Новая коронавирусная инфекция, поразившая мир в 2020 г., бросила вызов всему мировому медицинскому сообществу. В России, как и в других странах, пандемия COVID-19 изменила приоритеты в работе системы здравоохранения, усилия федеральных, городских, а также частных медицинских организаций сейчас направлены главным образом на противостояние распространению болезни. В кратчайшие сроки были проведены мероприятия по перераспределению соответствующих ресурсов, оборудования, обучению, в том числе и дистанционному, врачей разных специальностей для лечения пациентов с COVID-19. Разработка эффективных мер по организации медицинской помощи населению России в условиях пандемии и предупреждению распространения COVID-19 являются важнейшими задачами, стоящими перед клиницистами, учеными и представителями фармацевтической промышленности всего мира.
Коронавирусы (Coronaviridae) – это большое семейство РНК-содержащих вирусов, способных инфицировать как животных (их естественных хозяев), так и человека. По результатам серологического и филогенетического анализа коронавирусы разделяются на четыре рода: Alphacoronavirus, Betacoronavirus, Gammacoronavirus и Deltacoronavirus. У людей коронавирусы могут вызвать целый ряд заболеваний – от легких форм острой респираторной вирусной инфекции (ОРВИ) до тяжелого острого респираторного синдрома (ТОРС или SARS). В настоящее время среди населения циркулируют 4 сезонных коронавируса (HCoV-229E, -OC43, -NL63 и -HKU1), которые круглогодично присутствуют в структуре ОРВИ и, как правило, вызывают поражение верхних дыхательных путей легкой и средней степени тяжести, а также два высокопатогенных коронавируса – вирус ближневосточного респираторного синдрома (MERS) и новой коронавирусной инфекции SARS-CoV-2 [1].
Изначально SARS-CoV-2 воспринимался как «респираторный» вирус, поскольку в качестве основных «входных ворот» возбудителя рассматривался эпителий верхних дыхательных путей, а основным поражаемым органом – альвеолы, в частности альвеолоциты 2 типа, которые осуществляют синтез сурфактанта, лизоцима, интерферона, нейтрализацию оксидантов, транспорт воды и ионов и ряд других важных функций. Несмотря на то что альвеолоциты-2 занимают только 1/20 поверхности альвеол, они определяют баланс воздушности и гидратации легочной ткани. Это наиболее метаболически активные клетки, что делает их привлекательными для репродукции вирионов COVID-19 и в итоге наиболее уязвимыми в процессе инфекционного воспаления с развитием острого респираторного дистресс-синдрома (ОРДС).
Однако очень быстро стало понятно, что инфекция поражает не только легкие, но и другие органы и ткани, а также запускает целый каскад патологических процессов в рамках системного воспалительного ответа с поражением других органов и систем, развитием в ряде случаев полиорганной недостаточности и летального исхода. Вероятность трансформации локального иммунного ответа на внедрение вируса в альвеолоциты в системную воспалительную реакцию с развитием «цитокинового шторма», по-видимому, опосредована целым рядом факторов (экзо- и эндогенных, в том числе генетических) и до настоящего времени не поддается точной оценке. Диффузное альвеолярное повреждение и повреждение сосудов легких приводит у 3–4% пациентов к дальнейшему скоплению жидкости, разрушению альвеолярных пузырьков, затруднению газообмена и манифестации ОРДС. Критическая форма коронавирусной инфекции, опосредованная развитием «цитокинового шторма» с гиперактивацией системы врожденного и приобретенного иммунитета и гиперпродукцией провоспалительных цитокинов, клинически протекает по типу синдрома активации макрофагов. Внелегочные проявления COVID-19 включают специфическое поражение различных отделов желудочно-кишечного тракта (ЖКТ), сосудов (эндотелия) с развитием диссеминированного тромбоза, а также нарушения со стороны миокарда, печени, почек, клеток иммунной системы, гипогликемию и кетоацидоз, неврологические и нейротоксические осложнения, кожные реакции [2–4]. Столь широкий спектр клинических проявлений связан не только с тем, что «мишенями» вируса служат клетки, содержащие рецептор ангиотензинпревращающего фермента 2 типа (АПФ- 2), широко представленный в различных органах и тканях, но и с особенностями самого вируса. Неструктурный белок коронавируса (ORF3a), непосредственно активирующий NLRP3 инфламмасому через NF-κB-зависимый путь [5], выводит иммунный компонент заболевания, наряду с поражением легких, на передний план.
На настоящий момент патогенез коронавирусной инфекции в основном предполагает два пути попадания SARS-CoV-2 в клетку: путем соединения спайк-белка S с рецептором к ферменту АПФ-2 или трансмембранным гликопротеином CD147. Трансмембранная сериновая протеаза 2 типа (TMPRSS2) ускоряет проникновение вируса в клетку [9]. Стоит отметить, что рецептор CD147 относится к семейству иммуноглобулинов, что может быть важно для разработки или выбора средств, блокирующих проникновение вируса в организм.
Путь проникновения вируса в клетки можно представить следующим образом [6]:
- S-белок коронавируса по своей структуре имитирует АПФ-2;
- благодаря этому вирусные частицы успешно связываются с рецепторами АПФ-2 (их много на поверхности клеток легких — альвеолоцитов);
- после этого вирусные частицы впрыскивают свою РНК внутрь клетки;
- взаимодействие вируса с этими рецепторами осуществляется посредством субъединицы S2 через гептад-повторы 1 и 2 (HR1 и HR2);
- молекулы, обеспечивающие инвагинацию клеточной мембраны с комплексом вирус-рецептор, не известны.
