ВВЕДЕНИЕ
Современная медицина рассматривает атеросклероз как заболевание с вариабельной комбинацией изменений внутренней оболочки артерий эластического и мышечно-эластического типа, в основе которого лежат обменные нарушения, характеризующиеся аккумуляцией липидов, пролиферацией гладкомышечных клеток сосудов, воспалительными и некроапоптотическими процессами [1, 2]. В патогенетических механизмах атеросклероза ключевая роль принадлежит нарушению обмена липидов, эндотелиальной дисфункции, воспалительным и иммунным патологическим процессам, которые взаимосвязаны между собой. Как в условиях физиологической нормы, так и при развитии патологических процессов, в первую очередь связанных с формированием редокс-дисбаланса, в том числе при атерогенезе, центральное место занимает гомеостазирующая регуляторная система антиоксидант-респонсивного элемента Keap1/Nrf2/ARE [3].
Во время развития и прогрессирования атеросклероза опосредованная транскрипционным фактором Nrf2 передача сигналов модулирует многие физиологические и патофизиологические процессы: редокс-регуляцию, воспаление, липидный обмен, образование пенистых клеток, поляризацию макрофагов и др. Установлена связь некоторых полиморфизмов Nrf2 со способностью сосудов к вазодилатации, а также уровнем систолического и диастолического давления; в свою очередь, на активность системы Keap1/Nrf2/ARE могут влиять некоторые микроРНК [4]. Известно, что окислительное повреждение эндотелиальных клеток сосудов увеличивает риск атеросклероза [5]. Развитие окислительного стресса сопровождается стабилизацией в них Nrf2, который реализует свое защитное действие путем увеличения транскрипции целевых генов, в том числе антиоксидантных и связанных со II фазой детоксикации [6].
Nrf2 выполняет противовоспалительную функцию в эндотелиальных клетках [7], подавляет экспрессию молекул адгезии и цитокинов, ассоциированных с воспалением, на ранних этапах атерогенеза. Индукция системы Keap1/Nrf2/ARE Nrf2 в эндотелиальных клетках ингибирует ФНО-α-индуцированную экспрессию моноцитарного хемоаттрактантного белка-1 (MCP-1) и молекулы адгезии сосудистых клеток 1 (VCAM-1) [8, 9]. Эндотелиальный Nrf2 активируется ламинарным током крови и оказывает антиатерогенное действие [10]. В целом можно заключить, что Nrf2 эндотелиоцитов действует как антиатерогенный фактор за счет индукции антиоксидантных генов и угнетения экспрессии провоспалительных генов в ответ на различные сигналы, включая ламинарный сдвиг.
Повышенный уровень в крови общего холестерина (ХС), в основном за счет ХС липопротеидов низкой плотности (ЛНП) и триглицеридов (ТГ), наряду с низкой концентрацией ХС липопротеидов высокой плотности (ЛВП), увеличивает риск развития атеросклероза [12]. В то же время липопротеиды крови представляют собой гетерогенный спектр частиц, различающихся по плотности, размеру, электрическому заряду, химическому составу [13–16]. Критическими этапами атерогенеза являются поглощение окисленно модифицированных ЛНП макрофагами интимы и превращение последних в пенистые клетки [17].
Роль Nrf2-опосредованной регуляции в развитии и прогрессировании атеросклероза неоднозначна: выявлены ряд как антиатерогенных (снижение окисления ЛНП и их токсического действия, усиление синтеза антиоксидантных ферментов гемоксигеназы-1 и глутатионпероксидазы, повышение экспорта из клеток холестерина через транспортер АВСА1), так и проатерогенных (рост содержания ХС в крови, повышение экспрессии скэвинджер-рецепторов CD36 и миграции моноцитов в интиму, а также перевод макрофагов в проатерогенное состояние Mox) эффектов активации системы Keap1/Nrf2/ARE [3, 12].
