Бронхиальная астма (БА) – одно из самых распространенных хронических заболеваний человека, которое в большинстве случаев дебютирует в детском возрасте, но может развиться у взрослых и пожилых пациентов. Согласно отчету глобальной сети астмы (The Global Asthma Network), в настоящее время ей страдают 334 млн человек, из которых 14 % – дети [1]. Несмотря на сходные клинические симптомы (экспираторная одышка, сухой приступообразный кашель, свистящее дыхание), БА характеризуется большой гетерогенностью клинических форм, вариабельностью течения и персистенцией на протяжении жизни. Гетерогенность БА состоит в разной выраженности бронхиальной обструкции, разной тяжести течения и частоте обострений, разном ответе на бронходилататоры и препараты для длительного контроля. Современная терапия БА требует глубокого анализа факторов, ответственных за ее прогрессирование и развитие обострений, а также определения пациентов, подходящих для целевой терапии БА с учетом клинических и биологических фенотипов болезни.
Современная цель терапии БА – достижение контроля симптомов и предотвращение обострений. Несмотря на понимание гетерогенности БА, современная ступенчатая фармакотерапия астмы основывается на принципе «один размер подходит всем». Основу контролирующей фармакотерапии БА составляют ингаляционные глюкокортикостероиды (ИГКС) в виде монотерапии или в комбинации с длительно действующими β2-агонистами (ДДБА) и(или) антагонистами лейкотриеновых рецепторов (АЛТР). И, надо сказать, что большинство пациентов, страдающих БА, хорошо отвечают на эту традиционную терапию и при условии соблюдения оптимальной приверженности и адекватной техники ингаляции достигают и поддерживают контроль заболевания. Вместе с тем существует не очень большая (3–10%) доля больных, которые имеют тяжелую БА [2–4], и им унифицированный принцип лечения не приносит должной пользы. Они, как правило, рефрактерны к традиционной терапии, имеют высокую частоту обострений БА, незапланированных визитов к врачу и обращений за неотложной медицинской помощью, госпитализаций. Именно тяжелая БА (ТБА) поглощает 50% всех экономических затрат на заболевание в целом [2–4].
Долгое время для таких больных единственным вариантом поддерживающей терапии и избегания обострений было применение оральных кортикостероидов (ОКС). Ситуация кардинальным образом изменилась с появлением генно-инженерных биологических препаратов (ГИБП) для лечения ТБА. В настоящее время в мире, в том числе в нашей стране, уже зарегистрированы 5 ГИБП для лечения ТБА: омализумаб, меполизумаб, реслизумаб, бенрализумаб и дупилумаб. Однако применение этих таргетных препаратов требует строгого отбора пациентов, чтобы получить оптимальный ответ на лечение. Существующая биологическая терапия БА направлена против основных провоспалительных цитокинов или иммуноглобулина Е (IgE), участвующих в формировании и персистенции эозинофильного Т2-воспаления у пациентов с ТБА. Важный момент – выявление пациентов именно с ТБА: как показывают клинические исследования и опыт реальной практики, существенная часть пациентов, которым назначается лечение биологическими препаратами, имеют неконтролируемые симптомы астмы вследствие разных модифицируемых причин, а некоторые вовсе не имеют диагноза БА. Кроме того, ТБА тоже гетерогенна, и не для всех ее фенотипов пока существует таргетная терапия, поэтому определение подходящего пациента и подбор нужного ему ГИБП является довольно сложной задачей, требующей знания фено-/эндотипической гетерогенности ТБА, биомаркеров разных фенотипов ТБА, механизма действия различных молекул ГИБП, их клинической эффективности и профиля безопасности. Настоящая статья посвящена характеристике тяжелой и трудной для контроля БА, алгоритму выявления пациентов с ТБА, их характеристикам и фенотипам. Основной акцент сделан на аллергической ТБА как наиболее частом фенотипе, роли IgE и возможностям анти-IgE терапии с помощью препарата омализумаб.
ТРУДНАЯ ДЛЯ КОНТРОЛЯ И ТЯЖЕЛАЯ БРОНХИАЛЬНАЯ АСТМА
Прежде чем рассматривать возможность биологической терапии пациентов с ТБА, нужно подтвердить, что у пациента имеется тяжелая астма и вообще астма. Как уже отмечалось и каким бы курьезным ни казался этот факт, но исследования показывают, что довольно значительная часть пациентов, которые считались пациентами с тяжелой астмой, вообще не имеют диагноза БА. Так, в недавнем исследовании, выполненном в Канаде, среди 701 пациента, получавшего лечение по поводу астмы различной степени тяжести, у 31,1% пациентов при тщательном обследовании в специализированной клинике этот диагноз не подтвердился [5]. Поэтому врачу необходимо убедиться, что симптомы и функциональные нарушения связаны именно с БА, а не с хронической обструктивной болезнью легких (ХОБЛ) или эмфиземой или не обусловлены низкой приверженностью лечению, неправильной техникой ингаляции.