При попадании SARS-CoV2 внутрь клетки запускается процесс репликации. Вначале синтезируются вирусные полипротеины, которые кодируют комплекс репликаза-транскриптаза. Далее посредством (при участии) фермента РНК-зависимой-РНК-полимеразы происходит синтез вирусной РНК и синтезируются структурные белки, что приводит к завершению сборки и высвобождению вирусных частиц [10–12]. Вирус собирается несколькими независимыми частями, после этого везикулы, содержащие вирион, сливаются с плазматической мембраной и происходит выделение вируса. По неопубликованным данным, вирус также использует лизосомы клетки для активного экзоцитоза, предварительно защелачивая их, чтобы избежать деструкции РНК в кислой среде.
В отличие от других патогенных коронавирусов и РНК-вирусов, вызывающих сезонное ОРВИ и грипп, SARS-CoV-2 имеет длительный инкубационный период (до 14 дней), размножаясь на эпителиоцитах верхних дыхательных путях первоначально бессимптомно. Через несколько суток латентного периода в эпителиоцитах и альвеолоцитах развиваются нарушения метаболических процессов, постепенно приводящие к их дисфункции и гибели с развитием ОРДС [7]. При этом параллельно с уменьшением процессов репликации вируса нарастает тяжесть клинических симптомов, иммунные и тромбогенные нарушения. Обобщенная схема развития коронавирусной инфекции представлена на рисунке 1.
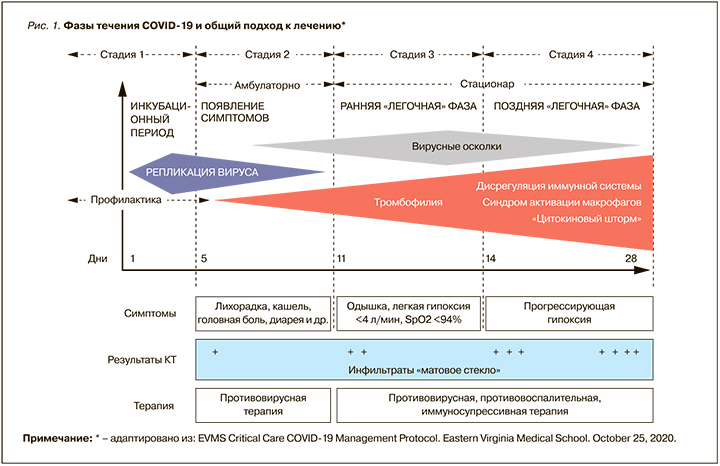
Таким образом, симптомы COVID-19 проявляются лишь при высоком уровне вирусной нагрузки, что определяет необходимость как можно более раннего начала противовирусной терапии для снижения риска развития осложнений.
ОСОБЕННОСТИ ЭТИОТРОПНОЙ ТЕРАПИИ COVID-19
В условиях отсутствия на фармрынке лекарственных препаратов, разработанных специально для лечения новой коронавирусной инфекции, единственным вариантом этиотропной терапии COVID-19 является «репозиционирование» имеющихся противовирусных средств и выбор наиболее перспективных молекул с учетом механизма их действия, структурных и молекулярных характеристик, а также особенности жизнедеятельности SARS-CoV-2 в организме. Перспективными мишенями для лекарственного воздействия, по мнению ведущих экспертов, служат неструктурные белки (например, 3-химотрипсин-подобная протеаза, папаиноподобная протеаза, РНК-зависимая РНК-полимераза), сходные с белками других новых коронавирусов (nCoV). Дополнительно рассматриваются мишени, препятствующие проникновению вирусов и молекулы, регулирующие степень иммунного ответа [13, 14] (рис. 2).
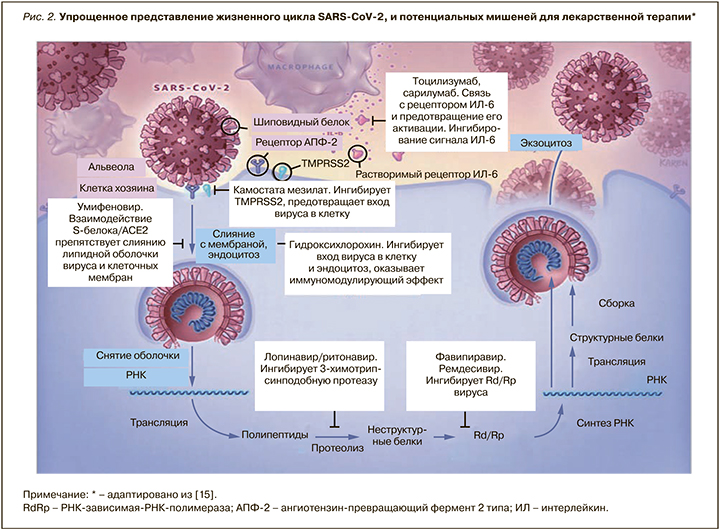
Особый интерес в качестве мишени для направленной противовирусной терапии представляет РНК-зависимая РНК-полимераза; она отсутствует в животных клетках, и ее ингибирование должно обеспечить селективных противовирусный эффект. Ремдесивир – ингибитор РНК-зависимой РНК-полимеразы (РзРп) – одобрен для лечения COVID-19 тяжелого течения и должен применяться у кислород-зависимых пациентов. Однако внутривенный путь введения и высокая стоимость ограничивают его массовое использование.
Другим ингибирующим вирусную РНК препаратом, чья эффективность в настоящее время интенсивно изучается по всему миру, является фавипиравир. Направленное действие в отношении вирусной РНК и удобный пероральный способ приема позволяют отнести это лекарственное средство к наиболее перспективным препаратам для борьбы с пандемией коронавирусной инфекции [2].