Миграция, пролиферация и апоптоз клеток гладких мышц сосудов (КГМС) тесно связаны с развитием атеросклероза [18]. На поздней стадии атеросклероза КГМС мигрируют в интиму и секретируют белки внеклеточного матрикса для стабилизации бляшек. Хотя неизвестно, модулирует ли Nrf2 функцию КГМС во время атерогенеза, ряд исследований предполагает возможное антиатерогенное действие Nrf2 на КГМС. Окислительный стресс влияет на некоторые функции КГМС, такие как пролиферация, миграция и выработка воспалительных цитокинов [19]. Показано, что индукция системы Keap1/Nrf2/ARE снижает окислительный стресс и ингибирует пролиферацию КГМС и экспрессию MCP-1 [20, 21], а снижение стационарной концентрации Nrf2 малыми интерферирующими РНК увеличивает индуцированную фактором роста тромбоцитов миграцию КГМС [20, 21].
Неоднозначность данных об участии Nrf2-опосредованных редокс-чувствительных сигнальных путей в атерогенезе и прогрессировании атеросклероза определяет необходимость поиска новых сведений о механизмах причинно-следственных взаимосвязей данных процессов и позволяет предположить возможность разработки персонализированного подхода для диагностики и прогнозирования развития атеросклероза.
Цель исследования – изучить особенности клинико-лабораторных маркеров атеросклероза и уровень экспрессии генов системы антиоксидантной защиты NRF2, HMOX1, NQO1, GSTP1 во взаимосвязи с эхоскопически верифицированной степенью стенозирования сонных артерий.
МАТЕРИАЛ И МЕТОДЫ
В исследование были включены 160 пациентов (54 мужчины, 106 женщин) клиники Федерального исследовательского центра фундаментальной и трансляционной медицины, проходивших обследование и лечение в период с 2016 по 2021 г. Возраст больных составил от 45 до 78 лет (в среднем 61,9±8,7 года). Исследование проводилось в соответствии со стандартами Хельсинкской декларации Всемирной медицинской ассоциации «Этические принципы проведения медицинских исследований с участием человека в качестве субъекта» и Правилами клинической практики в Российской Федерации, утвержденными Приказом Минздрава России от 19.06.2003 № 266. Все пациенты дали письменное информированное согласие на включение в исследование.
В исследовании были использованы данные клинической, лабораторной, функциональной, ультразвуковой диагностики, выполнялась оценка экспрессии генов, участвующих в формировании антиоксидантной зашиты.
Определение компонентов липидного спектра крови проводилось в сыворотке крови, взятой в утренние часы натощак; с помощью биохимического анализатора Konelab 30i (Thermo Clinical Labsystems, Финляндия) оценивалось содержание общего ХС, ХС ЛНП, ХС ЛВП и ТГ. Гиперхолестеринемию определяли при повышении содержания общего ХС в сыворотке крови более 5 ммоль/л, увеличение концентрации ХС ЛНП и ТГ – при значениях более 3 и 1,7 ммоль/л соответственно, снижение уровня ХС ЛВП – при его величине менее 0,9 ммоль/л.
Проводилось дуплексное сканирование сосудов шеи (Vivid E9, GE, США) с определением диаметра общих сонных артерий, внутренних сонных артерий, наружных сонных артерий, позвоночных артерий, толщины комплекса «интима-медиа», пиковой систолической скорости кровотока общих сонных, внутренних сонных, наружных сонных, позвоночных артерий, извитости артерий, индекса резистентности, процента стеноза брахицефальных артерий. У пациентов рассчитывался кумулятивный индекс коморбидности CIRS-G.
Для оценки экспрессии генов забирали цельную венозную кровь, получали лейкоциты, из которых впоследствии выделяли РНК с использованием TRIzol Reagent (Thermo Fisher Scientific, США). Для получения кДНК выполняли обратную транскрипцию с помощью набора реагентов iScript cDNA Synthesis Kit (BioRad Laboratories, США) в соответствии с инструкцией. Методом TaqMan ПЦР в режиме реального времени на амплификаторе CFX96 (Bio-Rad Laboratories) изучали изменение экспрессии мРНК гена, кодирующего транскрипционный фактор Nrf2 (NRF2), и мРНК контролируемых им генов белков NAD(P)H:хиноноксидоредуктазы 1 (NQO1), гемоксигеназы 1 (HMOX1), глутатион-S-трансферазы P1 (GSTP1); в качестве референсного использовали ген домашнего хозяйства GAPDH.
