Вирус Эпштейна–Барр (ЭБВ), названный в честь своих первооткрывателей Майкла Энтони Эпштейна (Эпстайна) и его аспирантки Ивонны Барр, также известен как вирус герпеса человека 4 типа (HHV-4) и относится к представителям субсемейства Gammaherpesviridae. Этим ДНК-вирусом инфицировано более 95% взрослого населения на земном шаре.
ЭПИДЕМИОЛОГИЧЕСКИЕ ДАННЫЕ
ЭБВ передается через слюну, инкубационный период колеблется от 4 до 8 нед. Первичной его мишенью служат В-лимфоциты, эпителиальные клетки слизистой ротоглотки и протоков слюнных желез; важную роль в патогенезе инфекции играет также инфицирование макрофагов и дендритных клеток [1, 2]. Человек в данном случае является единственным резервуаром вируса.
Источником ЭВБ-инфекции выступает больной или пациент с бессимптомной формой инфекции. Заражение происходит воздушно-капельным, контактным, реже половым, алиментарным и трансфузионным (трансплантационным) путем. Первичная встреча с вирусом может повлечь за собой острую инфекцию, называемую инфекционным мононуклеозом (ИМ), «поцелуйной болезнью» или «железистой лихорадкой» (ранее используемые термины), а может обернуться бессимптомной формой, приводящей к сероконверсии. Существует некоторая закономерность относительно возрастных групп риска по заболеваемости ЭБВ-инфекцией: так, в развивающихся странах чаще болеют дети младшего, дошкольного возраста, а в развитых – младшие школьники и подростки [3]. Согласно данным серологических скрининговых исследований, частота определения антител к ЭБВ возрастает с возрастом обследуемых (к 35 годам – более 90–95%), имеет тенденцию к большей встречаемости среди женщин, представителей неевропеоидной расы и лиц, проживающих в неблагоприятных социоэкономических условиях [4, 5].
Более поздний возраст первичного инфицирования ЭВБ ассоциирован с более частой регистрацией клиники инфекционного мононуклеоза и более тяжелым течением острой инфекции [6]. Типичным для представителей семейства герпесвирусов является установление пожизненной латентной инфекции после первичного инфицирования, при этом генетический материал вируса способен реплицироваться наряду с геномом организма-хозяина.
В настоящее время ЭБВ рассматривают как потенциального участника патогенеза целого ряда аутоиммунных заболеваний и неоплазм, таких как разнообразные лимфомы, лимфопролиферативные заболевания и эпителиальные опухоли [7, 8] (табл.).
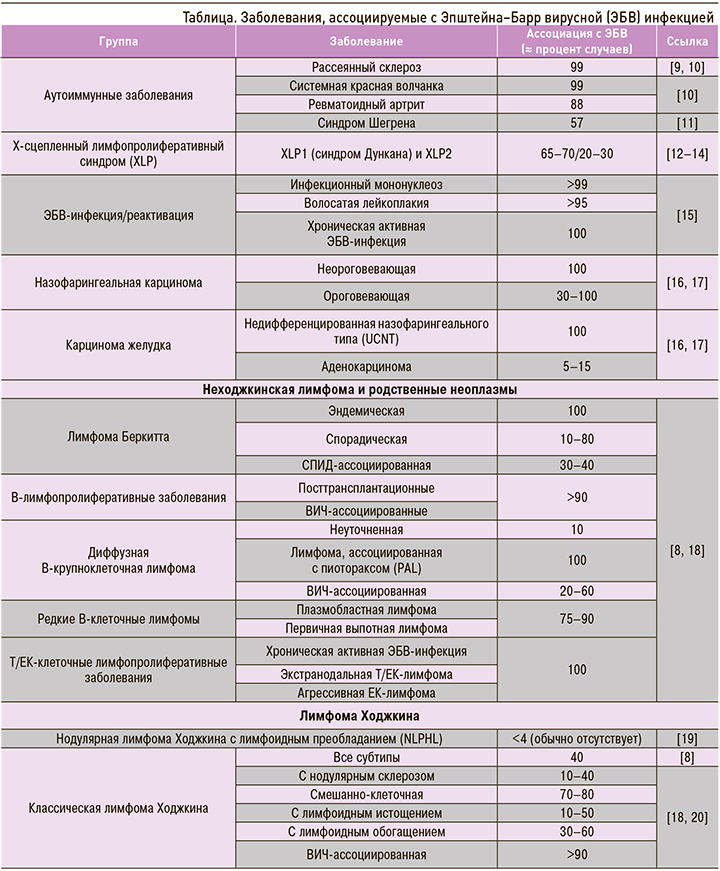
Интересно то, что ЭБВ-ассоциированные злокачественные опухоли распределены географически неравномерно [21]. Подобные вариации, по крайней мере частично, исследователи объясняют генетическими особенностями хозяев (человек), факторами окружающей среды (климат, распространенность малярии), локальными особенностями диеты [7].