Сопутствующие заболевания также могут повлиять на течение БА и эффективность проводимой противоастматической терапии. Неконтролируемый аллергический ринит или хронический риносинусит с полипами или без усугубляют течение БА, но могут быть и дополнительными критериями в пользу выбора биологической терапии. Например, если пациент с БА страдает аллергическим ринитом, то это дополнительный клинический маркер аллергической астмы Т2-эндотипа. Дисфункцию голосовых связок часто трудно диагностировать и дифференцировать от БА, учитывая, что пациент может иметь оба заболевания. Часто с астмой сосуществует и рефлюкс-эзофагит, который также может ухудшать симптомы БА. Наконец, приблизительно 20% пациентов с БА курят, а это тоже может усугублять тяжесть заболевания и снижать ответ на ИГКС [4].
Согласно Федеральным клиническим рекомендациям по диагностике и лечению БА от 2019 г. [6], а также GINA 2019 [2] и карманному руководству GINA по диагностике и лечению трудной для лечения и тяжелой БА [7], трудная для контроля БА – это астма, которая не контролируется, несмотря на применение 4-й или 5-й ступени по GINA (рис. 1) (например, применение ИГКС в средней или высокой дозе со вторым контроллером в виде ДДБА или АЛТР или поддерживающей терапии ОКС), или же астма, которая требует такого лечения для поддержания хорошего контроля симптомов и уменьшения риска обострений. Во многих случаях БА может быть трудной для лечения из-за модифицируемых факторов, таких как неправильная техника ингаляции, плохая приверженность лечению, курение, сопутствующие заболевания, или же из-за неправильного диагноза.
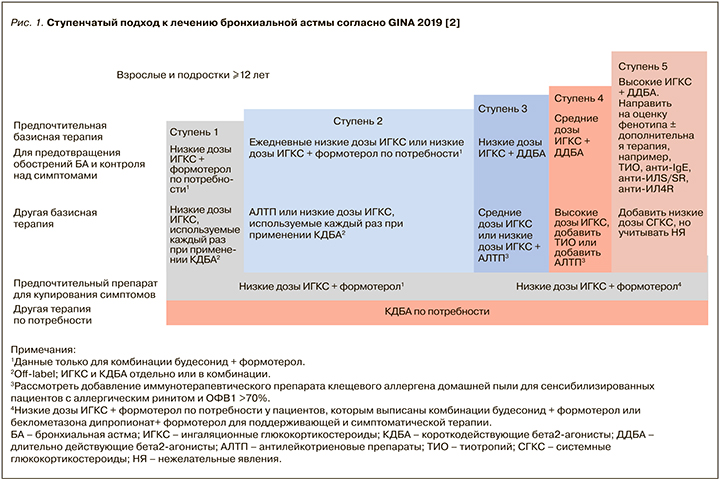
ТБА является подгруппой, трудной для контроля астмы, и означает ту астму, которая остается неконтролируемой, несмотря на приверженность пациента к максимально оптимизированной терапии и лечению сопутствующих заболеваний, или ухудшается, когда высокие дозы ИГКС уменьшаются. Таким образом, в настоящее время «тяжелая астма» – ретроспективный диагноз. Иногда ее называют «тяжелая рефрактерная астма», поскольку она характеризуется относительной устойчивостью к высоким дозам ИГКС. Однако с появлением биологической терапии слово «рефрактерная» больше не подходит для характеристики заболевания [5]. Неконтролируемая БА характеризуется:
- плохим контролем симптомов (критерии – частые симптомы и/или применение препаратов для их купирования, ограничение активности пациента из-за БА, ночные пробуждения из-за БА);
- частыми обострениями (≥2 в год), требующими ОКС, или серьезными обострениями (≥1 в год), требующими госпитализации [7].
