Гепатит дельта вызывается РНК-содержащим вирусом (вирус гепатита дельта, или HDV) и является наиболее тяжелым и трудно поддающимся лечению заболеванием печени вирусной этиологии [1]. Развитие HDV-инфекции в организме-хозяине возможно только при наличии второго вируса – вируса гепатита В (HBV) [2]. Острая форма инфекции, вызванная одновременным заражением и HBV, и HDV (коинфекция), может либо спонтанно разрешиться, либо прогрессировать до фульминантного гепатита, но при этом достаточно редко принимает хроническую форму [3]. Напротив, хроническая HDV-инфекция, как правило, связана с суперинфекцией, которая возникает при попадании HDV в организм больного вирусным гепатитом В. Хроническая HDV-инфекция приводит к более агрессивной форме заболевания печени по сравнению с хронической HBV-моноинфекцией и связана с большим риском развития гепатоцеллюлярной карциномы (ГЦК), более ранним началом цирроза печени (ЦП) и его декомпенсацией. Приблизительно у 70–80% пациентов с хронической HDV-инфекцией развитие ЦП происходит в течение 5–10 лет [4]. В настоящее время известно о существовании восьми генотипов HDV, среди которых наиболее тяжелое течение заболевания печени определяют генотипы 1 и 3 [5].
Несмотря на то что вирусный гепатит дельта известен уже более 40 лет, доступность адекватных методов лечения остается серьезной проблемой при хронической HDV-инфекции. Изучение молекулярной вирусологии HDV привело к появлению новых подходов к терапии. В настоящее время клинические испытания проходит множество многообещающих методов лечения, нацеленных на пути индукции интерферон-стимулированного гена (пегилированный интерферон-лямбда), проникновение вируса в клетку (булевиртид (Myrcludex B), REP2139-Ca), сборку/секрецию субвирусных частиц (REP 2139-Ca/REP 2139-Mg) и сборку вирусов (лонафарниб) [3].
Пегилированный интерферон альфа обладает ограниченной эффективностью при терапии гепатита дельта и сопровождается значительными побочными эффектами, которые ограничивают его рутинное использование [6]. Длительная терапия этим препаратом рекомендуется только при хорошей переносимости пациентом для улучшения показателей фиброза [7]. Myrcludex B – первый в своем классе ингибитор проникновения вируса в клетку, который представляет собой липопептид, извлеченный из pre-S1 домена оболочки вируса HВV, селективно блокирующий рецептор HBV (пептид, котранспортирующий натрия таурохолат, NTCP) и тем самым подавляющий проникновение HBV и HDV в гепатоцит. Препарат показал сильное влияние на уровни РНК HDV в сыворотке крови и вызывал нормализацию аланинаминотрансферазы (АЛТ) при монотерапии. Синергетическое противовирусное действие на РНК HDV и ДНК HBV показало преимущество комбинированного применения ингибитора проникновения вируса в клетку с пегилированным интерфероном альфа-2a для лечения больных хроническим гепатитом дельта [8]. В настоящее время в России продолжаются дополнительные исследования по изучению булевиртида (Myrcludex B) в комбинации с пегинтерфероном альфа [9].
К сожалению, применение каких-либо схем терапии для лечения хронической дельта-инфекции практически недоступно для пациентов в Республике Тыва, которая является эндемичной территорией по HDV. В республике 8% общей популяции жителей инфицировано HBV, а примерно половина из них имеет маркеры инфицирования HDV [10, 11].
На основании исследования уникального клинического материала, полученного в результате многолетнего наблюдения за когортой пациентов с хронической дельта-инфекцией в Республике Тыва (наблюдение ведется с 2009 г.), нами ранее было показано, что это заболевание имеет различное течение и исходы [11]. Ввиду отсутствия эффективного и доступного для населения республики лечения одним из вариантов развития болезни становится быстрый прогресс с возникновением декомпенсированного ЦП и его осложнений (комы, кровотечений), сопровождающихся высокой частотой летальных исходов [12]. Однако данные об особенностях и динамике клинико-биохимических и вирусологических показателей у пациентов с летальным исходом гепатита дельта крайне ограничены.
Цель исследования – представить демографические, клинико-биохимические и вирусологические факторы, которые характеризуют течение и прогрессирование хронического гепатита дельта с летальным исходом, у пациентов, наблюдавшихся в динамике на протяжении 10 лет и проживавших на территории эндемичного по данному заболеванию региону России (Республика Тыва).
