Эндотелиальная дисфункция служит важным пусковым механизмом формирования и прогрессирования как сердечно-сосудистой, так и бронхолегочной патологии [1–4]. Функция эндотелия хорошо изучена у больных, имеющих одно заболевание [1–4]. Однако в последние годы все большее количество больных имеет не одно, а сразу несколько заболеваний, связанных единым патогенетическим механизмом. В большей степени это относится к хронической обструктивной болезни легких (ХОБЛ), число больных которой постоянно и значительно увеличивается [5–11].
По прогнозам экспертов, ХОБЛ к 2020 г. станет не только одним из самых распространенных заболеваний, но и войдет в число лидирующих причин смертельных исходов, а к 2030 г. займет 3-е место среди причин смерти после инсульта и инфаркта миокарда [11–15].
С современных позиций ХОБЛ определяется как заболевание, характеризующееся частично обратимым под воздействием лечения ограничением воздушного потока, которое носит, как правило, неуклонно прогрессирующий характер и связано с воспалительной реакцией легочной ткани на раздражение различными патогенными агентами и газами [9]. Кроме того, в последние годы все шире обсуждаются экстрапульмональные проявления ХОБЛ. Показано, что развитие внелегочных эффектов ХОБЛ имеет большое клиническое и прогностическое значение [13, 15]. В качестве потенциальных системных проявлений ХОБЛ рассматриваются прежде всего кардиоваскулярные эффекты, к которым относятся повреждение эндотелия с развитием эндотелиальной дисфункции, атеросклероз с формированием ишемической болезни сердца (ИБС), артериальная гипертензия [15–17].
Как при ХОБЛ, так и ИБС начальным звеном, запускающим каскад патологических реакций, выступает перекисное окисление липидов [18]. Его изучение позволит выявить самые ранние изменения при развитии коморбидности.
Целью настоящего исследования стала оценка окислительного стресса и эндотелиальной дисфункции у больных ХОБЛ, имеющих сопутствующую сердечно-сосудистую патологию.
МАТЕРИАЛ И МЕТОДЫ
Диагноз ХОБЛ устанавливался по данным анамнеза, клинической картины, функциональных методов диагностики в соответствии с программой GOLD, 2018. Основным показателем, позволяющим достоверно судить о наличии ХОБЛ, служило постбронходилатационное значение ОФВ1/ФЖЕЛ <0,70, подтверждающее наличие ограничения воздушного потока [10]. Степень дыхательной недостаточности определялась по выраженности одышки, для количественной оценки которой использовали шкалу Британского медицинского исследовательского совета (mMRC, 1999). Использовалась классификация ИБС по ВОЗ (1979) с поправками ВКНЦ АМН СССР (1984).
Критериями включения в исследование были наличие у пациента ХОБЛ легкой и средней степени тяжести вне обострения, ИБС, а также сочетание этих двух заболеваний у одного пациента. Из исследования исключались больные тяжелой ХОБЛ, так как вероятность наличия у них коморбидных сердечно-сосудистых заболеваний очень высока [4–15], а следовательно, такие больные не вошли бы в группу «чистой» ХОБЛ. Также критериями исключения служили нестабильная стенокардия, инфаркт миокарда, перенесенный в течение последних 6 мес, артериальная гипертония выше 3 степени, сахарный диабет, сердечная недостаточность выше III функционального класса, дыхательная недостаточность более II степени, ХОБЛ в стадии обострения, острые воспалительные и онкологические заболевания.