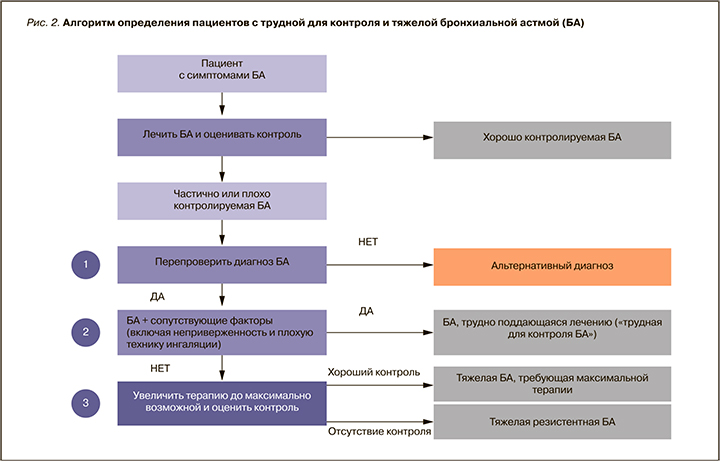
Таким образом, для того чтобы выявить пациентов с ТБА (рис. 2), необходимо:
- подтвердить диагноз БА;
- оценить и по возможности устранить влияние модифицируемых факторов (низкая приверженность контролирующей терапии, неправильная техника ингаляции, влияние сопутствующих заболеваний);
- оптимизировать контролирующую терапию согласно 5-й ступени.
Рекомендуется оценить контроль БА после 3–6 мес оптимизированной терапии и, если БА по-прежнему не контролируется или контролируется только на максимальных дозах ИГКС/ДДБА с тиотропия бромидом и/или АЛТП с/без ОКС, необходимо направить пациента к аллергологу-иммунологу или пульмонологу, занимающемуся лечением ТБА, для определения фенотипа и рассмотрения возможности назначения ГИБП.
Следуя данным международных исследований, только 3–10% пациентов соответствуют критериям тяжелой БА [2–5, 7]. В настоящее время в России по инициативе Российского респираторного общества проводится исследование по регистру пациентов с ТБА, которое позволит оценить распространенность этой формы заболевания, выявить основные ее фенотипы, а также особенности проводимого лечения и уровень контроля у больных. Это, в свою очередь, будет способствовать оптимизации терапии ТБА, в том числе с помощью ГИБП.
ГЕТЕРОГЕННОСТЬ ТЯЖЕЛОЙ БРОНХИАЛЬНОЙ АСТМЫ
ТБА, так же как и астма в целом, гетерогенна, что продемонстрировали недавно проведенные кластерные анализы, выполненные в когортах соответствующих пациентов [8, 9].
На рисунке 3 представлены основные фенотипы и эндотипы ТБА [10]. Выявление патобиологических механизмов, лежащих в основе формирования фенотипов БА или так называемых эндотипов БА, – насущная задача в рамках оптимизации терапии БА, особенно тяжелой. Согласно определению Anderson G.P. [11], эндотип заболевания – это субтип болезни, определяемый уникальным или отличительным функциональным или патофизиологическим механизмом. Один эндотип БА может лежать в основе нескольких фенотипов, так как эндотип – это молекулярная основа фенотипов. Большая часть пациентов с ТБА относятся к Т2-эндотипу, который характеризуется высокой экспрессией Тh2-лимфоцитов и цитокинов Тh2-профиля: интерлейкинов (ИЛ) 4, 5, 9 и 13. Этот механизм характерен преимущественно для тяжелой аллергической БА; он формирует соответствующий фенотип, проявляющийся эозинофильным воспалением нижних дыхательных путей.

Помимо Тh2-лимфоцитов, в формировании эозинофильного воспаления при БА принимают участие недавно открытые клетки врожденного иммунитета – ILC2 (врожденные лимфоидные клетки 2 типа), которые, как и Th2-лимфоциты, в избыточном количестве генерируют цитокины Т2-профиля: ИЛ-5, ИЛ-9 и ИЛ-13. Таким образом, название этого типа воспаления и эндотипа БА было изменено с Th2, что подразумевало продукцию этих цитокинов исключительно Th2-лимфоцитами, на воспаление 2-го типа (Т2-воспаление), которое соответственно лежит в основе Т2-астмы. Как видно из рисунка 3, в случае аллергической БА, поздней эозинофильной (в том числе аспириновой) БА воспалительной основой заболевания служит преимущественно T2-эозинофильное воспаление, тогда как в случае БА, ассоциированной с ожирением, астмы курильщиков и БА с очень поздним дебютом – не Т2-воспаление.
