Системная красная волчанка (СКВ) – хроническое аутоиммунное заболевание неизвестной этиологии, характеризующееся иммуновоспалительным повреждением различных тканей и внутренних органов. У 50% пациентов с СКВ и наличием антифосфолипидных антител (АФА) развивается вторичный антифосфолипидный синдром (АФС) [1, 2]. АФС – симптомокомплекс, включающий рецидивирующие тромбозы и/или акушерскую патологию (чаще привычное невынашивание беременности) и связанный с синтезом АФА: волчаночного антикоагулянта (ВА), антикардиолипиновых антител (аКЛ) и антител к β2-гликопротеиду I (анти-β2-ГП-I) [1].
Частота обнаружения АФА у больных СКВ колеблется от 12 до 44% для ВА и от 10 до 19% для анти-β2-ГП-I. В 2003 г. описан серонегативный вариант АФС, при котором в крови не определяются диагностически значимые титры «классических» антител, но могут присутствовать антитела к протромбину, аннексину V, фосфатидилэтаноамину, фосфатидилсерин-протромбиновому комплексу (аФС-ПТ) и к отрицательно заряженным фосфолипидам [3]. Роль этих антител в клинических проявлениях АФС до конца не определена.
АФС может быть первичным или развиваться на фоне другого заболевания, чаще всего СКВ.
В терапии СКВ основная роль отводится препаратам с иммуносупрессивными свойствами: глюкокортикостероидам (ГКС), цитостатикам (циклофосфамид, азатиоприн, метотрексат, микофенолатомофетил), аминохинолиновым средствам (хлорохин, гидроксихлорохин). ГКС короткого действия (преднизолон, метилпреднизолон) служат наиболее эффективными средствами для лечения СКВ. При высокой активности СКВ для достижения быстрого контроля за проявлениями заболевания возможно использование пульс-терапии (метилпреднизолон по 1000 мг внутривенно капельно последовательно 3 инъекции, затем ежемесячно) [4, 5].
Цитотоксические препараты используются в комплексных схемах терапии совместно с ГКС, что позволяет ускорить достижение ремиссии и снизить поддерживающую дозу ГКС. Циклофосфамид является препаратом выбора для индукции ремиссии при волчаночном нефрите и тяжелом поражении центральной нервной системы (ЦНС). Он используется совместно с ГКС в схеме комбинированной пульс-терапии и назначается по 1000 мг внутривенно капельно однократно в первый день [5].
Азатиоприн (по 50–150 мг/сут) применяется для поддержания индуцированной циклофосфамидом ремиссии люпус-нефрита, в лечении аутоиммунной гемолитической анемии, тромбоцитопении, поражений кожи, генерализованной СКВ.
Микофенолата мофетил (по 1000–3000 мг/сут) назначается для терапии люпус-нефрита. Метотрексат (15–30 мг/нед) служит препаратом выбора при волчаночном артрите и поражениях кожи. Назначение гидроксихлорохина (по 200–400 мг/сут) снижает риск тромбообразования, способствует поддержанию ремиссии и предупреждению рецидивов у больных СКВ и АФА [4]. Значение иммуносупрессивной терапии в динамике АФА у больных СКВ с АФС до сих пор не определено [5, 6].
Перспективы лечения больных СКВ с АФС связаны с использованием генно-инженерных биологических препаратов (ГИБП). Ритуксимаб – химерное моноклональное антитело против антигена CD20, экспрессируемого на В-лимфоцитах, которое вводится по стандартной схеме, включающей две внутривенные инфузии по 500–100 мг с интервалом в 2 нед каждые 6 мес. Получены данные о снижении уровня АФА на фоне терапии ритуксимабом у больных СКВ. Напротив, в исследовании D. Erkan et al. не обнаружено изменения в уровне АФА на фоне лечения этим лекарственным средством у больных СКВ и АФС [7, 8]. Несмотря на доказанную эффективность ритуксимаба в лечении СКВ, опыт его применения при СКВ с АФС весьма скромен и требует дальнейшего накопления.
В комплексном лечении СКВ с целью профилактики тромбозов у пациентов с АФС назначаются антиагреганты (ацетилсалициловая кислота, дипиридамол). При эпизодах тромбоза показаны антикоагулянты прямого действия (гепарин натрия с индивидуальным подбором дозы под контролем активированного частичного тромбопластинового времени, низкомолекулярный гепарин) с дальнейшим переводом на непрямые антикоагулянты: варфарин с контролем дозы по уровню международного нормализованного отношения [4].