ФАВИПИРАВИР: МЕХАНИЗМ ДЕЙСТВИЯ И ЭФФЕКТИВНОСТЬ ПРИ COVID-19
Фавипиравир (6-флуоро-3-гидрокси-2-пираз—инкарбоксамид) представляет собой синтетический селективный ингибитор РНК-зависимой РНК-полимеразы [16]. Он проявляет активность в отношении штаммов вируса гриппа типа А, В и С, включая резистентные к другим противовирусным средствам, таким как ингибиторы нейроаминидазы и М2 [17–20], а также в отношении других РНК вирусов, трудно поддающихся лечению [21–27]. Препарат был впервые синтезирован и одобрен в Японии в 2014 г. и используется в качестве резервного средства для лечения тяжелого гриппа и тромбоцитопенического синдрома.
В связи с высоким уровнем смертности, наблюдаемой при вспышке инфекций, вызванных новыми типами вирусов (53,5% для вируса гриппа А (H5N1) и 34,9% для вируса гриппа А (H7N9)) фавипиравир успешно применяли в виде монотерапии и в комбинации с осельтамивиром во время очередной эпидемии в Китае [28]. Во время эпидемии лихорадки Эболы экспериментальное применение фавипиравира позволило добиться значимого снижения смертности и повышения выживаемости при хорошей переносимости, в связи с чем этот препарат еще до завершения всех необходимых клинических исследований был выбран и успешно использован для постконтактной профилактики и лечения пациентов с лихорадкой Эбола в 2014 и 2016 г. [17].
Фавипиравир, будучи пролекарством, метаболизируется до рибофуранозил 5’-трифосфата (фавипиравир-РТФ) [29, 30]. Активная форма фавипиравира селективно взаимодействует с РзРп и действует в двух направлениях. С одной стороны, фавипиравир-РТФ может включаться в растущую цепь вирусной РНК или связываться с сохраненными доменами полимеразы, что предотвращает репликацию вирусной РНК. Этот комплекс РНК-фавипиравир-РТФ-(-РзРп) не восстанавливается корректирующим ферментом и утилизируется как ненужная РНК, что приводит к исчезновению вирусного генома и снижению эффективности заражения. С другой стороны, в исследованиях на вирусе гриппа было показано, что фавипиравир приводит к летальному мутагенезу вирусного генома, т.е. запускает процессы самоуничтожения вирусных частиц, резко снижая вирусные титры в культуре клеток и ускоряя элиминацию вируса [31]. Стоит отметить, что за все время проведения многолетних исследований данного вещества не выявлено ни одного вирусного штамма, резистентного к фавипиравиру. Исходя из выше описанного механизма действия, фавипиравир может индуцировать мутагенез, так же как и ингибирование РзРп, и в других РНК-содержащих вирусах, что делает его мощным, эффективным и универсальным ингибитором целой группы эпидемиологически значимых вирусов различных семейств, вызывающих серьезные, часто смертельные заболевания [32–34].
Для фавипиравира сначала на культурах клеток [35], а затем и в клинических исследованиях была продемонстрирована выраженная эффективность против SARS-CoV-2. Так, в Китае в рамках рандомизированного, контролируемого мультицентрового исследования с участием 240 пациентов с диагностированной пневмонией, ассоциированной с COVID-19, эффективность этого препарата оценивалась в сравнении с умифеновиром [36]. Больные первой группы в дополнение к стандартной терапии получали фавипиравир (по 1600 мг 2 раза/ сут в первый день, затем по 600 мг 2 раза/ сут в течение 10 дней), второй группы – умифеновир (по 200 мг 3 раза/сут 10 дней). Были получены значимые преимущества фавипиравира по таким параметрам, как количество пациентов, достигших выздоровления на 7-й день (61,2 против 51,6% среди всех больных и 71,43 против 55,86% среди пациентов без артериальной гипертензии и сахарного диабета), сокращение длительности лихорадки (разница 1,7 дней, р <0,0001) и кашля (разница 1,75 дня; р <0,0001) (рис. 3).
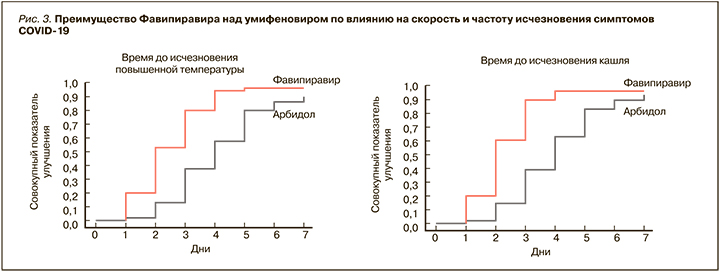
В группе пациентов, принимавших фавипиравир, включая больных с артериальной гипертензией и сахарным диабетом, также наблюдалась меньшая потребность в кислородной поддержке и неинвазивной механической вентиляции легких, реже регистрировались нарушения дыхания. Терапия обоими препаратами характеризовалась сопоставимым профилем безопасности. Не было отмечено серьезных нежелательных явлений, а большинство из них проходили до момента выписки пациентов из стационара [36].
В другом открытом сравнительном исследовании с участием 80 пациентов эффективность терапии фавипиравиром (по 1600 мг в два раза в 1 день, затем по 600 мг 2 раза/сут в течение 13 дней) сравнивалась с лопинавиром/ритонавиром [37]. В обеих группах пациенты дополнительно получали интерферон-альфа (ИФН-α) в виде ингаляций. Фавипиравир обеспечил более короткий период элиминации вируса (4 и 11 дней соответственно; p <0,001) в сочетании с более выраженными улучшениями состояния легких (91,43% пациентов против 62,22%, p <0,01). Авторы приводят в публикации результаты компьютерной томографии (КТ) органов грудной клетки 56-летней пациентки с COVID-19, принимавшей фавипиравир, иллюстрируя высокую эффективность препарата в плане уменьшения объема инфильтративных изменений в легких (рис. 4).