Разработка технологий высокопроизводительного секвенирования позволила выделять и секвенировать геномы ЭБВ из различных клинических образцов, таких как биоптаты различных опухолей и пробы слюны [22]. Результаты подобных работ позволили выдвинуть гипотезу, что конкретные ЭБВ-ассоциированные заболевания вызываются различными штаммами вируса [7, 23]. Например, эндемичная лимфома Беркита практически в 100% ЭБВ-позитивна и, как считают, неизменно ассоциирована с иммуносупрессивным состоянием на фоне ареала распространения малярийной инфекции [21, 24], т.е., весьма вероятно, этиологически зависит от факторов окружающей среды. Еще одна ЭБВ-ассоциированная опухоль – назофарингеальная карцинома (ЭБВ-позитивна более чем в 97% случаев) – наиболее распространена на юге Китая и в Юго-Восточной Азии [21, 25, 26]. При этом выделены генетические (отдельные HLA-аллели) [27], эпигенетические факторы, диетические особенности (высокий уровень употребления соленой рыбы), определяющие распространение в данных регионах определенных штаммов ЭБВ [7].
Еще одним типом подобных ЭБВ-ассоциированных опухолей является Т/ЕК-клеточная лимфома. Само по себе редкое заболевание, оно относительно часто встречается в Восточной Азии, включая Японию и Корею [28], где в то же время распространенность назофарингеальной карциномы относительно низкая. Таким образом, эндемические ареалы этих опухолей не совпадают. Подобные географические расхождения распространения опухолей на территории Азии окончательно не ясны. Предполагается, что онкогенные варианты ЭБВ, которые обнаруживают в образцах опухолей, отличны от таковых, выделяемых из лимфоидных клеток, и способны приводить к метилированию определенных участков хромосом хозяина. Это, в свою очередь, может запускать канцерогенез через подавление генов-супрессоров опухолевого процесса. Подобный механизм изучался на примере ЭБВ-ассоциированного рака желудка [7].
ВИРУС ЭПШТЕЙНА–БАРР И ЕГО ЖИЗНЕННЫЙ ЦИКЛ
Геном ЭБВ представлен линейной двухцепочечной ДНК (dsDNA) размером порядка 175 килобаз (175 kb – 175 тыс. пар нуклеотидов) (рис. 1). Эволюционируя параллельно с человеком, ЭБВ выработал множество стратегий выживания в клетках. Вирус способен копировать ключевые клеточные механизмы, такие как рецепторные сигнальные пути, репликация ДНК и транскрипция генов, для собственных нужд [29].
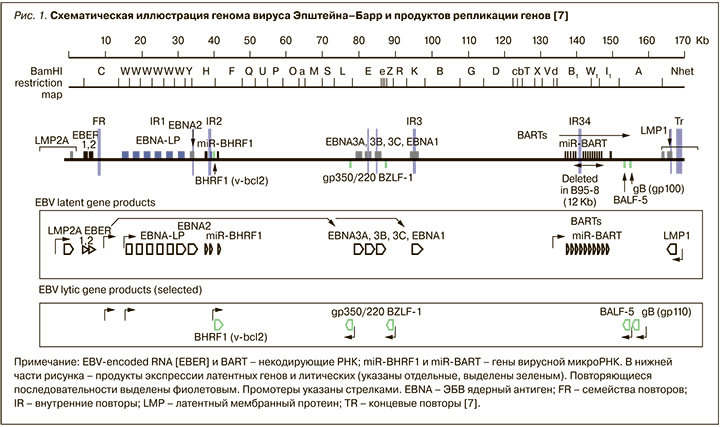
Гены вируса, которые экспрессируются на протяжении продуктивных циклов репликации, называются ЭБВ-литическими генами (EBV lytic genes). Гены этого типа кодируют вирусные факторы транскрипции (BZLF1), вирусную ДНК-полимеразу (BALF5), вирусные гликопротеины (gp350/220 и gp110) и структурные белки (капсидные, белки тегумента). В латентной фазе инфекции экспрессируются ЭБВ-латентные гены. Среди продуктов экспрессии этих генов выделяют 4 протеина EBNA (EBNA1, EBNA2, EBNA3A и EBNA3C) и LMP1 (латентный мембранный протеин), которые считаются ключевыми факторами В-клеточной трансформации [7].
