальция с пищей [1, 2].
• Эффективность приема кальция и витамина D сильно коррелирует с комплаенсом. Так, в метаанализе 17 исследований, продолжавшихся до трех лет, более 50 000 пациентов получали монотерапию препаратами кальция или в сочетании с витамином D. В результате изолированного приема кальция было отмечено снижение риска переломов разных локализаций, включая позвонки, бедренную кость и предплечье, на 12% (ОР – 0,88, 95%ДИ, 0,83–0,95). Добавление витамина D в среднем не приводило к существенному снижению риска переломов: на фоне приема кальция риск снижался на 10%, в комбинации с витамином D – на 13%. Однако в исследованиях с хорошим комплаенсом (>80%) снижение риска переломов при приеме кальция и витамина D достигало 24% [1, 2].
2.
• Бисфосфонаты (БФ) преимущественно подавляют костную резорбцию (антирезорбтивное действие) [1, 2].
• Алендроновая кислота снижает частоту переломов позвонков и бедренной кости в течение 3 лет лечения у пациентов с предшествующим переломом позвонков или у пациентов с ОП (Т-критерий ≤-2,5) в области бедренной кости. Также она повышает МПК при ОП у мужчин и глюкокортикоидном ОП у женщин и мужчин [1, 2].
• Алендроновая кислота выпускается в виде препаратов для перорального приема, в том числе в фиксированной комбинации с витамином D [12].
• Похожие результаты в исследованиях показала и ризедроновая кислота [1, 2], имеющая тот же спектр показаний, что и алендроновая кислота. Она также представлена в России препаратом в пероральной форме.
• Золедроновая кислота (формы для инфузионного применения) снижает частоту переломов тел позвонков (со значительным снижением риска уже за 1 год лечения), переломов бедренной кости и внепозвоночных переломов в течение 3 лет у пациентов с предшествующим переломом тела позвонка или снижением МПК в области бедренной кости, соответствующим ОП. Препарат продемонстрировал противопереломную эффективность для лечения ОП у мужчин, а также для повышения МПК при глюкокортикоидном ОП [1, 2].
• Золедроновую кислоту в дозе 5 мг 1 раз в год дополнительно рекомендуется назначать после хирургического лечения по поводу патологического перелома проксимального отдела бедренной кости (минимум через 2 нед после операции) для лечения ОП и улучшения выживаемости пациентов [1, 2].
• Ибадроновая кислота (выпускается в пероральной и внутривенной формах) снижает частоту переломов тел позвонков в течение 3 лет, но не влияет на снижение риска внепозвоночных переломов при первичном анализе данных исследования BONE [13]. Другие дозы ибандроновой кислоты (150 мг 1 раз в месяц и 3 мг 1 раз в 3 мес внутривенно) подтвердили свою сходную эффективность. Эффективность препарата для предупреждения внепозвоночных переломов была подтверждена результатами метаанализа. Ибадроновая кислота 150 мг 1 раз в месяц исследовалась при глюкокортикоидном ОП (n=140; Т-критерий >-2,0) и у мужчин (n=132; Т критерий ≤-2,0 в шейке бедренной кости) и была эффективна для повышения МПК и снижения маркеров костного ремоделирования. Однако официально эти показания не зарегистрированы и назначение ибадроновой кислоты при глюкокортикоидном ОП и ОП у мужчин не прописано в инструкции.
• Средняя продолжительность непрерывного лечения ОП таблетированными БФ составляет 5 лет, внутривенными – 3 года. Максимальная изученная продолжительность непрерывной терапии БФ – 10 лет. Режим применения этой группы лекарственных средств при ОП приведен в таблице.

• Нежелательные явления со стороны желудочного тракта (трудности при глотании, эзофагит и гастрит) встречаются при применении пероральных препаратов из группы БФ, внутривенные БФ (ибандроновая кислота, золедроновая кислота) не оказывают влияния на желудочно-кишечный тракт.
• Все БФ выводятся в неизмененном виде почками и противопоказаны пациентам с СКФ ниже 30–35 мл/мин. Для выявления пациентов группы риска необходимо контролировать уровень креатинина крови до начала лечения [1]. Для внутривенных БФ характерна гриппоподобная реакция (возможна и при приеме таблеток, но реже) в ответ на введение препарата, симптомы которой, как правило, исчезают спустя 3 дня после введения БФ. Прием НПВП (ибупрофена) и парацетамола облегчает симптомы гриппоподобной реакции.