Более выраженная положительная динамика на фоне терапии фавипиравиром была отмечена и после поправки на наличие отягощающих прогноз коморбидных состояний (возраст, артериальная гипертензия, сахарный диабет и др.) и тяжести состояния. Терапия фавипиравиром характеризовалась лучшей переносимостью и меньшей частотой возникновения нежелательных явлений (11,43% по сравнению с 55,56%; p <0,01). Авторы работы сделали вывод о большей клинической эффективности фавипиравира в лечении COVID- 19 в отношении прогрессирования заболевания и клиренса вируса и рекомендовали включить это лекарственное средство в клинический протокол лечения новой коронавирусной инфекции [37].
Регистрационное исследование эффективности применения фавипиравира у пациентов с COVID-19 завершилось в сентябре в Японии. В нем также было показано, что применение фавипиравира у пациентов с неосложненной COVID-19 ассоциированной пневмонией, клинически значимо ускоряет время до выздоровления (ОР 1,593; 95% ДИ 1,024–2,479; p=0,0136) [38].
Помимо результатов оценки эффективности фавипиравира в клинических исследованиях, ограниченных рамками протокола, отдельный интерес представляет изучение его применения в реальной клинической практике. В Японии получены предварительные результаты применения фавипиравира у 2158 пациентов с новой коронавирусной инфекцией различной степени тяжести, в том числе с сопутствующими сердечно-сосудистыми заболеваниями, сахарным диабетом, хронической болезнью легких и др. Улучшение клинического статуса наблюдалось на 7-й день лечения у 73,8; 66,6 и 40,1% пациентов с легким, среднетяжелым и тяжелым течением болезни соответственно. При этом уровень летальности в течение месяца от начала терапии фавипиравиром составил 12,7 и 31,7% для среднего и тяжелого течения соответственно. В то же время у интубированных пациентов летальность, по мировым данным, обычно достигает около 80%. Таким образом, логично предположить снижение потребности в искусственной вентиляции легких (ИВЛ) и уменьшение частоты летальных исходов при терапии фавипиравиром. Среди наиболее частых нежелательных явлений препарата отмечали гиперурикемию (15,52%) и изменение концентрации печеночных ферментов (7,37%) [39].
Схожие результаты были получены при анализе результатов терапии 247 пациентов с COVID-19 (из которых 63 получали фавипиравир), госпитализированных в 5 лечебных учреждений в Таиланде, где препарат был одобрен к применению в феврале 2020 г. В исследуемой группе 42,9% пациентам на старте терапии требовалась кислородная поддержка, включая использование ИВЛ. На 7-й день лечения среди всех пациентов, получавших фавипиравир на фоне стандартной терапии, клинического улучшения достигли 66,7%, в том числе 92,5% пациентов, не нуждавшихся в кислородной поддержке, и 47,2% пациентов с кислородной поддержкой. На 14-й день терапии аналогичные показатели составили уже 85,7; 100 и 75% соответственно. Среди пациентов, нуждавшихся в кислородной поддержке, клиническое улучшение зарегистрировано у 96,1% на 28-й день терапии. На фоне терапии фавипиравиром не было отмечено серьезных нежелательных явлений. Авторы признают, что фавипиравир – перспективный препарат для лечения COVID-19. Более того, в мае 2020 г. регуляторные органы Таиланда одобрили его применение не только при тяжелом, но и легком течении пневмонии (наличие поражения легких по данным рентгенографии, но удовлетворительная сатурация), так как раннее начало направленной противовирусной терапии является одним из предикторов успешного лечения [40].
Стоит обратить внимание на вопросы безопасности применения фавипиравира. Препарат прошел все необходимые исследования и относится к 5-й (высшей) категории безопасности, согласно классификации в Глобальной гармонизированной системе (GHS) [30, 41]. Синтез РНК – неотъемлемая часть жизни человеческой клетки. В отличие от вирусов у людей нет РзРп, однако есть ДНК-зависимая РНК-полимераза (ДзРп), а также ДНК-зависимая ДНК-полимераза. Было показано, что фавипиравир-РТФ блокирует RdRp гриппа при IC50 0,341 µмоль/л, но не оказывает никакого ингибирующего действия на ДНК-полимеразу α, β, γ в концентрациях до 1000 µмоль/л. В концентрациях 637 µмоль/л in vitro в клетках MDCK фавипиравир не блокировал ни синтез клеточной ДНК, ни синтез клеточной РНК, что говорит о его высокой эффективности и безопасности [17].
В клинических исследованиях показано, что фавипиравир обладает предсказуемым и сопоставимым профилем безопасности с другими широко применяемыми противовирусными средствами. Так, при анализе 29 клинических исследований, включавших в общей сложности 4299 больных, было продемонстрировано, что у пациентов, принимавших фавипиравир, и пациентов, принимавших такие лекарственные средства, как осельтамивир, умифеновир, комбинация лопинавир + ритонавир (группа сравнения), частота возникновения нежелательных явлений составила 28,2 и 28,4% соответственно, частота прекращения лечения из-за нежелательных явлений – 1,1 и 1,2%, а частота возникновения серьезных нежелательных явлений – 0,4% для обеих групп. При этом на фоне приема фавипиравира отмечалось значительно меньшее по сравнению с другими противовирусными средствами число нежелательных явлений со стороны желудочно-кишечного тракта (тошнота, диарея, рвота и др.): 8,7 и 11,5% соответственно [42]. Стоит отметить, что обнаружено лекарственное взаимодействие фавипиравира с парацетамолом, поэтому рекомендация по консервативной терапии для пациентов, принимающих фавипиравир, заключается в ограничении суточной дозы парацетамола до 3,0 г и менее (обычно максимальная доза этого простого анальгетика составляет 4,0 г/сут) [43].