In vitro ЭБВ инфицирует преимущественно непролиферирующие человеческие В-лимфоциты, находящиеся в стадии покоя. После инфицирования происходит их активация, неконтролируемая пролиферация, и далее наступает стадия латентной инфекции В-клеток. Внутри вирусных частиц ЭБВ ДНК не связана с гистонами, и метилирование CpG-участков отсутствует. Во время инфекционного процесса вирусная ДНК попадает в клетки-мишени и персистирует внутриклеточно в виде внутриядерных экстрахромосомных копий плазмид. Именно здесь ДНК вируса соединяется с гистонами, образует нуклеосомы и подвергается метилированию по CpG. Как и когда происходит связывание вирусного материала с клеточным хроматином и какие клеточные факторы управляют процессом, в настоящее время изучено недостаточно [29].
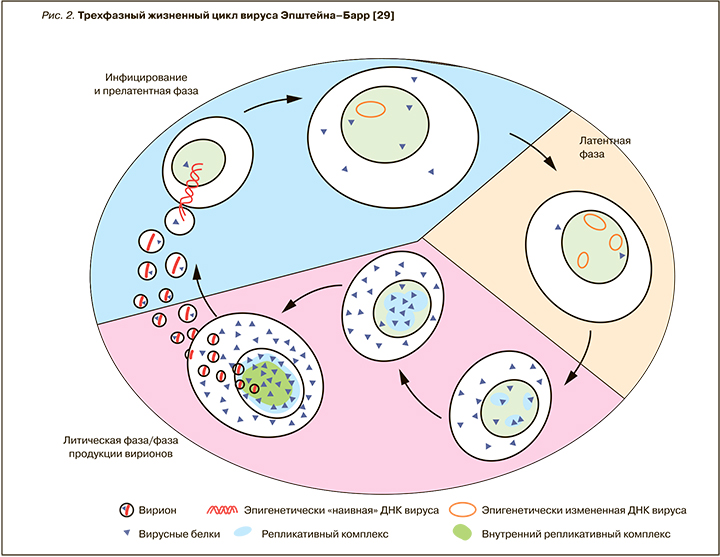
В так называемую прелатентную фазу вирусной инфекции [30], продолжающуюся первые 8 дней (рис. 2), вирус репрограммирует покоящиеся B-клетки в активированные и пролиферирующие бласты [31], при этом отмечается высокий уровень транскрипции и экспрессии вирусных генов. В противоположность данной фазе в латентно инфицированных клетках активно очень ограниченное число генов. Исследователи считают, что образование объединенного комплекса вирусной ДНК и клеточного хроматина, которое происходит в прелатентную фазу, выступает важным моментом контроля дальнейшей программы вирусной транскрипции, гарантирующим выживание ЭБВ и постоянную пролиферацию инфицированных клеток на длительный период времени [29]. В результате этого процесса появляется так называемая лимфобластоидная линия/клон клеток (lymphoblastoid cell lines, LCLs), в которой вирус вызывает состояние латентной инфекции. Как известно, латентная инфекция является парадигмой всех герпесвирусов, но для ЭБВ – это предпочтительный «стиль» существования. У здоровых инфицированных лиц вирус формирует латентный резервуар инфекции, в основном представленный В-лимфоцитами, а именно малым компартментом долгоживущих В-клеток памяти (одна клетка на 104–106 В-лимфоцитов). Состояние латентной инфекции B-клеток изучалось ex vivo на примерах опухолевых клеток из биоптатов тканей, устойчивых (стабильных) клеточных линий опухолей и лимфобластоидных линиях клеток, причем именно из последних двух источников были получены многие данные насчет молекулярных механизмов латентности ЭБВ-инфекции. Отдельные клеточные линии, такие как линия клеток лимфомы Беркитта, Akata, Raji и P3HR1, изучались на предмет механизмов «выскользания» ЭБВ из латентного состояния [32–34]. Это привело к идентификации функции вирусного гена-переключателя BZLF1, способного переключать латентно инфицированные клетки в состояние «фабрик вируса», которые высвобождают многочисленные новые вирусные частицы, т.е. реализуют переход в литическую фазу инфекции (см. рис. 2). При индукции третьей, или литической, фазы экспрессируется полный набор из примерно 80 вирусных генов, ДНК ЭБВ автономно реплицируется, и синтезируется пул вирусных частиц-потомков. Эти новые инфекционные вирусные частицы высвобождаются из клеток, происходит горизонтальное распространение вируса с поражением других чувствительных клеток и инфицирование других индивидуумов. Таким образом, вирус использует эпигенетические механизмы управления экспрессией разных паттернов генов, связанных с тремя разными фазами его жизненного цикла [29], но конкретные этапы этого процесса переключения фаз еще в полной мере не исследованы.