В структуре тяжелой неконтролируемой БА частота эозинофильного эндотипа воспаления достаточно высока. По данным цитологического анализа образцов мокроты больных тяжелой неконтролируемой БА из Бельгийского регистра, 55% пациентов характеризуются эозинофильным типом воспаления дыхательных путей (эозинофилы ≥3%), у 21% определяется нейтрофильный фенотип (нейтрофилы ≥76%), у 18% – малогранулоцитарный и у 6% – смешанный тип воспаления дыхательных путей (эозинофилы ≥3% и нейтрофилы ≥76%) [12]. Согласно этому исследованию, 57% пациентов в Бельгийском регистре ТБА имели эозинофильную (2-го типа) астму и 43% неэозинофильную (не 2-й тип) астму [12].
Эозинофильный эндотип БА ассоциируется с большей выраженностью симптомов, наличием атопии, иногда с поздним развитием заболевания и назальными полипами. Преобладающая доля пациентов с этим типом воспаления дыхательных путей хорошо отвечает на ИГКС; они имеют легкую или среднетяжелую БА, тогда как пациенты с тяжелой эозинофильной БА характеризуются сниженным ответом/отсутствием ответа на лечение ГКС. У пациентов с тяжелой эозинофильной БА наблюдается неконтролируемое течение заболевания с частыми и тяжелыми обострениями.
В результате длительной персистенции эозинофильного воспаления в дыхательных путях пациентов с ТБА развиваются необратимые структурные изменения, называемые ремоделированием бронхов, которые включают бокаловидноклеточную гиперплазию желез подслизистого слоя бронхов, гиперплазию и гипертрофию гладкой мускулатуры, гиперваскуляризацию подслизистого слоя бронхов, накопление коллагена в зонах, расположенных ниже базальной мембраны, и субэпителиальный фиброз. Эти изменения коррелируют с тяжестью БА и, наряду с частыми обострениями, приводят к прогрессивному снижению функции легких у больных БА. Традиционная терапия БА, включая ИГКС, практически не оказывает влияния на этот процесс [13].
ТЯЖЕЛАЯ АЛЛЕРГИЧЕСКАЯ БРОНХИАЛЬНАЯ АСТМА: РОЛЬ IgЕ
Пациенты, относящиеся к фенотипу тяжелой аллергической БА (см. рис. 3), составляют 40–50% всех больных ТБА [14]; согласно результатам анализа Moore W.C. et al., проведенного в ходе программы по изучению ТБА в США [15], этот фенотип может развиваться из легкой и среднетяжелой атопической БА, начавшейся в детстве. Результаты отечественных исследований также свидетельствуют о высокой представленности аллергического фенотипа ТБА. По данным Сергеевой Г.Р., Емельянова А.В. с соавт., у 77% больных ТБА присутствует фенотип атопической БА, ассоциированный с эозинофильным воспалением дыхательных путей [16]. Данные национального регистра по ТБА показывают наличие аллергического фенотипа у 68% пациентов [17]. Пациенты, принадлежащие к этому фенотипу тяжелой БА, имеют широкий спектр сенсибилизации к аллергенам и соответственно положительных кожных проб и/или специфических IgE в сыворотке крови, а также повышенный уровень общего IgE в крови. Как правило, они страдают сопутствующим аллергическим ринитом и отмечают отягощенный семейный анамнез по аллергии и БА. Как известно, IgE-сенсибилизация к клещам домашней пыли (КДП) затрагивает до 25–30% населения мира и представляет собой основной фактор риска развития аллергической астмы.
IgE-обусловленные реакции составляют основу патогенетического механизма многих, если не большинства случаев БА. В фазу сенсибилизации дендритные клетки захватывают ингаляционные аллергены (АГ), процессируют их до линейных пептидов и представляют наивным CD4+ Т-клеткам в региональном лимфоузле, превращая их в Th2, которые обусловливают переключение В-клеток на синтез АГ-специфических IgE (рис. 4) [18]. Последние, образованные в избытке при контакте с АГ у предрасположенных к атопии лиц, фиксируются на высокоаффинных рецепторах к IgE (FcεRI) тучных клеток. Это приводит к сенсибилизации клеток и слизистой оболочки дыхательных путей.