При лечении лихорадки Эболы в связи с высокой вирусной нагрузкой и высоким риском летального исхода фавипиравир применялся в очень высоких дозах (6000 мг в день 1 и 2400 мг со 2 по 9 день). При этом ни одному пациенту не потребовалось прекращение лечения из-за возникновения нежелательных явлений, что свидетельствует о хорошей переносимости препарата [25].
Важно отметить, что в экспериментах на животных было обнаружено негативное влияние фавипиравира на жизнеспособность и подвижность сперматозоидов, а также увеличение степени преимплантационных потерь у самок, получавших фавипиравир [30]. В связи с полученными данными фавипиравир противопоказан к применению у беременных и кормящих женщин. Перед использованием препарата необходимо получить отрицательный тест на беременность и пользоваться надежными контрацептивными средствами весь период приема препарата и в течение 1 мес для женщин и 3 мес для мужчин после окончания курса терапии. Подобные противопоказания характерны для многих лекарственных средств, в том числе противовирусных (более 300 противовирусных препаратов относятся к категориям С, D и X по классификации FDA). В свете же текущей пандемии новой коронавирусной инфекции стоит отдельно отметить, что широко применяемый антималярийный препарат хлорохин также обладает доказанным негативным действием на плод (ретино-, ото- и нейротоксичностью), что ограничивает его прием у рассматриваемой категории женщин [44, 45]. Таким образом, мировые данные показывают высокую эффективность и предсказуемый профиль безопасности терапии фавипиравиром.
В нашей стране фавипиравир рекомендован в качестве этиотропной терапии коронавирусной инфекции как в амбулаторно-поликлинической практике, так и в условиях стационара при любой степени тяжести заболевания в соответствии с Временными методическими рекомендациями «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)» (версия 9) Минздрава России. Важно отметить, что фавипиравир сочетается со всеми препаратами патогенетической терапии, указанными в этих рекомендациях.
В Российской Федерации зарегистрировано несколько торговых наименований фавипиравира, в том числе препарат Арепливир (таблетки 200 мг) [46]. Эффективность и безопасность приема Арепливира, в сравнении со стандартной терапией у пациентов, госпитализированных с COVID-19, доказана в рамках многоцентрового клинического исследования, которое было проведено в 5 городах России (Москва, Саранск, Смоленск, Санкт-Петербург, Рязань) на базе лечебных учреждений, занимающихся лечением новой коронавирусной инфекции [47]. В исследовании приняли участие 200 пациентов мужского и женского пола в возрасте от 18 до 80 лет включительно, госпитализированные с диагнозом «коронавирусная инфекция, вызванная SARS-CoV-2 (подтвержденная)» и рандомизированные в основную и контрольную группы после подписания формы информированного согласия.
У 101 пациента (49,03%), включенного в исследование, были выявлены различные сопутствующие заболевания, включая артериальную гипертензию (n=57), ишемический инсульт и инфаркт в анамнезе (n=12), сахарный диабет 2 типа (n=18), заболевания ЖКТ (n=15), бронхиальная астма и легочный саркоидоз (n=10) и др. Таким образом, в исследование, наряду с другими больными, вошли пациенты с высоким риском развития жизнеугрожающих состояний и ухудшения прогноза. У 32,7% пациентов основной группы и 47,1% пациентов контрольной группы наблюдались отклонения от нормы по ЭКГ. Сравнительный анализ сопутствующих заболеваний и общего состояния пациентов также не выявил межгрупповых различий.
Пациенты основной группы принимали Арепливир по схеме: в 1-й день терапии по 1600 мг (8 таб.) 2 раза/сут, во 2–14-й дни по 600 мг (3 таб.) 2 раза/сут с интервалом в 12 ч. Пациенты контрольной группы получали препараты стандартной терапии в соответствии с рекомендованными схемами лечения, представленными во Временных методических рекомендациях Минздрава России по профилактике, диагностике и лечению новой коронавирусной инфекции (COVID-19) (в основном гидроксихлорохин в комбинации с азитромицином и комбинация ритонавир/лопинавир).
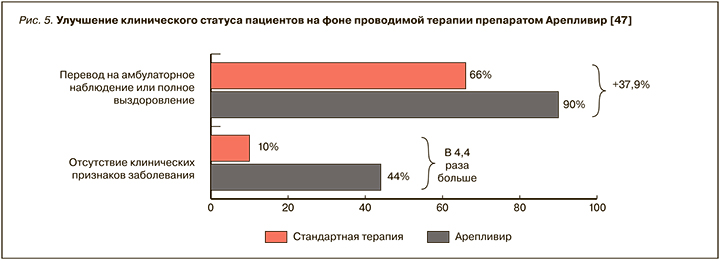
Полученные результаты показывают, что Арепливир позволяет достичь улучшения клинического статуса пациентов по категориальной шкале ВОЗ на 4 дня раньше, чем стандартная терапия (p <0,0001), и обеспечить высокую скорость элиминации вируса (по данным ПЦР) у 98% пациентов. Отсутствие клинических признаков заболевания на 10-й день терапии в основной группе больных наблюдалось в 4,4 раза чаще, чем в контрольной группе. В связи со значительным улучшением состояния 90% пациентов основной группы были выписаны из стационара, тогда как в контрольной группе этот показатель составил 66% (рис. 5).
