ВВЕДЕНИЕ
В последние несколько десятилетий сердечно-сосудистые заболевания (ССЗ), включая ишемическую болезнь сердца (ИБС), инсульт, артериальную гипертензию (АГ) и сердечную недостаточность, признаны ведущей причиной смертности в мире [1]. По данным Всемирной организации здравоохранения, опубликованным в 2019 г., в мире на тот момент было зарегистрировано 17,9 млн смертей, причиной которых стала кардиоваскулярная патология, что составило 32% от общей смертности. Этот показатель продолжает расти параллельно с увеличением продолжительности жизни населения [2, 3].
Лечение, восстановление и реабилитация больных с сердечно-сосудистой патологией – серьезная медико-социальная проблема, требующая больших экономических затрат государства и семьи больного. Для эффективной профилактики ССЗ важно проводить оценку факторов кардиоваскулярного риска (КВР), поскольку их выявление позволяет своевременно осуществлять профилактические мероприятия [4].
Известно, что 90% популяционного риска развития инфаркта миокарда определяется такими факторами, как АГ, гиперхолестеринемия, курение, недостаточное употребление фруктов и овощей, избыточная масса тела и ожирение, потребление алкоголя, стресс, сахарный диабет [5]. Последние исследования демонстрируют связь микробиоты кишечника и ее метаболитов с развитием ССЗ, и некоторые из них указывают на высокую вероятность того, что измененный кишечный микробиом выступает дополнительным фактором КВР.
СОВРЕМЕННЫЕ ПРЕДСТАВЛЕНИЯ О МИКРОБИОТЕ КИШЕЧНИКА
Стремительное развитие методов метагеномного секвенирования и метаболомики расширило понимание структурных и функциональных возможностей микробиома. Высокая производительность, скорость и доступность геномных исследований, использование биоинформационных подходов, применяемых для анализа полученных данных, открыли перед учеными новые перспективы в области изучения структуры микробиоценозов. Стало известно, что микробиота кишечника состоит в симбиозе со своим хозяином, включает более 100 трлн микробов и содержит как минимум в 150 раз больше генов, чем геном человека [6]. Состав кишечной микрофлоры у каждого человека стабилен, индивидуален и адаптирован к его потребностям. Путем метагеномного секвенирования микробиома кишечника методом shotgun-sequencing (метод «дробовика») обнаружено 1952 неклассифицированных вида бактерий в дополнение к 553 видам, ранее выделенным из кишечника человека [7].
Микроорганизмы взаимодействуют между собой, конкурируют за питательные компоненты, паразитируют, приспосабливаются к среде обитания и усиливают функции друг друга. Микробиота кишечника продуцирует ферменты, участвующие в метаболизме углеводов, липидов, нуклеиновых кислот, синтезе витаминов, короткоцепочечных жирных кислот (КЦЖК), антимикробных веществ, гормонов, аминокислот, а также в иммуномодуляции, детоксикации, поддержании барьера слизистой оболочки, эпителия кишечника и эвакуаторной функции желудочно-кишечного тракта (ЖКТ) [8]. Микрофлора толстой кишки представлена следующими типами бактерий: Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria. У взрослых доминируют Bacteroidetes и Firmicutes, на долю которых приходится более 90% популяции кишечных микроорганизмов [9].
Разнообразие бактерий выше в содержимом просвета кишечника, чем в слое пристеночной слизи [10] за счет факультативной микробиоты, поступающей с пищей; ее количество изменяется даже в течение суток. В слое пристеночной слизи состав микробиоты относительно стабилен, но во многом зависит от содержимого химуса и пищевых привычек хозяина. Те пищевые волокна, сахара и белки, которые не перевариваются ферментами макроорганизма в тонкой кишке, ферментируются микробиотой. Продуктами ферментации пищевых волокон бактериями становятся КЦЖК – ацетат, пропионат и бутират [11]. Основная функция этих веществ заключается в поддержании целостности кишечного барьера, участии в метаболизме глюкозы и липидов, регуляции артериального давления, модуляции иммунной системы и реализации противовоспалительного эффекта [12].