Представленные данные свидетельствуют об отсутствии единой точки зрения на ведение больных СКВ с АФС.
Цель исследования – оценка влияния различных схем иммуносупрессивной терапии, включая применение ГИБП в варианте блокаторов пролиферации зрелых B-лимфоцитов (ритуксимаб), на клинико-иммунологическую активность и динамику АФА у больных СКВ и СКВ с АФС.
МАТЕРИАЛЫ И МЕТОДЫ
В исследование было включено 52 больных СКВ, проходивших лечение в Клинической ревматологической больнице № 25 (Санкт-Петербург), из них 49 (94%) женщин и 3 (6%) мужчин. Средний возраст пациентов составил 45 [18; 56] лет, средняя продолжительность заболевания – 12 [0,5; 18] лет. Медиана активности СКВ по шкале SELENA SLEDAI была высокой и составила 12,2 [5,8; 16].
Пациенты были распределены на две группы: основную группу составили 26 больных СКВ с наличием АФА, из них 19 (70%) пациентов с СКВ и АФС и 7 (30%) – с СКВ без АФС. В контрольную группу вошли 26 пациентов с СКВ без АФА. Группы были сравнимы по полу, возрасту, длительности и активности заболевания.
В зависимости от проводимой иммуносупрессивной терапии пациенты основной и контрольной групп были распределены на две подгруппы: подгруппа А (основная – 7 больных, контрольная – 7 человек) на первом этапе получала комбинированную пульс-терапию метилпреднизолоном по 1000 мг внутривенно капельно № 3 и циклофосфамидом по 1000 мг внутривенно капельно однократно. Два пациента с СКВ и АФС из основной группы получили ритуксимаб по 1000 мг внутривенно капельно 2 введения с перерывом в 2 нед.
В последующем всем пациентам подгруппы А назначался преднизолон внутрь в дозе 0,5 мг/сут с постепенным снижением до 0,2 мг/сут, плаквенил по 200 мг/сут и азатиоприн по 100 мг/сут. Подгруппа B (основная – 17 больных, контрольная – 19 человек) исходно получала комбинированную терапию внутрь: преднизолон по 1 мг/кг/сут в течение 4 нед со снижением дозы до 0,2 мг/кг/сут, плаквенил по 200 мг/сут и азатиоприн по100 мг/сут. Все пациенты с АФС (19 больных) дополнительно получали дезагрегантную терапию в виде ацетилсалициловой кислоты по 100 мг/сут.
Длительность наблюдения составила 6 мес.
В динамике оценивалась активность СКВ по шкале SELENA SLEDAI. Методом иммунофлуоресценции определялся уровень антител к двуспиральной ДНК, уровень компонентов комплемента С3 и С4 оценивался методом простой радиальной иммунодиффузии. С помощью иммуноферментного анализа проводилось определение уровня АФА: аКЛ Ig G и IgM, анти-β2-ГП-IIgGAM, волчаночного антикоагулянта, аФС-ПТ и антител к аннексину V IgG/IgM.
Анализ результатов исследования проводился с помощью программы STATISTICA 6.0, использовались методы непараметрической статистики (критерий U-теста Манна–Уитни). Уровень значимости был принят за 0,05.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Через 6 мес терапии в основной и контрольной группах показатели клинической активности СКВ по шкале SELENA SLEDA достоверно снизились по сравнению с исходными значениями и свидетельствовали о низкой активности болезни (табл. 1). В основной группе медиана активности СКВ по SLEDAI оказалась выше, чем в группе контроля (5,0 и 4,0 баллов соответственно), однако статистических различий по этому показателю выявить не удалось. Отмечены достоверно более низкие значения показателей С4 и С3 компонентов комплемента у больных СКВ и АФА по сравнению с группой СКВ без АФА (р <0,05). Напротив, уровни АФА (аКЛIgG и М, анти-β2-ГП-I и аФС-ПТ) оказались достоверно выше в основной группе, чем в группе контроля (р <0,05), хотя и не выходили за референсные значения нормы. Полученные изменения свидетельствовали о сохраняющейся иммунологической активности у больных СКВ и АФА после проведенного лечения.