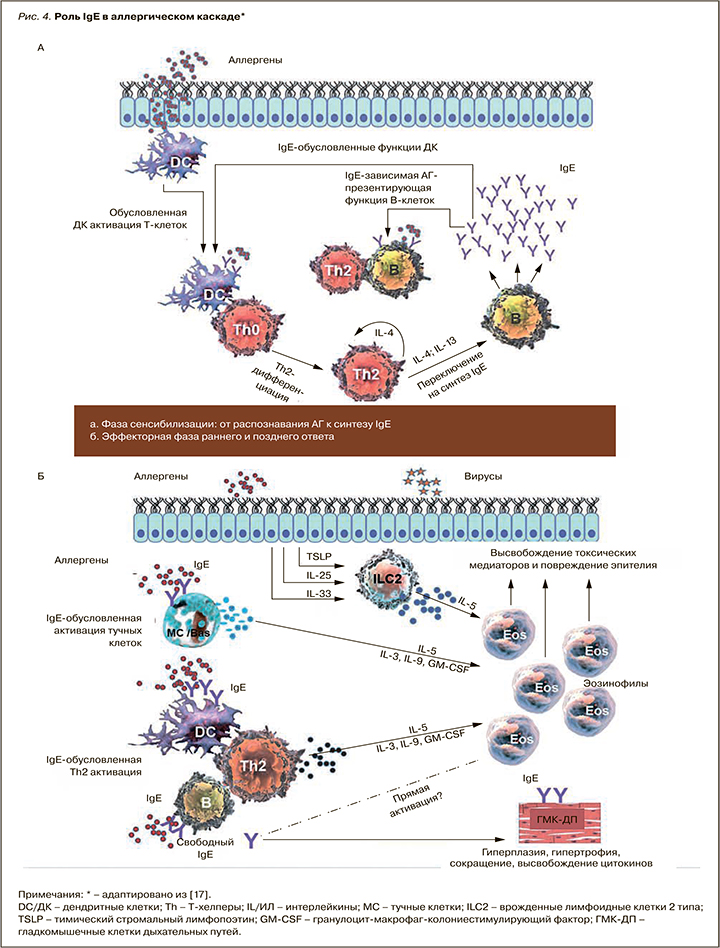
При последующем контакте АГ связывается со специфическими к нему IgE на поверхности тучной клетки и базофилов, приводя к активации этих клеток и высвобождению из них биологически активных медиаторов – гистамина, триптазы, цистеин-лейкотриенов и фактора активации тромбоцитов. Эти медиаторы вызывают отек слизистой дыхательных путей и бронхоконстрикцию – ключевые симптомы раннего аллергического ответа.
События, вызванные медиаторами тучных клеток и базофилов во время раннего ответа, приводят к выработке и высвобождению цитокинов, таких как ИЛ-3, ИЛ-4, ИЛ-5, ИЛ-13, хемокинов и гранулоцитарно-макрофагального колониестимулирующего фактора (ГМ-КСФ), которые рекрутируют нейтрофилы, эозинофилы и базофилы, Т-клетки и макрофаги в область воспаления (см. рис. 4) [18]. Этот процесс, известный как поздний аллергический/астматический ответ, развивается спустя часы после контакта с АГ и способствует гиперсекреции слизи, воспалению дыхательных путей и развитию бронхиальной гиперреактивности (БГР) – основному клиническому маркеру БА. Поздний ответ способен переходить в хронический воспалительный ответ, который может быть вызван повторными воздействиями специфического АГ, стимулирующего АГ-специфические Th2 и тучные клетки, которые, в свою очередь, способствуют увеличению эозинофилии и дополнительной продукции IgE. IgE регулирует экспрессию FcεRI на тучных клетках и базофилах: высокий уровень IgE в сыворотке крови приводит к высокой экспрессии рецепторов и активации клеток в ответ даже на небольшой антигенный стимул [18]. Помимо запуска и поддержания аллергического воспаления в нижних дыхательных путях при БА, IgE через FcεRI плазмоцитоидных дендритных клеток редуцирует продукцию интерферона (ИНФ) I типа, снижая антивирусный иммунитет.
Участие IgE в формировании аллергического воспаления осуществляется не только через активацию высокоаффинного (FcεRI), но и низкоаффинного (CD23 или FcεRII) рецептора, расположенного преимущественно на В-лимфоцитах. Взаимодействуя с FcεRII на В-клетках, IgE активирует их способность захватывать АГ и таким образом усиливать аллергическую реакцию, через FcεRII IgE способствует выживаемости, повышает миграцию эозинофилов, индуцирует высвобождение ФНО-альфа [19].
Таким образом, активация аллергического каскада IgE при постоянной стимуляции аллергеном приводит к возникновению хронического аллергического воспаления в дыхательных путях у пациентов с астмой. При этом именно IgE выступает ключевым поддерживающим элементом этого порочного круга.