Помимо бактерий, микробиота толстой кишки здорового человека состоит из вирусов, грибов, архей и протистов, которые являются не менее важной частью экосистемы кишечника [13]. Благодаря полученной информации, анализу и накопленным знаниям о структурных и функциональных возможностях микробиома кишечника доказана его связь с физиологией и метаболизмом макроорганизма как в норме, так и при патологических процессах.
ВЛИЯНИЕ МИКРОБИОТЫ КИШЕЧНИКА И ЕЕ МЕТАБОЛИТОВ НА РАЗВИТИЕ СЕРДЕЧНО-СОСУДИСТЫХ ЗАБОЛЕВАНИЙ
Значительное внимание и усилия исследователей разных стран в настоящее время сосредоточено на выявлении взаимосвязей между микробиотой кишечника и ССЗ, а также факторами риска их возникновения. На 81-м конгрессе Европейского атеросклеротического общества в Лионе в 2013 г. обсуждалась теория «атеросклеротического микробиома», которая отражает ассоциацию между развитием атеросклероза с особенностями микрофлоры кишечника. Различные исследовательские группы продемонстрировали, что изменения в соотношении Firmicutes и Bacteroidetes и, следовательно, нарушение баланса микробных метаболитов, таких как КЦЖК, N-оксид триметиламин (ТМАО), коррелируют с патогенезом ССЗ [14–16].
Вклад микробиоты в синтез проатерогенных веществ состоит в способности некоторых микроорганизмов кишечной микрофлоры к превращению лецитина, L-карнитина и холина, содержащегося в продуктах животного происхождения, в триметиламин, который, в свою очередь, в печени человека под действием флавинсодержащей монооксигеназы 3 трансформируется в ТМАО [17, 18]. Роль этого соединения в развитии атеросклероза обусловлена тем, что оно может ингибировать обратный транспорт холестерина, регулировать экспрессию генов эндотелиальных и иммунных клеток, стимулировать образование пенистых клеток из макрофагов, повышать адгезию тромбоцитов [19]. К микроорганизмам, достоверно повышающим уровень ТМАО, относится тип Firmicutes, а именно роды Clostridium, Enterococcus, Streptococcus [20]. Исследования показали, что уровни TMAO в плазме крови связаны с риском ССЗ [18]. В опубликованном в 2021 г. исследовании Tang W.H.W. et al. рассматривается возможность использования ТМАО как маркера риска коронарного атеросклероза независимо от традиционных факторов риска [21].
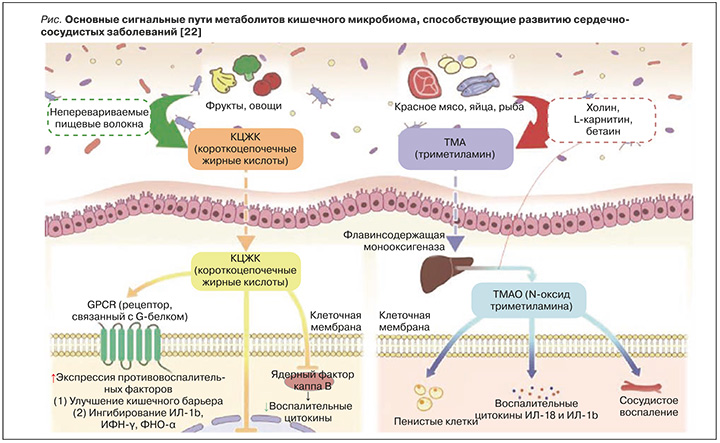
Помимо этого, недавние исследования продемонстрировали, что КЦЖК оказывают существенное влияние на возникновение и течение ССЗ, в частности ИБС (рис.) [22]. Количество вырабатываемых КЦЖК определяется объемом потребления пищевых волокон наряду с составом и количеством КЦЖК-продуцирующих микробов в микрофлоре кишечника. Изменение разнообразия, состава и количества кишечных бактерий сказывается на синтезе КЦЖК и, как следствие, влияет на развитие ССЗ, ассоциированных с системной воспалительной реакцией и эндотелиальной дисфункцией [12]. КЦЖК выполняют функции сигнальных молекул, связываясь с рецепторами GPR41 и GPR43, которые в основном экспрессируются в эпителиоцитах кишечника и фактически во всех иммунных клетках [23]. GPR41 и GPR43 могут быть активированы