Добавим, что, помимо улучшения состояния легких, в основной группе отмечалась нормализация уровня насыщения крови кислородом уже на 5-й день терапии (SpO2 >96%), что позволяет считать минимально возможным ухудшение прогноза и риск развития ОРДС-синдрома. В группе стандартной терапии подобный уровень сатурации наблюдался только к 10-му дню терапии.
Динамика клинических показателей может считаться более объективным показателем эффективности этиотропной терапии, чем данные об элиминации вируса, поскольку даже при отсутствии возбудителя в ротоглотке может иметь место прогрессирование пневмонии и ухудшение общего состояния. На фоне терапии фавипиравиром снижение температуры тела ниже 37,2 °C у большинства пациентов наблюдалось уже на 3 день лечения (p=0,008), причем результаты сравнительного анализа показывают, что при межгрупповом сравнении различия являются клинически значимыми. Ускорение избавления от лихорадки и общее улучшение клинического статуса в основной группе способствуют повышению качества жизни пациента, что, безусловно, является важным и для улучшения прогноза заболевания. Также ускорение нормализации температуры тела позволяет снизить потребность в приеме жаропонижающих средств, что способствует снижению риска возникновения НЯ со стороны печени и ЖКТ, и уменьшить лекарственную нагрузку на организм в целом.
Важным достижением является положительная динамика вплоть до нормализации лабораторных показателей, в частности С-реактивного белка, т.е. снижения уровня воспаления. Стоит отметить, что большинство пациентов отмечали улучшение состояния уже после первого дня применения препарата. Это может быть связано с тем, что высокая доза препарата, принимаемая в первые сутки, обеспечивает эффективное снижение виремии и «отражение» массивной вирусной атаки.
Результаты исследования подтвердили гипотезу о преимуществе применения фавипиравира у пациентов с COVID-19 по сравнению со стандартной схемой терапии. Лечение исследуемым препаратом характеризовалась положительным профилем безопасности: не было выявлено межгрупповых различий в отношении наличия, частоты, тяжести нежелательных явления, причинно-следственной связи с терапией и исходом. Ни у одного пациента основной группы не были выявлены серьезные нежелательные явления, связанные с приемом указанного препарата. Не было обнаружено значимых различий в показателях артериального давления, частоты сердечных сокращений в начале и конце терапии и при их анализе между группами, а также не отмечено связанных с приемом фавипиравира отклонений от нормы показателей ЭКГ, в частности удлинения интервала QTс. Также в настоящем исследовании не было зарегистрировано повышения уровня мочевой кислоты на фоне лечения фавипиравиром.
Таким образом, в приведенном исследовании была доказана эффективность терапии Арепливиром в отношении основных клинических точек терапии (скорость и частота элиминации вируса, улучшение лабораторных показателей, состояние легких по данным КТ, ускорение выписки из стационара и выздоровления), а также продемонстрировано, что польза от приема препарата превышает возможные риски.
НЕОБХОДИМОСТЬ НАЗНАЧЕНИЯ СОЧЕТАННОЙ ТЕРАПИИ ПРИ COVID-19
Учитывая, что SARS-CoV-2 приводит к полиорганным нарушениям, вызывая тотальную разбалансировку иммунной регуляции и системное воспаление, терапия такого состояния с полиорганными поражениями невозможна без воздействия на механизмы и системы, функционирование которых было нарушено за счет деятельности вируса в организме. Этого можно достичь с помощью использования иммуносупрессоров и медиаторов противовоспалительной активности интерлейкинов, таких как тоцилизумаб (антагонист рецептора интерлейкина-6) [48].
Некоторые авторы говорят о возможности применения в составе комплексной терапии эпигаллокатехин-3-галлата – ингибитора синтеза интерлейкина-6 (ИЛ-6), значительно понижающего его выработку и активность синовиальных фибробластов, поддерживающих системный иммунный ответ [49].
В недавних клинических исследованиях в одном из госпиталей Нью-Йорка была показана эффективность нового препарата сарилумаба (sarilumab), напрямую ингибирующего ИЛ-6 на этапе, когда макрофаги только запускают полномасштабный иммунный ответ, и вирус «раскручивает» инфламмасомную сигнализацию в организме [50]. Также в настоящее время доказано, что морфологическим маркером перехода заболевания в стадию тяжелого течения является фиброз и эндотелиолит, прогрессирующие в тромбоз, что делает необходимым применение пероральных и инъекционных антикоагулянтов в качестве патогенетической терапии при любой тяжести болезни [1, 51].