• На фоне длительного применения БФ для лечения ОП были зарегистрированы случаи остеонекроза челюсти. Это осложнение более распространено при лечении онкологических заболеваний, при введении высоких доз БФ. Риск развития остеонекроза челюсти при лечении остеопороза увеличивается, если продолжительность терапии БФ составляет более 5 лет.
• К редким осложнениям при длительном лечении БФ (более 5 лет) относятся низкотравматические атипичные переломы бедренной кости. При возникновении таких переломов необходимо прекратить лечение БФ [1, 2].
3.
• В отличие от других антирезорбтивных препаратов деносумаб (человеческое антитело к RANKL) уменьшает образование остеокластов, а не нарушает функцию зрелых клеток. Кроме того, будучи биологическим препаратом, деносумаб не накапливается в костной ткани и не оказывает отсроченного влияния с полным обратным развитием эффекта после отмены лечения [1, 2].
• Согласно данным РКИ, деносумаб, по сравнению с плацебо, вызывал снижение риска переломов тел позвонков на 68%, бедренной кости – на 40%, внепозвоночных переломов – на 20%. В ходе того же РКИ была обнаружена дополнительная польза препарата для предупреждения падений: 175 (4,5%) в группе лечения против 219 (5,7%) в группе плацебо (p=0,02). Следствием этого, по видимости, было уменьшение случаев ушибов: 1 в группе лечения (<1%) против 11 (0,3%) в группе плацебо [14]. Повышение МПК, сходное с полученным у женщин, наблюдалось у мужчин со сниженной костной массой (+8% в L1–L4 и +3,4% в шейке бедренной кости за 2 года лечения) [15, 16].
• Исследование эффективности деносумаба, по сравнению с ризедроновой кислотой у лиц с глюкокортикоидным ОП, включало 795 мужчин и женщин в возрасте ≥18 лет, получавших ≥7,5 мг преднизолона. Все включенные пациенты моложе 50 лет уже имели патологический перелом в анамнезе, среди лиц старше 50 лет были пациенты со снижением МПК ≤-2,0 по Т-критерию или перелом независимо от МПК. Пациенты были рандомизированы для получения инъекций деносумаба (60 мг 1 раз в 6 мес) или ризедроновой кислоты (5 мг ежедневно). По результатам лечения в течение 24 месяцев деносумаб превосходил ризедроновую кислоту в отношении увеличения МПК поясничного отдела позвоночника за 24 мес поясничный отдел позвоночника (за 24 мес деносумаб +6,2% против ризедроновой кислоты + 1,7%; р <0,001) и МПК бедренной кости во всех измеренных зонах (за 24 мес Total Hip деносумаб + 3,1% против ризедроновой кислоты 0,0%; р <0,001) [17].
• В отличие от БФ распределение деносумаба в костной ткани не зависит от активности костного ремоделирования в момент введения препарата, что позволяет добиться сопоставимой концентрации деносумаба в трабекулярной и кортикальной кости и постоянного присутствия препарата в любой зоне кровоснабжения костной ткани. Кроме того, клиренс деносумаба как белковой молекулы не зависит от функции почек (дополнительно рекомендуется лицам с компрометированной функцией почек) [1, 2].
• Деносумаб был более эффективен для прироста МПК при прямом сравнении со всеми БФ [18–20] и эффективно повышал МПК будучи назначенным как после БФ [21], так и после терипаратида [22].
• Лицам с переломами тел позвонков, бедренной кости или множественными переломами в анамнезе рекомендуется продолжать непрерывное лечение деносумабом в дозе 60 мг 1 раз в 6 мес до 10 лет независимо от МПК с последующим переводом на БФ при необходимости отмены деносумаба [1, 2].
• Лицам без переломов в анамнезе рекомендуется лечение деносумабом до достижения МПК -2,0 SD по Т-критерию и выше в шейке бедренной кости, а также поясничных позвонках. Во всех случаях отмены деносумаба необходим перевод на терапию БФ [1, 2].
• Таблетированные БФ могут быть рекомендованы через 6 мес после последнего введения деносумаба, а применение золедроновой кислоты оправдано примерно через 8 мес после последней инъекции [1, 2]. По результатам трехлетнего исследования с участием 7868 человек деносумаб продемонстрировал хороший профиль безопасности [14]. Атипичные переломы бедра редко ассоциированы с применением этого препарата [1, 2].