всеми основными КЦЖК, тогда как GPR109A наиболее эффективно активируется бутиратом [24]. Связывание КЦЖК с GPR вызывает противовоспалительный эффект, стимулируя дифференцировку регуляторных Т-клеток для выработки интерлейкина 10 (ИЛ-10), и восстанавливает целостность кишечного барьера через белки плотных контактов. Более ранние исследования продемонстрировали развитие атеросклероза и хронической сердечной недостаточности вследствие нарушения функции кишечного барьера, которое влечет за собой бактериальную транслокацию и циркуляцию провоспалительных бактериальных метаболитов; это лишний раз подчеркивает важность сохранения целостности эпителия кишечника [25].
Необходимо добавить, что КЦЖК вовлечены в образование глюкагоноподобного пептида 1. Последний стимулирует секрецию инсулина и ингибирует секрецию глюкагона, оказывает антагонистическое действие в отношении провоспалительных цитокинов (ИЛ-1β, интерферона-гамма и фактора некроза опухоли), подавляющих синтез инсулина [26].
Основными бактериями, производящими КЦЖК, являются виды Eubacterium, Roseburia, Faecalibacterium и Coprococcus [27]. Исследования указывают на изменения соотношения микробиоты, продуцирующей КЦЖК, у людей, страдающих атеросклерозом. Так, следует отметить уменьшение у них количества бутират-продуцирующей флоры (Roseburia intestinalis, Faecalibacterim prausnitzii) по сравнению со здоровыми людьми [28]. Считается, что бутират служит ключевым источником энергии для колоноцитов и энтероцитов, а также способствует нормальной дифференциации и пролиферации клеток [29]. Помимо этого, он способен ингибировать 3-гидрокси-3-метилглутарилкоэнзим А-редуктазу, что ведет к предотвращению синтеза холестерина [29]. В исследованиях на крысах было установлено, что пропионат ингибирует включение ацетата в жирные кислоты и холестерина в гепатоциты крыс, тем самым снижая уровень холестерина в сыворотке крови, ацетат же, наоборот, стимулирует синтез холестерина, так как выступает его субстратом [29]. Таким образом, по мнению авторов исследования, при рассмотрении указанных метаболитов как факторов риска ССЗ важно не столько их количество, сколько соотношение ацетата к пропионату.
Важная роль во влиянии микробиоты кишечника на липидный обмен принадлежит липополисахариду (ЛПС), входящему в состав наружной мембраны грамотрицательных бактерий. Это влияние проявляется даже при незначительном повышении содержания ЛПС в крови, получившем название «метаболическая эндотоксемия» [27]. Считается, что ЛПС способен выступать в качестве сигнальной молекулы для Toll-подобных рецепторов 4-го типа c активацией провоспалительных сигнальных путей [30], что вызывает формирование пенистых клеток и опосредованно увеличивает оксидативный стресс, как это было показано в исследовании Carnevale R. et al. в 2018 г. [31]. Данная работа подтверждает наличие корреляции между уровнем ЛПС и показателями липидного профиля, однако для дальнейшего изучения этого вопроса необходимы расширенный и углубленный анализ по возрасту и расе пациентов, а также увеличение численности выборки.