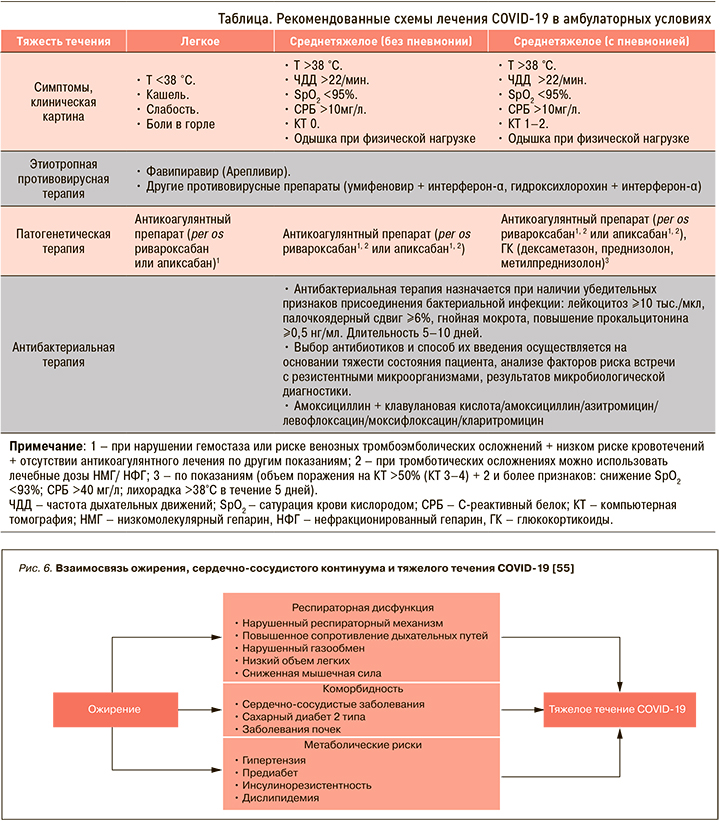
В настоящее время пациентов без определенных факторов риска не только с легким, но и среднетяжелым течением заболевания необходимо лечить в амбулаторных условиях. Рекомендованные схемы терапии представлены в таблице.
НЕКОТОРЫЕ АСПЕКТЫ ПРЕВЕНТИВНОЙ МЕДИЦИНЫ: МОДИФИЦИРУЕМЫЕ ФАКТОРЫ РИСКА БЫСТРО РАЗВИВАЮЩЕГОСЯ ИММУНОВОСПАЛИТЕЛЬНОГО СИНДРОМА
В рамках ведения пациентов с новой коронавирусной инфекции большое внимание должно уделяться модификации факторов риска развития и осложнений тяжелого течения заболевания. К заболеваниям, увеличивающим вероятность осложнений при COVID-19 и в несколько раз повышающим угрозу смерти, относится ожирение [52, 53].
За счет секреторной активности жировой ткани и ассоциированного хронического воспаления ожирение индуцирует и потенцирует множественные метаболические нарушения, приводя к развитию сердечно-сосудистых заболеваний и состояний, которые, в свою очередь, ухудшают прогноз пациентов с коронавирусной инфекцией (рис. 6) [54].
Французские исследования продемонстрировали, что риск тяжелого течения коронавирусной инфекции и потребность в нахождении в отделении реанимации и интенсивной терапии повышается в несколько раз у пациентов с ожирением по сравнению с общей популяцией. При этом частота использования ИВЛ более чем в 7 раз превышает показатели для лиц с индексом массы тела (ИМТ) >35 кг/м2 по сравнению с ИМТ <25 кг/м2 [56, 57].
Эти данные коррелируют с результатами других исследований. Так, анализ данных пациентов в возрасте до 60 лет в Нью-Йорке показал, что у лиц с ИМТ 30–34,9 и более 35 кг/м2 в 1,8 и 3,6 раз соответственно выше шансы попасть в отделение интенсивной терапии при COVID-19 в сравнении с пациентами с ИМТ <30 кг/м2 [58].
Обсуждаемая в научных кругах прямая связь между тяжелым течением COVID-19 и протромботической диссеминированной внутрисосудистой коагуляцией и большой частотой венозной тромбоэмболии также заставляет обратить внимание на ожирение и ассоциированное с этим состоянием повышение рисков тромбозов [59].
Ожирение и метаболический синдром общепризнано выступают источником хронического воспаления, поскольку сопровождаются продукцией провоспалительных цитокинов и увеличением уровня белков острой фазы. У пациентов с ожирением может наблюдаться интенсивное выделение вируса, что приводит к риску заражения других людей, в особенности когда несколько членов семьи имеют избыточный вес. Наблюдения указывают на потенциально неблагоприятную взаимосвязь между вирусом и иммунным ответом организма при ожирении. Иммунологическая дисрегуляция усиливается при эндокринных и метаболических нарушениях (нарушении чувствительности к инсулину, метаболизма липидов и жирных кислот) [60].
Одним из патогенетических механизмов ожирения является инсулинорезистентность, что впоследствии может приводить к нарушению функции бета-клеток, развитию нарушений углеводного обмена, в том числе к сахарному диабету, который напрямую связан с более тяжелым течением коронавирусной инфекции [61, 62]. COVID-19 способен разрушать бета-клетки поджелудочной железы, проникая в них посредством взаимодействия с AПФ-2.
Наконец, COVID-19 часто сопровождается гипокалиемией, что связано с подавлением легочной АПФ-2, снижением деградации ангиотензина-II и затем повышением секреции альдостерона. Гипокалиемия может ухудшать контроль гликемии у пациентов с сахарным диабетом 1 и 2 типа [63].
Понимание взаимоотягощающего влияния ожирения и COVID-19 определяет необходимость превентивных мер, направленных на уменьшение рисков не только неблагоприятных исходов коронавирусной инфекции, но и развития и прогрессирования хронических заболеваний и снижения общего иммунного ответа организма, а следовательно, актуализирует необходимость разработки комплексных программ, направленных на лечение ожирения и модификацию образа жизни.
Подход к лечению ожирения должен быть комплексным и включать изменение характера питания, сокращение размеров порций, калорийности еды и напитков, отказ от приема пищи непосредственно перед сном, избегание эпизодов компульсивного переедания [64].
Медикаментозная терапия показана пациентам с ИМТ >30 кг/м2 или больным с ИМТ >27 кг/м2 и сопутствующими заболеваниями. На современном фармрынке России для лечения ожирения одобрены орлистат, лираглутид 3 мг и сибутрамин (в том числе в форме фикисрованной комбинации с метформином) [65].