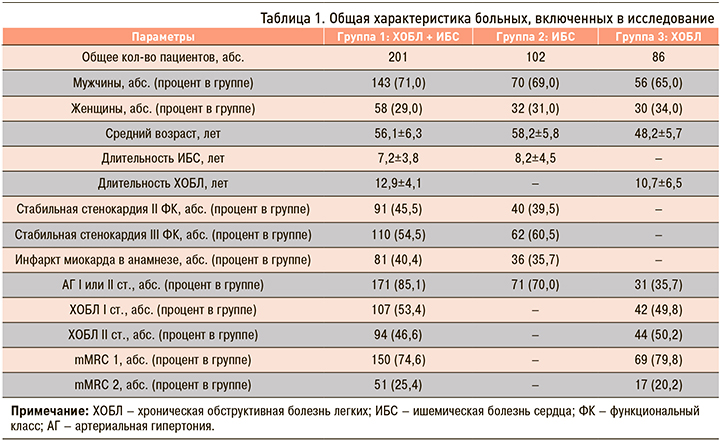
Обследовано 389 пациентов, находившихся на лечении в кардиологическом отделении Городской клинической больницы № 5 Нижнего Новгорода, которые были разделены на 3 группы. Группа 1 была представлена коморбидными больными, имеющими ХОБЛ в сочетании с ИБС. В группу 2 вошли пациенты с ИБС без ХОБЛ, в группу 3 – больные ХОБЛ, у которых не было указания на наличие каких-либо сердечно-сосудистых заболеваний, в том числе ИБС. Общая характеристика пациентов, участвовавших в исследовании, представлена в таблице 1.
Всем пациентам было проведено полное клинико-инструментальное обследование, для верификации ИБС – селективная коронарография. Внутрисосудистое вмешательство выполняли на рентгенохирургической установке Аdvantx LСV+ (General Electrics, Франция). С целью оценки степени тяжести ХОБЛ и ее верификации было осуществлено исследование функции внешнего дыхания (ФВД) на аппарате Spirosift 3000 (Япония).
Состояние свободно-радикального окисления оценивалось по данным индуцированной хемилюминесценции с помощью биохемилюминометра БХЛ-06: определялись максимальная интенсивность (Imax), отражающая уровень свободно-радикальной активности плазмы, и светосумма свечения (S), так как общую антиоксидантную активность плазмы характеризует величина, обратно пропорциональная S. Содержание первичных (диеновые и триеновые конъюгаты) и конечных (основания Шиффа) продуктов перекисного окисления липидов (ПОЛ) устанавливалось методом И.А. Волчегорского (1989).
Эндотелийзависимую вазодилатацию (ЭЗВД) оценивали посредством пробы с реактивной гиперемией, возникающей в плечевой артерии после ее кратковременного пережатия, по методике D.S. Celermajer et al. (1992). О концентрации оксида азота, также описывавшей функцию эндотелия, судили косвенно по количеству его метаболитов – нитрат-(NO3) и нитрит-(NO2) ионов, которые определяли в сыворотке крови колориметрическим методом по реакции Грисса.
Пациенты принимали дезагреганты, статины, бета-блокаторы, ингибиторы АПФ, бронхолитики, нитраты. С целью исключения влияния нитратов на результаты диагностических тестов за сутки до их проведения препараты этой группы были отменены, а также исключены другие возможные внешние источники этих химических соединений.
Статистическая обработка результатов исследования выполнялась при помощи лицензионной программы STATISTICA 10.0. Характер распределения анализируемых признаков оценивался критерием Шапиро–Уилка. Если распределение было нормальным, то результаты представлялись в виде М±sd, где M – среднее значение, а sd – среднее квадратичное отклонение. В этом случае для сравнения групп по количественному признаку использовался параметрический метод с вычислением t-критерия Стьюдента для независимых групп. При распределении, отличном от нормального, данные приводились в виде медианы и 25-го и 75-го перцентилей (Ме [25р;75р]). В этом случае о достоверности межгрупповых различий судили по U-критерию Манна–Уитни.
Достоверность отличий трех групп определяли по критерию Краскела–Уоллиса. Сравнение групп по качественному бинарному признаку производили с помощью вычисления относительных частот и доверительных интервалов для них.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Максимальные изменения всех показателей ПОЛ были выявлены у больных коморбидной патологией ХОБЛ и ИБС (табл. 2). Минимальные изменения Imax и S наблюдались у больных ХОБЛ, концентраций диеновых конъюгатов (ДК), триеновых конъюгатов (ТК) и оснований Шиффа (ОШ) – у пациентов с ИБС. Таким образом, у больных ХОБЛ с наличием сердечно-сосудистой коморбидности окислительный стресс был достоверно более выражен, чем у пациентов ИБС (р <0,01) и ХОБЛ (р <0,01).