В другом исследовании [32] было продемонстрировано, что пациенты с ИБС при сравнении с контрольной группой, сопоставимой по возрасту/полу/расе/индексу массы тела, имели отличительный микробный пейзаж кишечника. Он охарактеризован авторами как кишечный микробиом с уменьшенным относительно группы сравнения количеством специфических кишечных микробов (Lachnospiraceae NK4B4 и других представителей семейства Lachnospiraceae), продуцирующих бутират и ацетат, и увеличенным относительным количеством специфических кишечных микробов Ruminococcus gnavus, которые вырабатывают воспалительные полисахариды, индуцирующие синтез фактора некроза опухоли-альфа. В свою очередь, фактор некроза опухоли-альфа способствует рекрутированию лейкоцитов путем индуцирования эндотелиальной экспрессии VCAM-1, ингибирует эндотелиальную синтазу NO и повышает количество активных форм кислорода. Более того, увеличение Ruminococcus gnavus – надежный предиктор прогрессирования ИБС после корректировки на обычные факторы КВР, такие как гиперлипидемия и сахарный диабет. Другой таксон, который был менее распространен у пациентов с ИБС, Ruminococcus gauvreauii, в основном продуцирует ацетат в качестве конечного продукта ферментации.
Помимо синтеза проатерогенного ТМАО, некоторые представители микробиоты кишечника могут изменять метаболизм холестерина через модификацию желчных кислот (ЖК). Рассматриваются варианты действия микроорганизмов на обмен ЖК, заключающиеся в их деконъюгации [33]. Этот механизм реализуется с помощью фермента под названием гидролаза желчных солей (ГЖС), который имеется у грамположительных кишечных бактерий, включая Lactobacillus, Clostridium, Listeria и Bifidobacterium. Представители рода Bacteroides являются единственными грамотрицательными бактериями, обладающими активностью ГЖС. В результате деконъюгации формируются свободные холевые кислоты, плохо реабсорбирующиеся в кишечнике. Следствием избытка свободных холевых кислот становится активация фарнезоидных Х-рецепторов в печени, которая ведет к подавлению фермента 7-α-гидроксилазы (CYP7A1), ответственного за превращение холестерина в ЖК [34]. Данные реакции приводят к изменению соотношения холестерин/ЖК, в сторону повышения уровня холестерина.
НОВЫЕ СТРАТЕГИИ В ПРОФИЛАКТИКЕ СЕРДЕЧНО-СОСУДИСТЫХ ЗАБОЛЕВАНИЙ И МИКРОБИОТА
Среди нутрицевтических средств коррекции ССЗ в мире наиболее широко используется экстракт красного ферментированного риса (ЭКФР) [35] – традиционный продукт питания, с древних времен используемый в Китае, Японии и Корее. Его полезные свойства было описаны еще в энциклопедии лекарственных продуктов питания в 1578 г. «Беньцао Ганму» («Компендиум лекарственных веществ»), составленной китайским ученым Ли Шинчжэнем в эпоху династии Мин.
На данный момент доступны различные безрецептурные формы ЭКФР, получаемого посредством ферментации риса с использованием грибов Monascus purpureus и содержащего не менее 13 разновидностей монаколина (включая монаколин К, известный как ловастатин) и другие биологически активные молекулы [36]. В сравнительных исследованиях при употреблении ЭКФР наблюдалась меньшая частота развития статин-индуцированной миопатии, нежели при приеме собственно статинов [37]. ЭКФР проявляет антиоксидантные, противовоспалительные и антигиперлипидемические свойства, способствующие улучшению обмена углеводов, модуляции липидного профиля и снижению жесткости стенок сосудов [38].