МАТЕРИАЛ И МЕТОДЫ
Проанализированы клинико-биохимические и вирусологические данные лабораторных исследований 14 больных, скончавшихся от декомпенсированного ЦП класса В или С по Child–Pugh и ГЦК в исходе заболевания хроническим вирусным гепатитом дельта. Все пациенты состояли на учете в консультативном кабинете Инфекционной больницы Республики Тыва с 2009 по 2019 г. и наблюдались в динамике. Количество обследований на протяжении периода наблюдения варьировало от 1 до 9. Все пациенты принадлежали к одной этнической группе (тувинцы).
В ходе исследования анализировались демографические (пол, возраст на момент включения в исследование) характеристики, количество лет, прошедших от включения пациента в исследование до формирования ЦП и ГЦК, а также до летального исхода. У больных оценивались в динамике показатели активности аланиновой (АЛТ) и аспарагиновой (АСТ) аминотрансфераз, щелочной фосфатазы (ЩФ), гамма-глутамилтранспептидазы (ГГТП), содержание общего билирубина. Выявление РНК HDV и ДНК HBV в образцах сыворотки крови, а также определение генотипа HDV проводили молекулярно-биологическими методами, описанными ранее [11].
Для определения генотипа HBV применяли метод иммуноферментного анализа (ИФА), позволяющий выполнять генотипирование даже при отсутствии детектируемого уровня вирусной ДНК в сыворотке крови. Нами использовалась лабораторная версия теста с панелью моноклональных антител производства ЗАО «Вектор-Бест», позволяющих на основании серотипа HBsAg предсказывать генотип HBV [13, 14].
Для количественного определения уровня HBsAg HBV в сыворотке крови пациентов применялся набор реагентов «HBsAg – ИФА – БЕСТ-количественный» (ЗАО «Вектор-Бест») с аналитическим диапазоном измерения от 0,05 до 100 000 МЕ/мл. Исследования проводили в соответствии с инструкцией производителя.
Материалы исследования были подвергнуты статистической обработке с использованием методов параметрического и непараметрического анализа. Накопление и систематизация исходной информации и визуализация полученных результатов осуществлялись в электронных таблицах Microsoft Office Excel 2016. Анализ полученных результатов выполняли с помощью стандартной программы Excel 2010 и программы статистической обработки данных GraphPad Prism 4.
РЕЗУЛЬТАТЫ
В таблице 1 представлена демографическая характеристика и диагнозы 14 пациентов, наблюдавшихся в период с 2009 по 2019 г. и скончавшихся от осложнений хронической HDV-инфекции. Соотношение мужчин и женщин в наблюдавшейся когорте составило 1:1, средний возраст пациентов на момент включения в исследование – 40,93±10,1, средняя продолжительность наблюдения – 5,5±3,3лет, средний возраст на момент смерти – 44,69±9,13 лет. Прогрессирование заболевания на протяжении периода наблюдения было отмечено у всех пациентов с летальным исходом хронического гепатита дельта. У 2 больных ЦП класса С привел к развитию ГЦК и гибели. Еще 12 человек скончались от осложнений, связанных с декомпенсированным ЦП. Из 14 человек только у трех при первом визите был выявлен хронический гепатит В+D с последующим развитием ЦП от класса В до класса С, остальные пациенты уже при первом визите имели ЦП классов В (n=7) или С (n=4). Нужно отметить, что ни у одного из больных нами не было зафиксировано ЦП класса А. Вероятно, это обусловлено отсутствием возможности регулярных наблюдений и быстрым прогрессированием заболевания. Среди наблюдавшихся пациентов среднее количество лет от включения в исследование до формирования ЦП составило 3,65±2,3 года, до летального исхода – 4,5±3,25 года. При этом данный показатель не имел статистически значимых различий у мужчин и женщин (4,57 и 4,43 года соответственно).