ИММУННЫЙ ОТВЕТ
В иммунный ответ при первичной ЭБВ- инфекции вовлечены реакции как врожденного, так и приобретенного иммунитета. В течение примерно 6 нед инкубационного периода активная вирусная репликация происходит в полости рта. Вирус инфицирует В-лимфоциты и клетки эпителия, затем проникает в кровоток. Как и когда конкретно этот процесс осуществляется, в настоящее время не совсем ясно, но установлено, что ДНК ЭБВ может обнаруживаться в крови за 2 нед до появления симптоматики [6]. Также за 2 нед до манифестации инфекции инициируется экспрессия генов интерферонов I типа (первая линия защиты от вирусных инфекций), затем в дело вступают и другие цитокины. ДНК и вирусные белки идентифицируются паттерн-распознающими рецепторами (Толл-подобными рецепторами, TLRs), что запускает экспрессию генов интерферонов, активирование естественных киллеров (ЕК) и далее реакции адаптивного иммунного ответа [35].
В тканях миндалин у пациентов с инфекционным мононуклеозом повышаются уровни провоспалительных цитокинов, таких как фактор некроза опухоли-α (ФНО-α), интерлейкин-6 (ИЛ-6), ИЛ-1, также есть работы, указывающие на возрастание уровней цитокинов в сыворотке больных в острую фазу. Особую роль отводят интерферону-γ (ИНФ- γ), который в основном продуцируется активированными Т-клетками и ЕК. Повышается уровень и неоптерина, синтезируемого моноцитами под влиянием ИНФ-γ. Высокие уровни ИНФ-γ считают ответственными за такие симптомы инфекционного мононуклеоза, как лихорадка, головная боль, слабость [35]. Увеличение уровня ИЛ-2 совпадает с увеличением пула CD8+ лимфоцитов.
Также в сыворотке больных инфекционным мононуклеозом определяются иммуносупрессивный цитокин ИЛ-10 и трансформирующий фактор роста бета (TGF-β). Интересно, что ген-регулятор латентной фазы вирусного цикла BCRF1 функционирует как ИЛ-10, и по аминокислотной последовательности продукт экспрессии этого гена на 84% идентичен человеческому ИЛ-10. На протяжении острой первичной фазы инфекции в сыворотке пациента могут обнаруживаться как вирусный, так и человеческий ИЛ-10 [35]. Этот цитокин способен подавлять пролиферацию Т-клеток и ингибировать продукцию ИНФ-γ, т.е. выступать контрагентом; это согласуется с наблюдением, что при более высоких уровнях ИЛ-10 длительность симптоматики укорачивается [35, 36].
ЕК-клетки также выступают важным компонентом иммунного ответа и считаются одним из ключевых регуляторов при хронических вирусных инфекциях [37]. Показано, что их дефицит связан с повышенной предрасположенностью организма к ряду вирусных (и бактериальных) инфекций, включая ЭБВ-инфекцию [38]. Количество ЕК увеличивается при инфекционном мононуклеозе [35, 39], причем, что интересно, их уровень находится в обратной корреляции с тяжестью болезни; это предполагает участие данной клеточной популяции в контроле вирусной репликации [35]. Также in vitro продемонстрирована способность ЕК уничтожать инфицированные ЭБВ-клетки при переходе инфекции в литическую фазу и подавляюще воздействовать на В-лимфоциты. Инкубация особой популяции ЕК – CD56bright CD16- с B-клетками в присутствии вируса in vitro приводила к меньшей трансформации В-лимфоцитов. То есть, вероятно, ЕК могут подавлять другую клеточную популяцию как прямым цитолизом, так и опосредованно, через ИФН-γ [6].