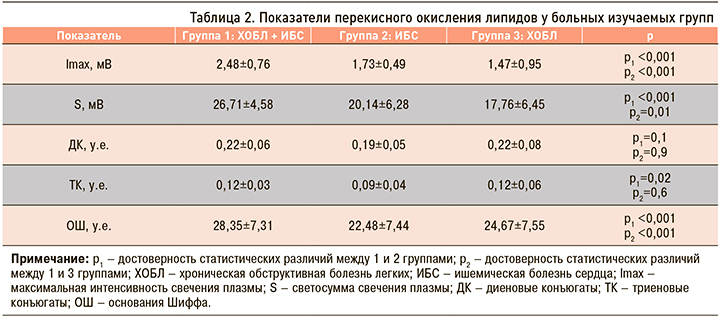
Количество первичных продуктов ПОЛ (ДК и ТК) у больных групп 1 и 3 было снижено, но достоверно не отличалось между собой (ДК р=0,9; ТК р=0,6), однако количество конечных продуктов ПОЛ (ОШ) у пациентов группы 1 оказалось достоверно больше, чем у больных группы 3 (р=0,006). У пациентов группы 2 ДК, ТК и ОШ превышали норму, но были достоверно ниже, чем в группе 1 (р <0,05) и чем в группе 3 (р <0,05). Следовательно, у коморбидных больных ХОБЛ и ИБС имеется не только избыточная продукция свободных радикалов с образованием большого количества конечных продуктов ПОЛ, но и их замедленная элиминация.
Инициация ПОЛ при ИБС обусловлена снижением активности естественных антиоксидантных ферментов и дефицитом антиоксидантов, а также наличием дислипидемии, при которой содержащиеся в высокой концентрации в крови атерогенные липиды служат легким субстратом для свободно-радикального окисления [15]. Первичные продукты ПОЛ запускают системную воспалительную реакцию: происходит усиление агрегации тромбоцитов и адгезии нейтрофилов к эндотелию, нарушение синтеза оксида азота и развитие вазоконстрикции, снижение содержания ненасыщенных жирных кислот, цитотоксическое повреждение эндотелиоцитов [1].
У пациентов с ХОБЛ в результате воспалительной реакции в бронхах активизируются макрофаги, Т-лимфоциты, нейтрофилы, под воздействием которых образуется большое количество свободных радикалов [2, 9, 12].
Среди отрицательных эффектов свободных радикалов можно назвать ингибирование протеаз, активацию ядерного фактора (Nuclear Factor-кB), фактора некроза опухоли α (Tumor Necrosis Factor α) и интерлейкина-8 (Interleukin-8), которые вызывают активацию нейтрофилов и способствуют прогрессированию заболеваний [1, 2].
Таким образом, наши данные показали, что у пациентов изучаемых групп независимо от нозологической формы заболевания наблюдаются однонаправленные изменения в системе «оксиданты – антиоксиданты» в виде нарушения баланса в пользу оксидантов. У коморбидных пациентов с ХОБЛ и ИБС окислительный стресс выражен максимально.
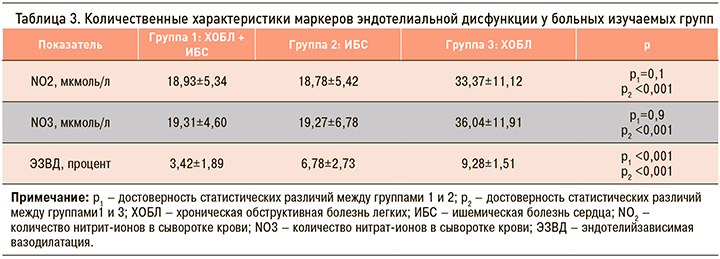
При исследовании функционального состояния эндотелия получены следующие результаты: у больных группы 1 (ХОБЛ и ИБС) увеличение диаметра плечевой артерии после ее декомпрессии составило в среднем 3,42+1,32%, что было достоверно ниже, чем в группе 2 и 3 (табл. 3).
При пробе с реактивной гиперемией после декомпрессии манжеты возникает механическое напряжения на эндотелиоцитах из-за резкого усиления линейной и объемной скорости кровотока, что приводит к увеличению содержания ионов кальция в цитоплазме и в результате к синтезу эндотелийзависимого релаксирующего фактора – оксида азота, вызывающего дилатацию сосуда [1]. Фактически эта проба позволяет оценить способность эндотелия к быстрому синтезу оксида азота. Количественное определение метаболитов NO лабораторным методом также дает возможность изучить содержание NO, но постоянно синтезируемого и распадающегося в организме, а не индуцированного. Таким образом, эти пробы дополняют друг друга.