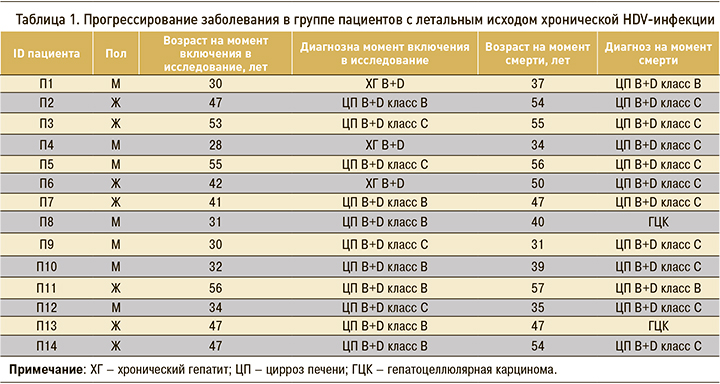
В таблице 2 приведены клинико-биохимические данные пациентов с летальным исходом хронической HDV-инфекции, которые свидетельствовали о незначительном превышении основных показателей активности печеночного процесса. Так, показатели верхней границы нормы (ВГН) АЛТ и АСТ были превышены у этих больных в 1,8 и 1,6 раза соответственно. Значения других маркеров функционального состояния печени – ГГТП, ЩФ и общего билирубина – не превышали 3 ВГН.
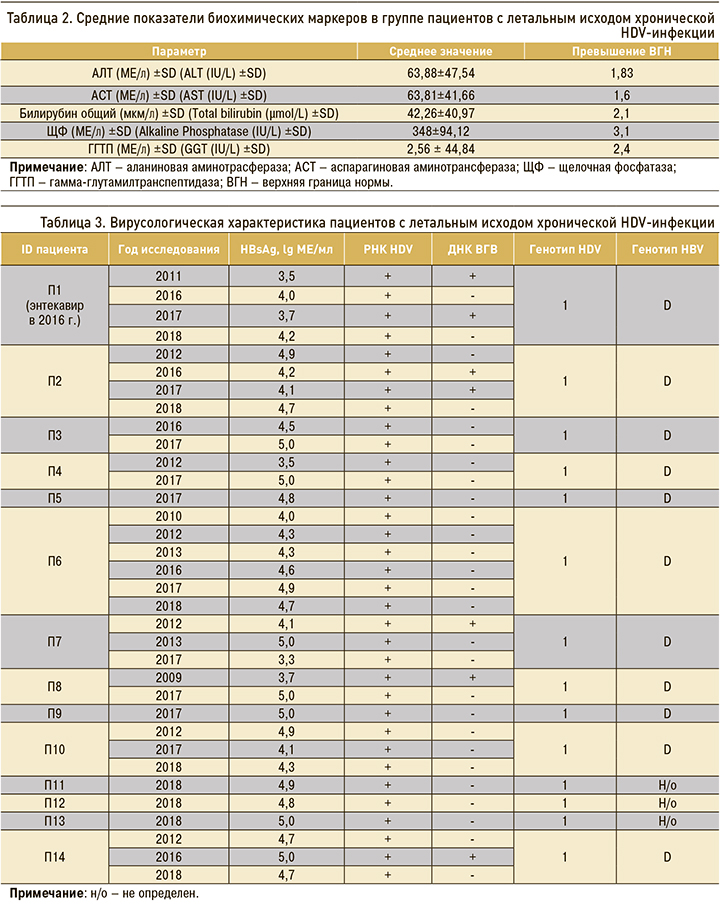
В таблице 3 показаны результаты динамического наблюдения изменения уровня HBsAg в сыворотке крови пациентов с летальным исходом. Данные представлены для каждого пациента по годам, когда это исследование проводилось, в десятичных логарифмах концентрации антигена. У 5 пациентов количественное определение HBsAg было выполнено однократно, поскольку они скончались в тот же или на следующий год после первого визита. Это еще раз указывает на очень позднее обращение в лечебное учреждение больных с этой инфекцией и быстрое прогрессирование заболевания, приводящее к летальному исходу. У этих пациентов (П5, П9, П11, П12 и П13 в таблице 3) были отмечены наиболее высокие концентрации HBsAg (4,86–5 lg МЕ/мл). Наибольшее число наблюдений имели три пациента (П1, П2 и П6 – 4, 4 и 6 точек наблюдения соответственно), 11 человек наблюдались три раза и менее. Наблюдаемые в динамике уровни HBsAg у всех пациентов были нестабильны и менялись от минимального значения (3,3 lg) до максимального (5 lg). У пациентов П1, П3, П4, П8, П10 и П14, наблюдавшихся в динамике несколько лет, уровень HBsAg увеличивался до максимальных значений свыше 4 lg в последний год исследования и превышал предыдущие показатели. У пациентов П2 и П10 в последний год также отмечалось повышение концентрации антигена до значений 4,65 и 4,28 lg, однако более высокие уровни выявленного HBsAg были обнаружены при первом определении и составили 4,86 и 4,85 lg соответственно. Пациент П1 наблюдался в динамике на протяжении четырех лет, при этом концентрации HBsAg у него оставались невысокими, незначительно меняясь от 3,5 до 4,2 lg в последний год наблюдения (2018). Данный пациент в 2016 г. получал противовирусную терапию (энтекавир). Прием препарата определил относительно невысокие уровни концентрации HBsAg, однако не остановил прогрессирование заболевания после его отмены. Вероятно, это и определило летальный исход от осложнений, связанных с декомпенсированным ЦП класса В (пациент скончался в 2018 г.).