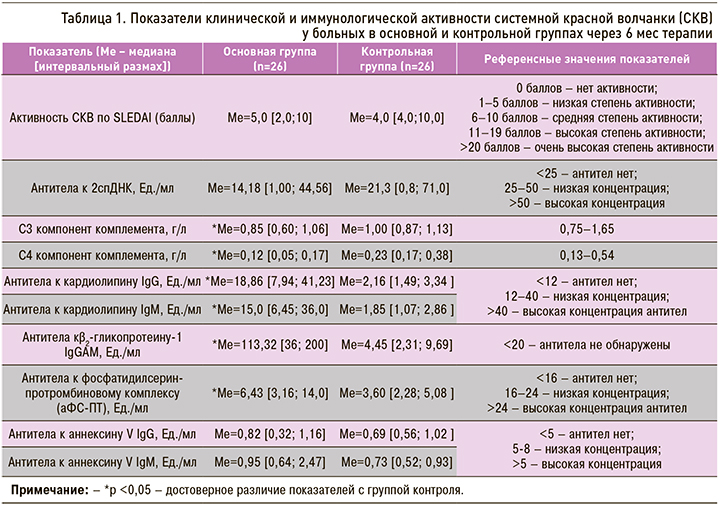
Важно отметить, что через 6 мес лечения у больных СКВ с бессимптомным носительством АФА (n=7) по сравнению с больными СКВ и АФС (n=19) были выявлены статистически более низкие показатели клинико-иммунологической активности по SLEDAI (Mе=2,0 [1,0; 6,0] и Mе=8,0 [5,0; 10,0] соответственно; р=0,04), уровня аКЛ IgG (Mе=4,08 [3,31; 10,52 ] и Mе=34,78 [11,00; 62,00] Ед./мл соответственно; р=0,0004) и анти-β2-ГП-I (Mе=27,80 [12,00; 200,00] и Mе=124,00 [41,00; 200,00] Ед./мл соответственно; р=0,025). Следовательно, у больных СКВ с АФС по сравнению с больными СКВ и носительством АФА отмечается более активное течение болезни, несмотря на проводимое лечение. Этот факт свидетельствует об обоснованности применения агрессивной иммуносупрессивной терапии у пациентов с активным течением СКВ и АФС.
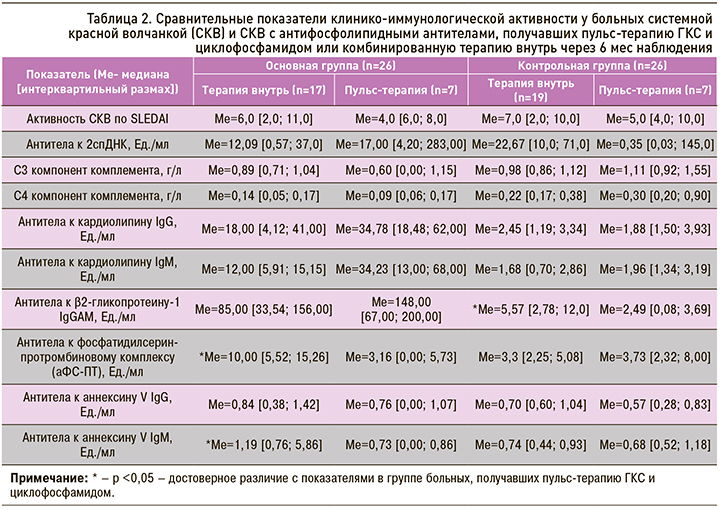
 Сравнительная оценка клинико-иммунологических показателей у больных СКВ и СКВ с АФА, в зависимости от проводимого лечения, позволила установить, что через 6 мес терапии у пациентов, исходно получавших пульс-терапию метилпреднизолоном и циклофосфамидом, по сравнению с больными, получавшими пероральную терапию, показатели клинической активности по шкале SELENA SLEDA оказались ниже, хотя достоверных различий по этому показателю в изучаемых группах получено не было. Важно отметить, что к концу наблюдения у больных СКВ с АФА, получавших пульс-терапию, уровень аФС-ПТ и антител к аннексину V IgM был достоверно ниже, чем у больных этой группы, получавших комбинированную терапию внутрь (p=0,01 и 0,02 соответственно) (табл. 2, рис. 1). Полученные данные позволили установить, что применение высокодозных схем комбинированной пульс-терапии эффективно снижает антителопродукцию у больных СКВ с АФА и, следовательно, приводит к уменьшению риска тромботических осложнений и улучшению прогноза этого заболевания.