У больных всех групп при проведении пробы с реактивной гиперемией наблюдалось уменьшение степени дилатации плечевой артерии после декомпрессии манжеты, что однозначно свидетельствовало о нарушении ЭЗВД и эндотелиальной дисфункции. В группе 1 (ХОБЛ + ИБС) нарушение ЭЗВД и, следовательно, эндотелиальная дисфункция были выражены максимально, в группе 2 (ХОБЛ) – минимально. Имелись достоверные различия между группами 1 и 2 (р <0,001), группами 1 и 3 (р <0,001), группами 2 и 3 (р <0,001).
Основываясь на этих результатах, следовало бы ожидать изменения метаболитов оксида азота в крови пропорциональные выраженности эндотелиальной дисфункции. Количество NO2 и NO3 у больных групп 1 и 2 было достоверно ниже нормы, но достоверные различия между ними по этому показателю не наблюдались (см. табл. 3). Вывод о том, что у этих больных была одинаковая степень нарушения функции эндотелия (снижение синтеза NO в равной степени) не подтвердился пробой с реактивной гиперемией (имелись высоко достоверные различия, р <0,001). У пациентов группы 3 содержание метаболитов оксида азота превышало норму и было достоверно выше, чем в группах 1 и 2 (р <0,001 и р <0,001 соответственно), что свидетельствовало о повышении синтеза NO, несмотря на имевшиеся нарушения ЭЗВД и эндотелиальную дисфункцию.
Объяснить это можно следующим образом. Как известно, синтез эндогенного NO регулируется NO-синтазой (NOS), а именно тремя ее изоформами: нейрональной (nNOS), индуцибельной или макрофагальной (iNOS) и эндотелиальной (eNOS) NO-синтазой [1]. Вазодилатирующие эффекты NO связаны с активностью eNOS [1, 2]. Установлено, что при ИБС дисфункция эндотелия обусловлена снижением активности именно изоформы eNOS и вследствие этого недостаточным синтезом NO [18, 19].
У больных ХОБЛ кинетика процесса отличается. В начальной стадии развития заболевания активность eNOS и, следовательно, уровень синтеза NO, отвечающего за вазодилатацию, прогрессивно снижается: равновесие вазодилатирующих и вазоконстрикторных механизмов смещается в пользу последних [19]. Дальнейший синтез NO уже происходит не под воздействием эндотелиальной NO-синтазы, а определяется макрофагальной NO-синтазой, активность которой возрастает под воздействием провоспалительных цитокинов и эндотоксинов [19]. Оксид азота, синтезируемый в больших концентрациях iNOS, а следовательно, и разлагающийся в увеличенных количествах (возрастание нитрита и нитрата азота у больных группы 3), не обладает свойством вазодилатации [19].
Мы считаем, что у коморбидных больных группы 1 общее количество оксида азота складывалось из значительно сниженного по сравнению с нормой «вазодилатирующего» NO, синтезируемого эндотелиальной NO-синтазой, и оксида азота, производимого макрофагальной NO-синтазой. У обследованных группы 3 (пациенты с ХОБЛ) увеличение концентрации оксида азота выше нормальных величин было обусловлено продукцией NO только макрофагальной NO-синтазой. Таким образом, у больных с коморбидной сердечно-легочной патологией, а также у пациентов с ХОБЛ уровни метаболитов оксида азота в крови не характеризовали истинное количество «вазодилатирующего» NO и не позволили достоверно судить о степени эндотелиальной дисфункции. Больным ХОБЛ при наличии у них сердечно-сосудистой коморбидности требуется комплексная оценка функции эндотелия.
ЗАКЛЮЧЕНИЕ
Общность патофизиологических проявлений окислительного стресса при ИБС и ХОБЛ может лежать в основе их сочетанного развития, а также определять взаимное влияние. При развитии сердечно-сосудистой коморбидности у больных ХОБЛ наблюдается усиление патологических реакций, что приводит к развитию выраженного окислительного стресса и усугублению эндотелиальной дисфункции, а значит, будет способствовать прогрессированию заболеваний.