Остальные вирусологические характеристики пациентов также отражены в таблице 3. Все больные, включенные в исследование, были инфицированы 1 генотипом HDV и положительны по РНК HDV в течение всего периода наблюдения. У 11 пациентов был обнаружен генотип D HBV, у трех пациентов (П11, П12 и П13) генотип HBV не был определен. Положительный результат выявления ДНК HBV наблюдали у 5 пациентов, из них у 2 в динамическом наблюдении ДНК HBV выявляли два и более раза, а у 3 – однократно. У остальных 9 пациентов отмечались недетектируемые уровни виремии HBV на протяжении всего периода наблюдения. Доля таких пациентов (64,3%) была достоверно выше по сравнению с долей пациентов с виремией HBV (p <0,05; точный критерий Фишера). У больного (П1), имевшего эпизод лечения энтекавиром, ДНК HBV определялась на детектируемом уровне как до, так и после курса терапии. Установлено, что у пациентов с детектируемой виремией HBV к последнему году жизни (последняя дата взятия материала) ДНК HBV в сыворотке крови исчезала.
ОБСУЖДЕНИЕ
Ранее нами было показано, что в эндемичном регионе России (Республика Тыва) хроническая HDV-инфекция имеет три различные формы течения – быстрое прогрессирование в ЦП с частым летальным исходом, непрогрессирующий в ЦП ХГ и медленно прогрессирующий ЦП класса А по Child–Pugh. [11]. Задачей настоящего исследования было представление динамических показателей клинико-биохимических и вирусологических маркеров HDV-инфекции в группе пациентов с летальным исходом этой инфекции (ЦП и ГЦК). В наблюдавшейся нами когорте наиболее частой причиной смерти становился ЦП, но не ГЦК. Считается, что HDV-инфекция увеличивает риск развития ГЦК по сравнению с моноинфекцией HBV, в первую очередь за счет окислительного стресса в результате тяжелого некровоспаления [15]. Однако эпидемиологические данные свидетельствуют, что наиболее частым клиническим исходом HDV-инфекции является печеночная декомпенсация, а не развитие ГЦК [16].
Ретроспективный анализ 962 пациентов с HBV, 82 из которых были коинфицированы HDV, показал одинаковую частоту ГЦК в обеих группах [17]. Наблюдения в германской когорте пациентов с гепатитом дельта продемонстрировали высокую частоту формирования ГЦК (16%, средний период наблюдения 3 года). Однако развитие ГЦК у пациентов в этой когорте было вторичными по отношению к ЦП [18]. Эти данные согласуются с результатами, полученными нами в тывинской когорте, где только у 2 пациентов из 14 (14,3%) развилась ГЦК. В то же время некоторые исследования подтверждают, что коинфекция HBV/HDV приводит к увеличению в 2 раза смертности и в 3 раза риска развития ГЦК по сравнению с пациентами с моноинфекцией HBV [19–21]. Таким образом, роль HDV в индукции и развитии ГЦК остается спорной и должна быть дополнительно изучена.
Мониторинг биохимических и вирусологических маркеров при HDV-инфекции позволяет выявить конкретные закономерности, связанные с активностью и стадией хронического гепатита дельта. В результате наблюдения за когортами пациентов с гепатитом дельта сделаны предположения, что значения концентраций HBsAg и РНК HDV, а также титров анти-HDV IgM, суммарных анти-HDV и суммарных анти-HBc позволяют прогнозировать течение и исход заболевания и определить кандидатов на лечение [22, 23]. По данным литературы, виремия HDV выступает основным независимым предиктором прогрессирования хронического гепатита дельта, печеночной декомпенсации и смерти [24, 25]. Эти данные подтверждаются результатами нашего наблюдения – РНК HDV в представленной группе пациентов выявлялась при каждом обследовании.