Однако роль IgE не исчерпывается только этим. Важный аспект патогенетического участия IgE в астме – влияние на процесс ремоделирования, причем не только косвенно, за счет индукции и поддержания эозинофильного воспаления дыхательных путей, но и непосредственного воздействия на этот процесс, обусловленного наличием FcεRI и FcεRII на гладкомышечных клетках дыхательных путей. В исследованиях in vitro на гладкомышечных клетках из биопсийного материала стенки бронхов больных БА, по сравнению с контролем (здоровыми лицами), было показано, что IgE дозозависимо повышает депозицию экстрацеллюлярного матрикса (ЭЦМ) и общего коллагена (особенно значимо коллагена I и III типа) у пациентов с БА [20, 21]. В этих же исследованиях было продемонстрировано, что IgE дозозависимо повышает депозицию фибронектина в образцах от больных БА и более значимо стимулирует пролиферацию гладкомышечных клеток больных БА по сравнению со здоровым контролем. Важно, что влияние на процесс ремоделирования IgE оказывал изолированно, без присутствия АГ; добавление АГ при этом не привело к усилению действия. При этом предварительная (30–60 мин) обработка клеток анти-IgE-антителами (омализумабом) уменьшает эти процессы у больных БА [20, 21].
Эти исследования in vitro были подтверждены клиническим исследованием, показавшим, что добавление омализумаба к обычной терапии астмы в течение 16 нед привело к значительному уменьшению толщины стенки дыхательных путей [22]. В другом исследовании сообщалось, что лечение омализумабом в течение одного года уменьшало толщину ретикулярной базальной мембраны, а также инфильтрацию эозинофилов у пациентов с астмой [23]. Недавнее исследование in vitro показало, что не связанный с АГ IgE достаточен для стимуляции пролиферации гладкомышечных клеток дыхательных путей и ремоделирования через up-регуляцию microРНК-21-5р [24].
АНТИ-IgE-ТЕРАПИЯ БРОНХИАЛЬНОЙ АСТМЫ: ИММУНОЛОГИЧЕСКИЕ ЭФФЕКТЫ ОМАЛИЗУМАБА И ВЛИЯНИЕ НА РЕМОДЕЛИРОВАНИЕ БРОНХОВ
Аллергическая БА, не контролируемая терапией, соответствующей 4–5 ступени GINA, в настоящее время рассматривается как целевой фенотип для анти-IgE-терапии. Омализумаб – гуманизированное моноклональное антитело, полученное на основе рекомбинантной ДНК и селективно связывающееся с иммуноглобулином (IgE). Омализумаб представляет собой IgG1 каппа антитело, которое содержит человеческую структурную основу с определяющими комплементарность участками мышиного антитела, связывающими IgE [25]. Остатки мышиного происхождения составляют менее 5% молекулы омализумаба, что минимизирует потенциал иммунного ответа и риск нежелательных побочных эффектов.
Основной механизм действия омализумаба заключается в связывании свободного IgE в крови, что способствует снижению экспрессии FcεRI-рецепторов на поверхности тучных клеток и базофилов. Предотвращение фиксации IgE с FcεRI на тучных клетках и базофилах позволяет избежать высвобождения медиаторов из этих клеток после контакта с аллергеном, а также приводит к:
- уменьшению числа самих рецепторов, сокращению количества базофилов и выживаемости тучных клеток;
- выключению IgE-опосредованного представления аллергена дендритными клетками T-лимфоцитам;
- снижению высвобождения ИЛ-4 и ИЛ-5 и, следовательно, уменьшению их концентрации [26–28].
Омализумаб обладает дополнительным антивирусным эффектом за счет регулирования экспрессии интерферонов плазмоцитоидным дендритными клетками посредством уменьшения перекрестного связывания IgE с FcεRI дендритных клеток [29, 30]. Доказано влияние этого ГИБП на эозинофильное воспаление дыхательных путей у больных БА: 16 недельная терапия омализумабом пациентов с легкой и среднетяжелой БА приводила к уменьшению уровня IgE в слизистой бронхов, экспрессии FcεRI и маркеров эозинофильного воспаления (числа эозинофилов в индуцированной мокроте и слизистой бронхов) [31].