В контроле вирусной репликации важная роль отведена популяции CD8+ лимфоцитов, которые способны напрямую взаимодействовать и уничтожать инфицированные ЭБВ В-лимфоциты. При инфекционном мононуклеозе среди всех лимфоцитов данный клеточный пул необычайно велик. CD8+ лимфоциты реагируют на антигены как ранней, так и латентной фазы вирусного цикла, в частности EBNA-2 и EBNA-3. Считается, что лимфоцитоз периферической крови при инфекционном мононуклеозе определяется именно популяцией CD8+ Т-клеток, специфичной к ЭБВ-антигенам литической фазы, но вместе с тем не исключается и активация неспецифичных Т-клеток [35].
Выраженность реакций адаптивного иммунного ответа обусловливает симптоматику инфекционного мононуклеоза. На примере исследования с небольшим количеством пациентов было показано, что тяжесть течения инфекции коррелировала с уровнем лимфоцитоза, а не с уровнем вирусной нагрузки [40]. Несмотря на то что, по данным литературы, увеличение популяции CD4+ лимфоцитов при инфекционном мононуклеозе не обнаружено, эта клеточная популяция также способна участвовать в контроле вирусной репликации. CD4+ Т-клетки могут распознавать ряд антигенов литической фазы жизненного цикла вируса посредством взаимодействий с тетрамерами MHC II, и такие клетки выявляются в крови не только при первичной ЭБВ-инфекции, но и (в меньшем количестве) при ее переходе в латентное состояние [6, 41].
ПРИНЦИПЫ ДИАГНОСТИКИ ЭПШТЕЙНА–БАРР ВИРУСНОЙ ИНФЕКЦИИ
Клеточный иммунный ответ, как составляющая адаптивного иммунитета, важен для контроля вирусной репликации и, как считается, может влиять на тяжесть течения инфекционного мононуклеоза. Гуморальный ответ (появление антител к вирусным белкам) положен в основу диагностики ЭБВ-инфекции. В дебюте заболевания ДНК вируса не всегда обнаруживается в крови, но обычно присутствует в большом количестве в слюне и клетках слизистой оболочки полости рта. Из крови ДНК ЭБВ обычно исчезает быстрее, чем со слизистой ротоглотки. Локальное выделение вируса может сохраняться месяцами и в последующем периодически вновь появляться. У большинства пациентов первыми обнаруживаются IgM антитела к капсидному антигену (VCA) с постепенным их снижением в течение 2–6 мес от начала заболевания. VCA IgG определяются самое раннее через 2 нед от дебюта инфекции и сохраняются пожизненно. EBNA-1 IgG (антитела к ядерному антигену) синтезируются через 3–6 мес после первичной инфекции и также персистируют всю жизнь [35]. Исследование антител к раннему антигену ЭБВ – EA IgG – обычно не рекомендуется для диагностики первичной инфекции (инфекционного мононуклеоза), так как они хоть и обнаруживаются в острую фазу у 60–80% пациентов, но вместе с тем могут определяться и у 20% клинически здоровых лиц [42]. Среди более редких методов диагностики можно упомянуть метод иммунного блоттинга и определение авидности специфических антител [6, 43].
В отдельных случаях для диагностики ЭБВ-инфекции могут использоваться различные варианты полимеразной цепной реакции (ПЦР), а материалом служат кровь, сыворотка, плазма, слюна, мазки и смывы, образцы тканей и др. «Золотым стандартом» для обнаружения ЭБВ в образцах тканей, особенно в латентной фазе вирусного цикла, считается гибридизация in situ, направленная на выявление кодируемых вирусом РНК, – EBER-ISH (EBV-encoded RNA in situ hybridization) [8].