К сожалению, в доступной литературе отсутствуют данные об исследованиях в динамике концентраций HBsAg в сыворотке крови пациентов с летальным исходом HDV-инфекции.
Коинфекция HDV может вызывать подавление репликации HBV до неопределяемых значений [26]. Наши результаты подтверждают это наблюдение: уровни ДНК HBV при чувствительности детекции 50 копий/мл не определялись у большинства пациентов с летальным исходом, что предполагает ингибирующий эффект HDV на репликацию HBV, сохраняющийся и на терминальной стадии инфекции. Патогенез многих инфекций, в том числе гепатитов В и дельта, может определяться генотипом вируса [27]. Выявленный в нашем исследовании у всех пациентов генотип 1 HDV считается, наряду с генотипом 3, наиболее патогенным и связанным с быстрым прогрессированием заболевания [5].
Данные о роли генотипа HBV в прогрессировании гепатита дельта ограниченны. Во многом это связано с отсутствием детектируемой виремии HBV у многих пациентов с гепатитом дельта, что затрудняет генотипирование молекулярными методами. У пациентов с HDV-инфекцией в сыворотке крови содержится достаточное количество HBsAg [28], поэтому для преодоления этого ограничения мы применяли в целях генотипирования HBV-метод ИФА, позволяющий определить серотип HBsAg и предсказать на его основании генотип вируса. В нашем исследовании у пациентов с летальным исходом HDV-инфекции был обнаружен только генотип D, что не позволяет определить влияние разных генотипов HBV на исходы HDV-инфекции.
Интересным фактом представляется отсутствие у наблюдавшихся в нашем исследовании пациентов с летальным исходом гепатита дельта значительного повышения показателей функционального состояния печени. Так, уровни АЛТ и АСТ превышали в ВГН менее чем в 2 раза, а уровни ГГТП, ЩФ и общего билирубина – не более чем в 3 раза. Результаты наблюдения китайских пациентов показывают, что коинфекция HBV/HDV связана с более высокими уровнями АЛТ и АСТ по сравнению с больными, имеющими моноинфекцию HBV [29]. Вероятно, невысокую активность воспалительного процесса в печени у наблюдавшихся нами пациентов можно объяснить сформировавшимся на момент включения в исследование ЦП и соответственно сравнительно небольшим числом живых клеток печени.
Очевидно, что позднее обращение в лечебное учреждение больных гепатитом дельта – негативный фактор, влияющий на летальность при данной инфекции. Полученные нами ранее данные указывают на то, что более 80% пациентов, проживающих на территории Республики Тыва, при первом обращении в лечебное учреждение уже имели далеко продвинутые стадии заболевания, включая ЦП класса С [30]. В настоящем исследовании из 14 пациентов с летальным исходом 5 (36%) скончались в тот же или на следующий год после первого визита. Это говорит, с одной стороны, о недостаточном уровне охвата больных ранней диагностикой и, с другой ‒ о быстром прогрессировании заболевания, приводящем к летальному исходу.
Терапия хронического гепатита дельта по-прежнему затруднена из-за отсутствия эффективных лекарств. Интерферон-альфа в настоящее время служит единственной доступной лицензированной терапией [31]. Наиболее часто используемым препаратом является пегилированный интерферон, но только около 25% пациентов сохраняют устойчивый вирусный ответ после 1 года лечения [32]. При этом уровни трансаминаз нормализуются только у 40–70% пролеченных пациентов, а рецидивы возникают у 60–97% [6]. Более того, опыт интерферонотерапии гепатита дельта в когорте пациентов, проживающих на территории Республики Тыва (этнические тувинцы), показал неэффективность схем лечения с применением интерферонов [30]. Таким образом, высокая степень летальности гепатита дельта, наряду с объективными патогенетическими факторами, объясняется поздней диагностикой и отсутствием доступной терапии.
ЗАКЛЮЧЕНИЕ
В эндемичном регионе (Республика Тыва) хроническая дельта-инфекция характеризуется быстрым прогрессирующим течением, развитием ЦП и ГЦК с летальным исходом. Значительная распространенность HDV-инфекции в регионе и отсутствие доступного эффективного лечения требуют более строгого клинического наблюдения инфицированных пациентов и определяют необходимость дальнейшего изучения этой проблемы.