Кроме того, как уже упоминалось выше, омализумаб может снижать выраженность ремоделирования бронхиальной стенки у пациентов с БА. Этот эффект препарата был доказан при минимальной продолжительности лечения 16 нед [22], однако у большего числа пациентов влияние на ремоделирование было показано через 1 год непрерывной терапии [23, 32]. Mauri P. et al. было установлено, что среди больных БА, получавших терапию омализумабом, есть те, у которых наблюдается редукция ремоделирования бронхиальной стенки (уменьшение толщины ретикулярной мембраны), и те, у которых этот процесс не наблюдается; при этом указанный эффект не коррелировал с клиническим улучшением, которое было отмечено у всех пациентов [33]. Исследователи выявили разницу между пациентами «ответчиками» и «не ответчиками» в отношении влияния на ремоделирование дыхательных путей: основные различия заключались в белках гладких мышц и внеклеточного матрикса. Омализумаб подавляет белки гладких мышц бронхов при тяжелой БА. Примечательно, что IgE-связывающий белок (галектин-3) был надежным, стабильным и прогностическим биомаркером модуляции ремоделирования бронхов [33].
Таким образом, все эти данные говорят в пользу того, что омализумаб может оказывать модифицирующее действие на компоненты ремоделирования дыхательных путей при тяжелой аллергической БА, а это, в свою очередь, позволяет предполагать болезнь-модифицирующее действие омализумаба. Вероятность такого эффекта показана в некоторых небольших клинических исследованиях, выполненных у детей с БА. Так, 4-летнее наблюдение за группой детей (n=7) с неконтролируемой средней/тяжелой астмой, принимавших участие в рандомизированном двойном слепом плацебо-контролируемом клиническом исследовании эффективности омализумаба, показало: после отмены анти-IgE терапии в течение первых 3 лет у них не было симп-томов астмы и необходимости применять ИГКС или препараты для купирования симптомов [34].
В ходе другого небольшого исследования изучались клинические и клеточные изменения у 18 пациентов, которые прекратили анти-IgE терапию примерно через 6 лет. В нем были получены доказательства возможности изменения прогрессирования астмы: клиническое улучшение сохранялось даже спустя 3 года после прекращения лечения омализумабом, у пациентов было отмечено снижение чувствительности базофилов к аллергену в течение 1 года [35].
Помимо улучшения контроля и профилактики обострений, омализумаб, по-видимому, имеет потенциал для изменения естественного течения аллергической БА, и этот эффект более вероятен при длительном (более 3–5 лет) лечении, особенно у детей, подростков и молодых взрослых. При этом, безусловно, требуется больше исследований, доказывающих возможность изменить естественное течение заболевания.
КЛИНИЧЕСКАЯ ЭФФЕКТИВНОСТЬ И БЕЗОПАСНОСТЬ ОМАЛИЗУМАБА ПРИ СРЕДНЕТЯЖЕЛОЙ И ТЯЖЕЛОЙ АЛЛЕРГИЧЕСКОЙ БРОНХИАЛЬНОЙ АСТМЕ
Омализумаб – единственный биологический препарат, зарегистрированный в России для лечения персистирующей атопической БА среднетяжелого и тяжелого течения, симптомы которой недостаточно контролируются применением ИГКС, у пациентов 6 лет и старше [25]. Это первый ГИБП для лечения БА, применение которого насчитывает более 15 лет в мировой общеклинической практике и более 10 лет в педиатрии.
Омализумаб показал высокую эффективность в рандомизированных клинических исследованиях и реальной практике. Обобщенные данные метаанализов и систематических обзоров применения омализумаба при БА у детей и взрослых показали [36–40]:
- уменьшение дневных и ночных симптомов БА и улучшение контроля;
- снижение частоты обострений и госпитализаций;
- улучшение функции легких;
- повышение качества жизни;
- возможность снижения или полной отмены ОКС;
- снижение дозы ИГКС.
При этом во всех исследованиях был отмечен благоприятный профиль безопасности препарата [36–40]. В частности, метаанализ исследований, посвященных реальному клиническому опыту применения омализумаба у взрослых пациентов с ТБА (25 исследований с участием 9213 пациентов из 32 стран), продемонстрировал, что более 70% пациентов ответили на терапию омализумабом, причем у всех у них было отмечено статистически значимое улучшение контроля астмы [36].
В недавнем систематическом обзоре (42 исследования реальной клинической практики, включившие 9377 пациентов из 35 стран и опубликованные в 2008–2018 гг.) была проведена оценка краткосрочной и долгосрочной эффективности омализумаба в терапии тяжелой аллергической астмы [41]. Согласно результатам этого обзора, через 23–32 мес терапии омализумабом [41]:
- доля ответивших на лечение составила 81%;
- на 60% уменьшилось число пациентов с обострениями БА, на 41% – число пациентов с дневными симптомами БА, на 69% – с ночными симптомами БА;
- у более половины пациентов улучшился контроль БА, оцененный по вопроснику АСТ (тест по контролю астмы);
- качество жизни возросло на 20% (вопросник по оценке качества жизни пациента с астмой, AQLQ);
- 57% пациентов отменили или снизили дозу ИГКС, 83% – ОКС.