В качестве метода диагностики в будущем ряд авторов рассматривает возможность обнаружения вирусных экзосом при помощи ПЦР. Экзосомы – это внеклеточные микровезикулы, окруженные двойной липидной мембраной, в которые заключены макромолекулы, например липиды, углеводы, белки, microРНК, мРНК, ДНК диаметром от 30 до 150 нм [44]. Экзосомы секретируются многими эукариотическими клетками, выступая посредниками межклеточной коммуникации и участниками большого количества процессов – от ангиогенеза до метастазирования опухолей. Показано, что эти частицы вовлечены в патогенез ряда вирусных инфекций, таких как герпесвирусные инфекции, ВИЧ-инфекция, гепатит С, а также онкологических заболеваний (лимфома, меланома, глиома, колоректальный рак, рак молочной железы, рак яичников и др.) [45]. ЭБВ-экзосомы содержат различные вирусные компоненты, такие как LMP1, LMP2A, BARF1, ДНК, мРНК, miРНК, и могут служить для диагностики различных заболеваний и состояний, ассоциированных с ЭБВ-инфекцией [8, 44, 45].
ОСОБЕННОСТИ ПЕРВИЧНОЙ ЭПШТЕЙНА–БАРР ВИРУСНОЙ ИНФЕКЦИИ (ИНФЕКЦИОННОГО МОНОНУКЛЕОЗА)
Первичная инфекция ЭБВ возможна в трех вариантах: а) бессимптомная; б) с неспецифической клинической картиной ОРВИ/ОРЗ (чаще у детей от 1 года до 3 лет); в) инфекционный мононуклеоз (дети от 3 до 14 лет, подростки и взрослые до 30 лет).
К типичным проявлениям инфекционного мононуклеоза относятся лихорадка, лимфаденопатия, тонзиллит и/или фарингит (симптоматическая триада), также регистрируются интоксикационный синдром, гепатоспленомегалия, возможны разнообразные кожные высыпания и развитие синдрома желтухи [46]. В большинстве случаев достаточно сложно выделить конкретную строгую последовательность появления симптомов, и большинство клиницистов рекомендуют опираться на наличие указанной триады наиболее частых симптомов: лихорадку, фарингит/тонзиллит, лимфаденопатию (чаще она цервикальная, но встречается аксиллярная и паховая, реже генерализованная). В случае присоединения бактериальной инфекции наблюдается появление налетов в лакунах миндалин, что сопровождается умеренным болевым синдромом. Обычно длительность клинической симптоматики составляет порядка 2–3 нед, но слабость и цервикальная лимфаденопатия персистируют дольше, в течение месяца и более.
Среди других, более редких симптомов инфекционного мононуклеоза, которые наблюдаются не у всех пациентов, можно отметить абдоминальную боль/дискомфорт, связанную(-ый) с внутрибрюшной лимфаденопатией или гепатомегалией, гепато- и спленомегалию, тошноту, рвоту, петехиальные кровоизлияния на слизистой мягкого нёба, отечность язычка, периорбитальный отек или общую пастозность лица, придающие довольно характерный вид пациенту, особенно в сочетании с некоторой гнусавостью голоса из-за увеличения миндалин и затруднения дыхания. Гепатит при острой ЭБВ-инфекции встречается примерно у 75% пациентов, но обычно протекает субклинически и выявляется по повышению уровня печеночных трансаминаз. В редких случаях возможно течение инфекции как острого гепатита с синдромом желтухи.
Экзантема при инфекционном мононуклеозе отмечается в 2 основных вариантах: неяркая пятнисто-папулезная сыпь, появляющаяся в первые дни болезни и сохраняющаяся примерно 24–48 ч (5–15% пациентов), или грубая пятнисто-папулезная (кореподобная) сыпь, иногда с геморрагическим компонентом, иногда с зудом кожи, чаще возникающая у пациентов, принимавших ампициллин (так называемая ампициллиновая сыпь). Интересно, что появление ампициллиновой сыпи может быть отсрочено от момента приема антибиотика (вплоть до 7–10 сут) [47]. После клинического выздоровления пациенты смогут принимать ампициллинсодержащие препараты без подобной симптоматики, т.е. данное явление не является истинной аллергической реакцией. Механизм временной ампициллиновой гиперчувствительности при инфекционном мононуклеозе до конца не изучен. У пациентов детского возраста могут регистрироваться более редкие варианты экзантем, например односторонняя латероторакальная экзантема (unilateral laterothoracic exanthema), синдром Джанотти–Крости (Gianotti–Crosti syndrome, детский папулезный акродерматит) [47, 48].
Осложнения инфекционного мононуклеоза достаточно редки. Среди них обструкция верхних дыхательных путей, паратонзиллярный абсцесс при присоединении вторичной бактериальной инфекции, гемолитическая анемия и тромбоцитопения, разрыв селезенки (менее чем у 1% пациентов). Реже наблюдаются миокардит, коагулопатия как следствие нарушения синтетической функции печени, интерстициальный нефрит, менингоэнцефалит, параличи черепно-мозговых нервов, мононейропатии, ретробульбарный неврит [6, 49].