• Терипаратид в России введен в список ЖВНЛП и ОНЛС и назначается по врачебной комиссии как дорогостоящее лечение на 24 мес. Он считается средством первой линии терапии у пациентов с двумя и более компрессионными переломами тел позвонков, а также при неэффективности предшествующей терапии [1, 2].
4.
• Терипаратид (генно-инженерный фрагмент молекулы паратгормона (1-34ПТГ)) относится к анаболической терапии ОП: он преимущественно действует на остеобласт, повышает продолжительность жизни костеобразующих клеток, уменьшает их апоптоз, увеличивает дифференцировку мезенхимальной стволовой клетки по направлению к остеобласту (через Wnt-сигнал), и, таким образом, усиливает костеобразования в каждом цикле костного ремоделирования, а также активирует моделирование в отдельных участках скелета [1, 2].
• Клиническая эффективность терипаратида в дозе 20 мкг 1 раз/сут доказана у женщин в постменопаузе с патологическими переломами тел позвонков в анамнезе независимо от исходного снижения МПК, предшествующих переломов и возраста, у мужчин со снижением МПК, в том числе вследствие гипогонадизма, и у пациентов с глюкокортикоидным ОП. Увеличение МПК и изменения костного обмена у мужчин (437 пациентов 30–85 лет средний Т-критерий в шейке бедренной кости -2,7 SD) соответствовали увеличению МПК и изменениям маркеров костного метаболизма у женщин, что позволяет предположить аналогичное влияние терипаратида на снижение риска переломов у мужчин [1, 2].
• Терипаратид был более эффективен для предупреждения переломов тел позвонков по сравнению с ризедроновой кислотой в РКИ у женщин в постменопаузе с двумя компрессионными переломами тел позвонков в анамнезе. Через 24 мес лечения новые переломы позвонков произошли у 28 (5,4%) из 680 пациентов в группе терипаратида и у 64 (12,0%) из 680 пациентов в группе ризедроновой кислоты (соотношение риска 0 44, 95% ДИ 0,29–0,68; р <0,0001). Клинические переломы произошли у 30 (4,8%) из 680 пациентов в группе терипаратида посравнению с 61 (9,8%) из 680 в группе ризедроновой кислоты (отношение рисков 0,48, 95% ДИ 0,32–0,74; р = 0,0009) [23]. Терипаратид также был более эффективен по сравнению с алендроновой кислотой для предупреждения переломов тел позвонков у лиц с глюкокортикоидным ОП. Ранее была показана лучшая эффективность терипаратида для прибавки МПК и для снижения болевого синдрома в спине по сравнению с другими БФ [1, 2].
• Максимально разрешенная продолжительность лечения терипаратидом составляет 24 мес (подкожные иньекции в дозе 20 мкг 1 раз/сут ежедневно). После окончания лечения терипаратидом рекомендуется перевод на антирезорбтивную терапию деносумабом для дальнейшего прироста МПК или БФ для сохранения терапевтического эффекта [1, 2].
• Наиболее частые нежелательные явления (<10% испытуемых) при использовании терипаратида – головокружение и судороги в ногах [1, 2].
5.
• Смена терапии производится в случае неэффективности предшествующего лечения: от таблетированных к парентеральным антирезорбтивным препаратам и анаболической терапии [1, 2, 24]. Вместе с тем терапия ОП может назначаться в любой последовательности (за исключением замены деносумаба на терипартид) на усмотрение врача и с учетом пожеланий пациента по режиму дозирования. У пациентов с впервые диагностированным тяжелым ОП наиболее предпочтительно начинать лечение с анаболической терапии и затем переходить на антирезорбтивную [1, 2, 23].
• Выбор таблетированных или парентеральных форм антирезорбтивной терапии как при первом назначении препарата, так и после анаболической терапии решается индивидуально [1, 2, 4, 5]. Нет оснований начинать лечение ОП именно с таблетированных форм, но преимущественное назначение парентеральных препаратов рекомендуется пациентам с патологией верхних отделов желудочно-кишечного тракта. Деносумаб может иметь дополнительное преимущество в качестве первой линии терапии при потере МПК в кортикальной кости и снижении функции почек. В случае исключения вторичных причин остеопороза, тяжелого дефицита витамина D, низкой приверженности пациента к лечению оправдан перевод пациента с таблетированных антирезорбтивных препаратов на парентеральные и/или с антирезорбтивной терапии на анаболическую терапию терипаратидом [1, 2].