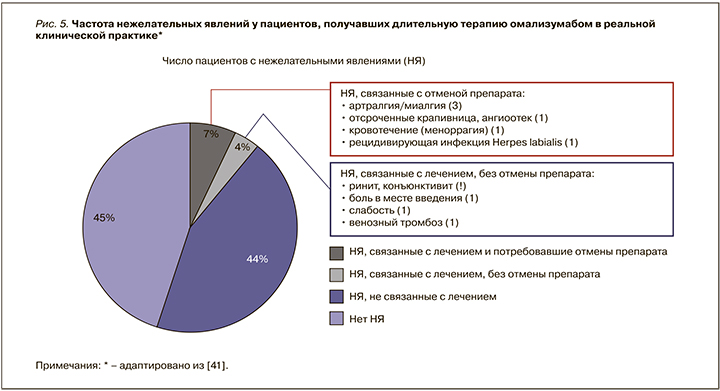
Частота развития нежелательных реакций у пациентов, получавших омализумаб, была сопоставима с таковой в группе плацебо. Из нежелательных явлений наиболее частыми (1–10%) были боль, отек, эритема и зуд в месте введения препарата, а также головные боли. Среди серьезных нежелательных эффектов препарата встречаются анафилактические реакции (0,2%) в течение 2 ч после введения препарата, что требует прекращения лечения [25, 39]. Оптимальный профиль безопасности омализумаба описан в 9-летнем исследовании реальной клинической практики, в которое был включен 91 пациент с трудно контролируемой астмой, получившие 10 472 инъекции омализумаба [42]. Максимальная продолжительность терапии омализумабом составила 9 лет. Частота различных нежелательных явлений, обнаруженных в процессе терапии омализумабом, представлена на рисунке 5. Важно, что после 10 472 инъекций не было отмечено ни одной реакции немедленного типа (анафилаксии). Что касается нежелательных явлений, не связанных с лечением и повлекших отмену препарата, то они включали низкую приверженность лечению, беременность, субъективное ощущение отсутствия эффективности, рецидив воспалительного заболевания кишечника [42].
ЗАКЛЮЧЕНИЕ
Доля пациентов с ТБА, рефрактерных к традиционной фармакотерапии, не превышает 10% в общей структуре заболевания, однако у них отмечается высокая частота обострений БА, незапланированных визитов к врачу, обращений за неотложной медицинской помощью и госпитализаций. Появление биологических препаратов, которые нацелены на основные цитокины или IgE, участвующие в формировании эозинофильного воспаления дыхательных путей, открыло новые возможности достижения контроля заболевания у этих пациентов.
Пациенты, относящиеся к фенотипу тяжелой аллергической БА, составляют по крайней мере половину всех больных ТБА. Ключевым запускающим и поддерживающим элементом каскада аллергического воспаления, развивающегося в нижних дыхательных путях при аллергической БА, выступает IgE. Помимо участия в ранней и поздней фазе аллергического воспаления, важным аспектом его патогенетического участия в астме является влияние на процесс ремоделирования.
В исследованиях in vitro показано, что IgE в дозозависимой манере повышает депозицию экстрацеллюлярного матрикса, общего коллагена (коллагена I и III типа) и депозицию фибронектина, стимулирует пролиферацию гладкомышечных клеток у пациентов с БА. При этом предварительная (30–60 мин) обработка клеток анти-IgE-антителами (омализумаб) уменьшает эти процессы у больных БА.
Испытания in vitro подтверждены клиническими исследованиями, показавшими, что добавление омализумаба к обычной терапии астмы приводило к значительному уменьшению толщины стенки дыхательных путей и ретикулярной базальной мембраны. Существующие данные свидетельствуют, что омализумаб может оказывать модифицирующее действие на компоненты ремоделирования дыхательных путей при тяжелой аллергической БА, что, в свою очередь, позволяет предполагать его болезнь-модифицирующее действие.
Омализумаб, помимо улучшения контроля и профилактики обострений, по-видимому, имеет потенциал для изменения естественного течения аллергической БА, и этот эффект более вероятен при длительном (более 3–5 лет) лечении, особенно у детей, подростков и молодых взрослых. Для изучения этого аспекта действия омализумаба требуется проведение большего количества исследований.