Для общего анализа крови при инфекционном мононуклеозе характерен лимфомоноцитоз, абсолютное количество лимфоцитов обычно более 4,0×109/л. Также характерно появление атипичных мононуклеаров (вироцитов) в периферической крови. Обнаружение таких клеток может регистрироваться при ряде вирусных инфекций, но при инфекционном мононуклеозе их содержание обычно более 10% от общего пула лейкоцитов [49]. Интересная особенность инфекционного мононуклеоза – появление в сыворотке гетерофильных антител, что послужило основой для разработки ряда тестов на их выявление. Эти серологические тесты достаточно просты в исполнении, но не являются строго специфичными. При их проведении возможны ложноположительные результаты при таких инфекциях, как вирусные гепатиты, краснуха, малярия, ВИЧ-инфекция, некоторые злокачественные и аутоиммунные заболевания [8, 50]. Важно отметить, что пациентов с инфекционным мононуклеозом обязательно обследуют на ВИЧ-инфекцию и рекомендуют повторное обследование в периоде реконвалесценции, так как острая ВИЧ-инфекция может манифестировать мононуклеозоподобным синдромом, а в части случаев антитела к ВИЧ обнаруживаются отсрочено.
На настоящий момент этиотропная терапия инфекционного мононуклеоза не разработана. Лечение сводится к симптоматической терапии (анальгетики, антипиретики, при необходимости регидратация и др.), соблюдению щадящего режима. По аналогии с другими герпесвирусами предполагалась эффективность при инфекционном мононуклеозе таких противовирусных средств, как ацикловир, валацикловир, валомацикловир, ганцикловир. Также имелись отдельные наблюдения о частичной подавляющей активности ацикловира в отношении ЭБВ in vitro. Однако Кохрейновский обзор (Cochrane review) 2016 г. содержал выводы о том, что эффективность этих препаратов при инфекционном мононуклеозе, по данным проведенных исследований, оказалась сомнительной. Уровень их доказательности был низким [51, 52], а валацикловир сокращал сроки выделения вируса, но не влиял при этом на выраженность и длительность симптоматики [49].
При присоединении бактериальной инфекции, появлении налетов на миндалинах пациентам может понадобиться антибактериальная терапия. При этом важно помнить, что препараты ампициллинового ряда при этом противопоказаны. Такие противомикробные средства, как метронидазол, за счет воздействия на анаэробную микрофлору полости рта могут способствовать более быстрому разрешению локального воспалительного процесса. Так, положительный эффект метронидазола на укорочение сроков пребывания в стационаре был продемонстрирован рандомизированным клиническим исследованием [53, 54].
Глюкокортикостероиды (ГКС) способны уменьшать выраженность воспалительного процесса в ротоглотке при инфекционном мононуклеозе, но только в первые 12 ч болезни, в дальнейшем их эффект не столь явно выражен. ГКС не влияют на общую тяжесть болезни и продолжительность симптоматики [49, 55].
Эффективной вакцины против инфекционного мононуклеоза, несмотря на попытки разработки, на сегодняшний день не существует [51].
Такие осложнения инфекционного мононуклеоза, как острая обструкция верхних дыхательных путей (чаще наблюдается у детей) и разрыв селезенки, могут потребовать инвазивного и хирургического лечения.
Жизненный цикл ЭБВ после фазы инфицирования (прелатентной) предполагает развитие латентной фазы с пожизненным персистированием в организме человека, что на клиническом уровне соответствует переходу фазы реконвалесценции (4–6 мес) в фазу паст-инфекции. Под влиянием триггеров, которые на сегодняшний день достоверно не известны, возможно возобновление репликации вируса, переход цикла в литическую фазу, что в части случаев клинически проявляется реактивацией ЭБВ-инфекции. При реактивации ЭБВ-инфекции регистрируется симптоматика как частично, так и полностью воспроизводящая клиническую картину инфекционного мононуклеоза, выявляется широкий диапазон серологических маркеров, также возможно обнаружение ДНК вируса методом ПЦР в слюне, крови (редко).