Реакцию амплификации проводили в следующих условиях: реакционная смесь ПЦР объемом 20 мкл содержала буфер для ПЦР, 2,5 мМ MgCl2, 0,2 мМ dNTP’s, 1,25 е. а. Taq-полимеразы. Амплификацию выполняли согласно следующей программе: 3 мин при 95 °С начальной денатурации, далее 40 циклов: 10 с при 95 °С для денатурации, 20 с при 60 °С для гибридизации праймеров, съем флуоресцентного сигнала, 20 с при 72 °С для элонгации. Подобранные пары праймеров приведены в таблице 1.

Уровень экспрессии мРНК генов рассчитывали согласно методу 2-ΔΔCT и нормировали относительно референсного гена GAPDH. Статистическую обработку результатов исследования проводили, вычисляя среднее арифметическое значение (М), ошибку среднего арифметического значения (m), и представляли в виде M±m (нормальность распределения данных оценивали по критерию Колмогорова–Смирнова). Различия между группами определяли с помощью критерия Стьюдента, статистически значимыми считали результаты при р <0,05. Связь между различными признаками в исследуемой выборке устанавливалась посредством корреляционного анализа величиной коэффициента корреляции Спирмена (r).
РЕЗУЛЬТАТЫ
В подгруппе из 92 обследованных (средний возраст 62,0±9,1 года, 25 мужчин, 67 женщин) анализировались ультразвуковые характеристики брахиоцефальных артерий во взаимосвязи с биохимическими показателями липидного обмена. По результатам обследования пациенты были разделены на 3 группы в зависимости от степени стенозирования сонных артерий:
1) менее 30%;
2) от 30 до 50%;
3) более 50%.
Распределение пациентов по степени стеноза представлена в таблице 2. Незначительное стенозирование сонных артерий наблюдалось у 60,9% обследованных, умеренное и выраженное – у 39,1%.

Также был проведен расчет средних значений скорости кровотока (табл. 3) и показателей липидного спектра пациентов (табл. 4). Выявлено, что в среднем исследуемые пациенты имели повышенный уровень общего ХС с тенденцией к повышению коэффициента атерогенности.

Выполненный корреляционный анализ позволил найти взаимосвязи между показателями скорости кровотока и липидного спектра (табл. 5). В частности, была выявлена прямая корреляция между показателями концентрации ТГ и показателями толщины комплекса «интима-медиа» (r=0,31; p <0,05). Обнаружена прямая корреляция между показателями общего ХС и скоростью кровотока во внутренней сонной артерии (r=0,32; p <0,05). Также была установлена обратная корреляция между показателями концентрации ЛВП и толщиной комплекса «интима-медиа» (r=-0,53; p <0,05). Была выявлена прямая корреляционная связь между показателями скорости кровотока в позвоночных артериях и концентрациями ТГ и ЛНП. Обнаружена прямая корреляция между показателями толщины комплекса «интима-медиа» и коэффициентом атерогенности (r=0,48; p <0,05), а также между скоростью кровотока в позвоночных артериях и коэффициентом атерогенности (r=0,37 и r=0,41; p <0,05). Установлена обратная корреляционная связь между показателем концентрации аполипопротеина А и толщиной комплекса «интима-медиа» (r= 0,49; p <0,05) и прямая корреляция между показателем концентрации аполипопротеина В и скоростью кровотока в позвоночных артериях (справа r=0,39; слева r=0,46; p <0,05).