Сравнительная оценка клинико-иммунологических показателей у больных СКВ и СКВ с АФА, в зависимости от проводимого лечения, позволила установить, что через 6 мес терапии у пациентов, исходно получавших пульс-терапию метилпреднизолоном и циклофосфамидом, по сравнению с больными, получавшими пероральную терапию, показатели клинической активности по шкале SELENA SLEDA оказались ниже, хотя достоверных различий по этому показателю в изучаемых группах получено не было. Важно отметить, что к концу наблюдения у больных СКВ с АФА, получавших пульс-терапию, уровень аФС-ПТ и антител к аннексину V IgM был достоверно ниже, чем у больных этой группы, получавших комбинированную терапию внутрь (p=0,01 и 0,02 соответственно) (табл. 2, рис. 1). Полученные данные позволили установить, что применение высокодозных схем комбинированной пульс-терапии эффективно снижает антителопродукцию у больных СКВ с АФА и, следовательно, приводит к уменьшению риска тромботических осложнений и улучшению прогноза этого заболевания.
Эта же тенденция наблюдалась у пациентов контрольной группы. У больных СКВ без АФА, исходно получавших пульс-терапию метилпреднизолоном и циклофосфамидом по сравнению с больными, получавшими терапию внутрь, уровень анти-β2-ГП-I через 6 мес лечения оказался достоверно ниже (2,49 [0,08; 3,69] и 5,57 [2,78; 12,0] Ед./мл соответственно; р <0,05) (табл. 2).
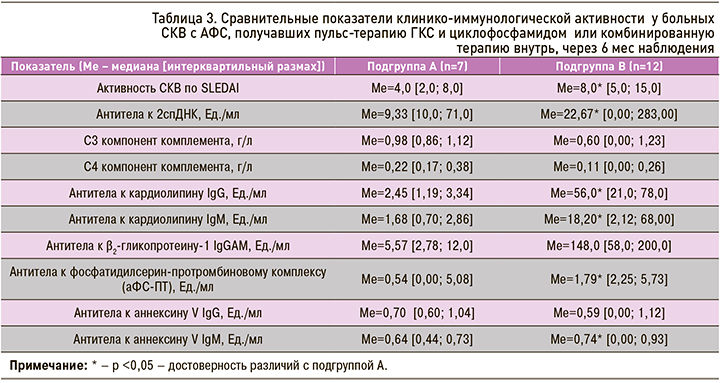
Пульс-терапия ГКС и циклофосфамидом, по сравнению с пероральной иммуносупрессивной терапией у больных СКВ с АФС через 6 мес наблюдения, способствовала более значимому снижению активности СКВ по шкале SLEDAI, антител к кардиолипину IgM, IgG, аннексину V IgM и аФС-ПТ (p <0,05 соответственно) (табл. 3).
Интересно отметить, что у двух больных СКВ с АФС, получавших ритуксимаб, после 2 инфузий показатели активности СКВ по шкале SLEDAI снизились более чем в 3 раза (с 14,2 до 4,6 и с 12,8 до 4,1 баллов), уровень аФС-ПТ снизился почти в 5 раз (с 4,97 до 0,00 и с 5,01 до 0,00 Ед./мл), а уровень антител к аннексину V IgM − более чем в 10 раз (с 1,12 до 0,00 и с 0,97 до 0,00 Ед./мл). Можно предположить, что применение ритуксимаба у больных СКВ с АФС является эффективным методом иммуносупрессивной терапии, однако эти данные требуют дальнейшего подтверждения на большем количестве наблюдений.
ЗАКЛЮЧЕНИЕ
- У больных СКВ с АФС, по сравнению с больными СКВ без АФС, наблюдается более значимая клиническая активность заболевания по шкале SLEDAI и высокий уровень аКЛ IgG и анти-β2-ГП-I.
- При активном течении СКВ с АФС применение интенсивных схем иммуносупрессивной терапии, включающих комбинированную пульс-терапию метилпреднизолоном и циклофосфамидом, способствует существенному снижению клинической активности болезни по шкале SLEDAI и уровня АФА (аФС-Пт, антител к аннексину IgM, анти-β2-ГП-I), что приводит к уменьшению риска тромботических осложнений и улучшению прогноза этого заболевания.
- У двух больных СКВ с АФС, получивших 2 инфузии ритуксимаба, через 6 мес наблюдения показатели активности СКВ по шкале SLEDAI снизились более чем в 3 раза, уровень аФС-ПТ – в 5 раз, а уровень антител к аннексину V IgM − более чем в 10 раз. Полученные данные о высокой эффективности ритуксимаба у больных СКВ с АФС требуют дальнейшего подтверждения на большем количестве наблюдений.