ХРОНИЧЕСКАЯ АКТИВНАЯ ЭПШТЕЙНА–БАРР ВИРУСНАЯ ИНФЕКЦИЯ
Хроническая активная ЭБВ-инфекция (CAEBV) относится к T- или ЕК-лимфопролиферативным заболеваниям. В 2016 г. она была занесена в классификацию ВОЗ, в раздел опухолей гематопоэтических и лимфоидных тканей. В последнее время к данной нозологии приковано все большее внимание исследователей. CAEBV отличает высокая частота летальных исходов, но некоторые пациенты демонстрируют длительную выживаемость после аллогенной трансплантации стволовых гематопоэтических клеток [56]. По мере прогрессирования заболевания происходит инфильтрация ЭБВ-инфицированными клетками различных органов и тканей, что приводит к нарушению функции последних. Фактически любой орган может стать мишенью ЭБВ.
В 2005 г. Okano M. et al. [57] предложили диагностические критерии для CAEBV: персистирующие (более 3 мес [51]) и рецидивирующие мононуклеозоподобные симптомы; нетипичный паттерн а-ЭБВ антител с повышением уровней anti-VCA и anti-EA антител; обнаружение большого количества фрагментов генома ЭБВ в пораженных тканях, включая периферическую кровь; наличие хронического заболевания, которое не может быть отнесено к другому известному состоянию/болезни. Отмечается, что чаще это заболевание встречается в Японии и Восточной Азии, что, вероятно, может быть связано с генетической предрасположенностью к нему местных жителей.
CAEBV имеет 2 основных составляющих: системное воспаление и неопластическое заболевание (лимфоидное). Однако солидные опухоли встречаются редко, и фактически основной клинической находкой является воспаление [56]. Интересно, что два заболевания с характерными кожными изменениями – тяжелая аллергия на укус комара (sMBA) и световая оспа (hydroa vacciniforme – HV, редкий фотодерматоз детского возраста) – тоже связывают с CAEBV. В биоптатах кожных изменений обнаруживают Т-лимфоциты и ЕК, инфицированные ЭБВ.
По мере прогрессирования хроническая активная ЭБВ-инфекция приводит к двум основным состояниям с высокой летальностью – гемофагоцитарному лимфогистиоцитозу или лимфоме из Т- или ЕК-клеток, резистентной к химиотерапии. Длительность болезни до развития данных состояний варьирует от нескольких месяцев до нескольких десятилетий.
Несмотря на наличие определенного количества наблюдений, патогенез CAEBV остается неясным. По невыясненным причинам в течение первичного инфицирования или позднее, в латентную фазу ЭБВ происходит инфицирование Т-лимфоцитов и ЕК с последующим прогрессированием. В части случаев достаточно сложно дифференцировать инфекционный мононуклеоз от CAEBV, так как с лабораторной точки зрения эти состояния имеют много общего. Отмечается, что у части пациентов с CAEBV отсутствуют антитела к EBNA.
При хронической активной инфекции происходит не только неконтролируемая пролиферация и инфильтрация тканей инфицированными клетками, но и гиперпродукция ряда провоспалительных цитокинов.
Лечение CAEBV окончательно не разработано, режимов эффективной химиотерапии не предложено. В ряде случаев помогает аллогенная трансплантация стволовых гематопоэтических клеток. С учетом гиперпродукции цитокинов перспективным представляется применение ингибиторов JAK/STAT молекулярного пути, например, руксолитиниба (ruxolitinib). Также изучаются индукторы апоптоза ЭБВ-инфицированных клеточных линий – бортезомиб (bortezomib) [56]. Очевидно, что молекулярные механизмы патогенеза CAEBV нуждаются в изучении, равно как и необходима разработка эффективных лечебных стратегий.
ЗАКЛЮЧЕНИЕ
Как известно, вирусы сосуществуют и коэволюционируют с человечеством очень давно. На сегодняшний день первичная ЭБВ-инфекция (инфекционный мононуклеоз) достаточно хорошо изучена и описана. Врачи самых разных специальностей могут столкнуться с этим заболеванием, и авторы этой статьи надеются, что изложенные в ней данные помогут лучше ориентироваться в проблеме. Вместе с тем нельзя не отметить, что чем более специализированными и изощренными становятся методы изучения патогенеза и особенностей жизненного цикла вирусов, в том числе и ЭБВ, тем больший горизонт неизведанного простирается перед исследователями, обрисовываются проблемы, о которых невозможно было и догадываться. И это удивительно, волнительно и интересно.