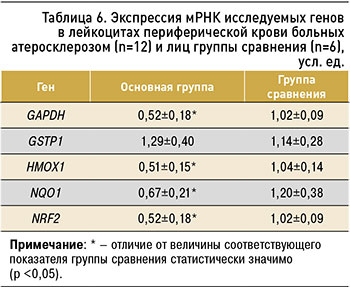
Далее были отобраны 12 больных (средний возраст 64,3±4,8 года) с верифицированным атеросклерозом брахиоцефальных артерий для исследования уровня экспрессии генов антиоксидантной защиты. Установлено, что у этих пациентов по сравнению с контролем (условно здоровых лиц сопоставимого пола и возраста) экспрессия генов GSTP1, NQO1 и NRF2 была снижена на 49, 51 и 44% соответственно (p <0,05), тогда как содержание мРНК гена HMOX1 изменено не было (табл. 6).
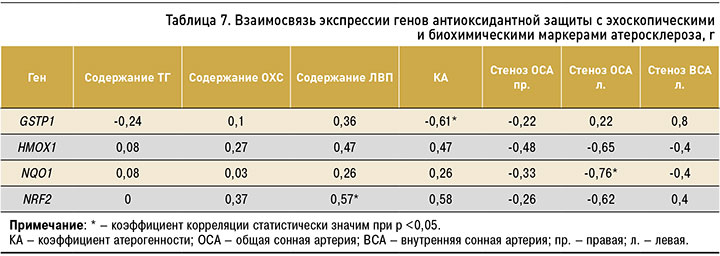
На следующем этапе исследования была выявлена взаимосвязь между выраженностью экспрессии генов GSTP1, HMOX1, NQO1, NRF2 и показателями липидного спектра крови, а также степенью стенозирования сонных артерий: обратная корреляция между экспрессией гена NQO1 и процентом стеноза в левой общей сонной артерии, прямая зависимость между уровнем мРНК гена NRF2 и концентрацией ЛВП, обратная корреляция между экспрессией гена GSTP1 и коэффициентом атерогенности (табл. 7).
В подгруппе из 68 пациентов (средний возраст 61,7±8,3 года; 29 мужчин, 39 женщин) была проанализирована взаимосвязь уровня полиморбидности с показателями липидного обмена. Была обнаружена умеренная обратная корреляция между содержанием общего ХС, ЛНП и индексом кумулятивной коморбидности (CIRS-G). r=-0,48 при р <0,05 и r=-0,45 при р<0,05 соответственно. Внутри групп с различной степенью стенозирования сонных артерий данная корреляция сохранялась. В группах с эхоскопически умеренным и выраженным (>30%), а также с незначительным (<30%) атеросклеротическим стенозированием сонных артерий концентрация общего ХС обратно коррелировала с индексом CIRS-G (r=-0,44 при р <0,05 и r=-0,46 при р <0,05 соответственно), уровень ЛНП – отрицательно (r=-0,41 при р <0,05, r=-0,43 при р <0,05 соответственно). Таким образом, в исследовании установлена и доказана статистически значимая обратная связь между индексом кумулятивной коморбидности (CIRS-G) и ЛНП у больных с атеросклерозом.
ОБСУЖДЕНИЕ
В проведенном исследовании обнаружено, что показатели липидного спектра могут влиять на скорость кровотока в брахиоцефальных артериях. В частности, повышенное содержание ТГ в крови влияет на толщину комплекса «интима-медиа» в общей сонной артерии, а также на увеличение скорости кровотока в позвоночных артериях. Помимо этого, повышенное содержание ЛНП также неблагоприятно сказывается на линейной скорости кровотока в позвоночных артериях и приводит к ее увеличению. Вместе с этим повышенный коэффициент атерогенности также влияет на линейную скорость кровотока в позвоночных артериях и приводит к увеличению последней. К тому же увеличенный коэффициент атерогенности влияет и на толщину комплекса «интима-медиа» в общих сонных артериях. Эти данные развивают представление о большой клинической значимости ультразвуковых показателей состояния брахиоцефальных артерий при комплексной оценке развития атеросклероза и его осложнений [22].