С учетом необходимости комплексного воздействия, которое направлено на компенсацию метаболических нарушений, ассоциированных с ожирением, необходимо дополнительное патогенетическое воздействие с целью снижения инсулинорезистентности, гиперинсулинемии, липо- и глюкозотоксичности. С патогенетической точки зрения препаратом первого выбора для лечения подобных состояний является метформин. Основные свойства этого препарата включают его способность влиять на инсулинорезистентность, глюконеогенез, уменьшать повышенную продукцию глюкозы печенью, тормозить всасывание глюкозы в тонком кишечнике. В результате терапии метформином улучшается чувствительность рецепторов к инсулину, уменьшается гиперинсулинемия, понижается аппетит, что также способствует снижению массы тела.
С учетом вышесказанного в качестве перспективного препарата для лечения ожирения и соответственно модификации одного из основных факторов риска развития осложненного течения новой коронавирусной инфекции можно рассматривать фиксированную комбинацию сибутрамина и метформина. В России зарегистрирован комбинированный препарат Редуксин® Форте, содержащий сибутрамин (10 и 15 мг) и метформин (850 мг) в одной таблетке. Сибутрамин представляет собой ингибитор обратного захвата нейромедиаторов серотонина (53%), норадреналина (54%) и дофамина (16%), вследствие чего на фоне его приема возрастает концентрация этих медиаторов в синаптическом пространстве. Вследствие такого двойного механизма действия препарат оказывает влияние на обе стороны энергетического баланса, т.е. усиливает и пролонгирует чувство насыщения, уменьшает поступление энергии за счет снижения количества потребляемой пищи и увеличивает ее расход вследствие усиления термогенеза [66]. Стоит отметить, что снижение веса происходит в большей степени за счет уменьшения количества висцерального жира; это особенно важно в аспекте превентивного воздействия на развитие метаболических нарушений [67].
В исследовании А.С. Аметова с соавт. (2020) была подтверждена клиническая обоснованность совместного применения сибутрамина и метформина для повышения эффективности снижения веса и обеспечения стойкого метаболического контроля [68]. Клинически значимого снижения веса на ≥5% за 6 мес терапии достигли 94% пациентов, при этом 91% пациентов удалось снизить вес на ≥10%. Уменьшение массы тела сопровождалось уменьшением окружности талии, атерогенных фракций крови, а также снижением уровня свободных жирных кислот, что говорит о снижении выраженности липотоксичности и оксидативного стресса. Более того, у пациентов с ранними нарушениями углеводного обмена наблюдалось достоверное снижение глюкозы крови натощак, постпрандиальной глюкозы крови и уровня гликированного гемоглобина на 12,9; 16 и 7,5% соответственно. При этом достижение нормальных значений параметров гликемии наблюдалось у 93,2% пациентов.
На фоне лечения препаратом Редуксин® Форте по данным показателей суточного мониторирования артериального давления (СМАД) достоверно увеличилось число dippers до 86,3%. В целом по популяции наблюдения отмечалось некоторое снижение систолического и диастолического АД и отсутствовали значимые изменения показателей частоты сердечных сокращений. Полученные результаты свидетельствовали о хорошей переносимости пациентами принимаемого препарата. Все побочные эффекты носили временный характер и не требовали прекращения лечения.
Таким образом, с одной стороны, разнонаправленное действие метформина и сибутрамина и их взаимодополняющие плейотропные эффекты обеспечивают потенцирование действия компонентов и достижение конечных точек терапии ожирения – устойчивого метаболического контроля, снижения рисков развития осложнений, повышения качества жизни. С другой стороны, снижение массы тела способствует восстановлению нормального иммунного ответа организма и снижению рисков развития осложнений COVID-19.
ЗАКЛЮЧЕНИЕ
Пандемия новой коронавирусной инфекции SARS-CoV-2 характеризуется высокой заболеваемостью и смертностью людей не только пожилого, но и молодого трудоспособного возраста. В связи с этим разработка эффективных схем лечения, направленных на ингибирование вируса, подавление вторичных эффектов «цитокинового шторма» и/или модуляцию иммунной системы организма, а также блокаду некоторых специфических звеньев патогенеза COVID-19 (например, гиперкоагуляции), является приоритетным направлением в клинических подходах к лечению этой комплексной патологии. Уже доказано, что основным подходом к терапии COVID-19 должно быть упреждающее назначение этиотропного лечения до развития полного симпмтомокомплекса жизнеугрожающих состояний. В условиях отсутствия лекарств, разработанных для лечения именно SARS-CoV-2, выбираемые препараты должны отвечать следующим требованиям: широкий спектр противовирусной активности, низкий потенциал развития резистентности, направленное действие в отношении влияния на механизмы жизнедеятельности вируса в организме, возможность успешного применения вне зависимости от времени начала терапии. Новый лекарственный препарат Арепливир (фавипиравир) обладает уникальным сочетанием высокой эффективности против новой коронавирусной инфекции и благоприятным профилем безопасности, а его наличие в арсенале российских клиницистов уже сейчас дает возможность проведения эффективной борьбы с существующими высокопатогенными вирусными возбудителями.
Интегрирование в клиническую практику препаратов, удовлетворяющих принципам трансляционной медицины, призвано помочь лечащему врачу повысить продолжительность и качество жизни пациентов и снизить социально-экономическое бремя глобальных инфекционных и неинфекционных эпидемий. А изменение образа жизни не только за счет повышения уровня гигиены и соблюдения карантинных мероприятий, но и посредством коррекции модифицируемых факторов риска развития и осложненного течения инфекции, таких как ожирение, может стать решающим шагом в борьбе с COVID-19.