В нашем исследовании изучена экспрессия гена транскрипционного фактора Nrf2 – мастер-регулятора гомеостаза – и контролируемых им генов белков антиоксидантной защиты HMOX1, NQO1, GSTP1. То, что развитие атеросклероза сопровождается снижением экспрессии генов антиоксидантной защиты, в целом не вызывает сомнений [10, 23, 24], однако данных об участии в атерогенезе регуляторной системы Keap1/Nrf2/ARE относительно немного, а имеющиеся сведения крайне противоречивы. Полученные в настоящем исследовании результаты подтверждают гипотезу о наличии такой взаимосвязи: так, у пациентов с верифицированным атеросклерозом брахиоцефальных артерий снижена экспрессия не только гена NRF2, но и активируемых им генов GSTP1 и NQO1. Кроме того, наличие обратной корреляционная связи экспрессии гена NQO1 со степенью стеноза общей сонной артерии позволяет предположить, что одной из причин увеличения атеросклеротический бляшки в сонных артериях может быть уменьшение активности системы Keap1/Nrf2/ARE, хотя зависимость между выраженностью экспрессии NRF2 и стенозом сонных артерий не установлена. Необходимо отметить, что система Keap1/Nrf2/ARE регулируется главным образом на посттранскрипционном уровне, один из ключевых моментов ее индукции – внутриклеточное перераспределение Nrf2 (транспорт в ядро) с последующей активацией подконтрольных генов, поэтому изменение экспрессии Nrf2 не является обязательным свидетельством его активности [4, 6, 25, 26].
Корреляционный анализ также позволил выявить прямую корреляционную связь между экспрессией гена NRF2 и концентрацией ХС ЛВП. Таким образом, снижение или увеличение экспрессии NRF2 может влиять на изменение содержания ЛВП в периферической крови, что, в свою очередь, также влияет на развитие атеросклероза, поскольку, согласно литературным данным [2, 27, 28], эта группа липопротеидов выполняет уникальную антиатерогенную функцию. Напомним, что ЛВП способствуют обратному транспорту холестерина, гидролизуют окисленные липиды, тем самым угнетая свободно-радикальные окислительные процессы. Кроме того, входящий в их состав апопротеин A1 действует как антиоксидант, защищая ЛНП от окислительной модификации.
Наличие обратной зависимости между экспрессией гена GSTP1 и коэффициентом атерогенности также свидетельствует об угнетении антиоксидантной защиты при атеросклерозе и увеличении риска развития атеросклеротической бляшки, поскольку, в соответствии с литературными данными [2, 10, 11, 27], коэффициент атерогенности служит показателем, отражающим степень риска развития атеросклероза.
В результате полученные данные об экспрессии генов GSTP1, HMOX1, NQO1, NRF2 могут позволить в будущем разработать персонализированный подход для диагностики и прогнозирования развития атеросклероза, а также открывают перспективы разработки антиатеросклеротических препаратов нового поколения, мишенью которых будет выступать регуляторная система Keap1/Nrf2/ARE и опосредованно система антиоксидантной защиты.
ЗАКЛЮЧЕНИЕ
1. У пациентов с верифицированным атеросклерозом брахиоцефальных артерий экспрессия гена GSTP1 снижена на 49% по сравнению с контролем, экспрессия гена NQO1 – на 51%, экспрессия гена NRF2 – на 44%, в то время как экспрессия гена HMOX1 не изменена.
2. Выявлены взаимосвязи между выраженностью экспрессии генов GSTP1, HMOX1, NQO1, NRF2 и биохимическими показателями липидного спектра, а также степенью стенозирования сонных артерий, а именно: обратная корреляция между экспрессией гена NQO1 и процентом стеноза в левой общей сонной артерии, прямая зависимость между уровнем мРНК гена NRF2 и концентрацией ЛВП, обратная корреляция между экспрессией гена GSTP1 и коэффициентом атерогенности.
3. Обнаружены прямые корреляционные связи между концентрацией ЛНП, ТГ, аполипопротеина B в крови и скоростью кровотока в позвоночных артериях, а также обратная корреляционная связь между содержанием ЛВП и толщиной комплекса «интима-медиа» в общих сонных артериях.