Пробиотические бактерии Вifidobacterium longum ВВ536 (В. longum ВВ536) изучаются с момента их открытия в 1969 г. К настоящему времени продемонстрирована способность этого штамма повышать концентрацию КЦЖК (фекального бутирата, биотина) [39], снижать в крови уровень липопротеидов низкой плотности (ЛПНП) и, напротив, повышать концентрацию липопротеидов высокой плотности (ЛПВП) [40]. Основной механизм гиполипидемического действия B. longum BB536 сводится к гидролизу глико- и тауроконъюгированных солей желчных кислот до глицина/таурина и свободных (деконъюгированных) солей ЖК [41]. Деконъюгированные соли ЖК менее эффективно реабсорбируются, чем их конъюгированные аналоги, и поэтому выводятся с калом. Они в большей степени адсорбируются бактериальными клетками и пищевыми волокнами в кишечнике, что также способствует увеличению их экскреции с калом [42]. Таким образом из энтерогепатической циркуляции выводится больше желчных солей, которые заменяются в печени желчными солями, вновь синтезируемыми из холестерина, что, в свою очередь, приводит к снижению уровня холестерина в сыворотке крови. В исследовании ученых Саудовской Аравии и Египта, представленном в 2021 г. [43], было показано синергическое положительное воздействие верблюжьего молока (концентрация холестерина в котором выше, чем в коровьем молоке), ферментированного с использованием B. longum BB536, на липидный профиль и показатели метаболического синдрома у крыс с ожирением, находившихся на высококалорийной диете.
Исходя из данных метаанализа [44], к другим специфическим холестеринснижающим штаммам пробиотиков относятся Bifidobacterium lactis и Lactobacillus acidophilus, VSL#3 и Lactobacillus plantarum. В метаанализе Wu Y. et al. выраженный эффект в плане снижения общего холестерина наблюдался на фоне приема пробиотиков, содержащих штамм Lactobacillus plantarum, а наибольшее уменьшение уровня ЛПНП отмечалось при применении того же Lactobacillus plantarum и Lactobacillus reuteri [45]. Другое исследование продемонстрировало, что штамм Lactobacillus acidophilus снижает общий холестерин и ЛПНП у лиц с умеренной гиперхолестеринемией [46].
Исследования, касающиеся влияния пробиотиков на снижение уровня ТМАО в сыворотке крови, пока что ограничены. Использование штамма Lactobacillus plantarum (ZDY04) снижало уровень ТМАО в сыворотке крови мышей за счет модуляции кишечной микробиоты, увеличив численность Lachnospiraceae на 78,8%, Bacteroidaceae – с 1,84±2,55 до 3,56±3,47% и снизив относительную численность рода Mucispirillum на 67%. Кроме того, этот штамм проявлял ангиопротективный эффект у ApoE/мышей (получавших холин) путем подавления развития ТМАО-индуцированного атеросклероза [47]. В другом исследовании было обнаружено, что Enterococcus faecium (WEFA23) увеличивает разнообразие кишечной микробиоты у крыс, получавших продукты с высоким содержанием жиров, а также снижает уровни ТМАО и общего холестерина [48].
Штамм Roseburia intestinalis (тип Firmicutes, класс Clostridia, порядок Clostridiales, семейство Lachnospiraceae) назван в честь американского микробиолога Теодора Роузбери, внесшего новаторский вклад в микробиологию. Впервые этот штамм был выявлен S. Duncan et al. (Великобритания) в фекалиях младенцев в 2001 г. с помощью секвенирования 16S pРНК и внесен в 2002 г. в немецкую коллекцию микроорганизмов и культур клеток Института Лейбница. Среди 20 наиболее распространенных бактерий в микробиоме кишечника на долю кластера Roseburia intestinalis приходится 0,9–5,0% (среднее значение 2,3%) от общего состава и количества микробиоты [49]. Об антиатерогенном действии Roseburia intestinalis впервые заговорили в 2018 г. [50]. Эта бактерия продуцирует бутират и взаимодействует с пищевыми растительными полисахаридами, снижая системное воспаление и атерогенез. Помимо этого, в 2023 г. было опубликовано исследование, в котором сообщается, что содержание Roseburia intestinalis в кале больных колоректальным раком значительно снижено по сравнению со здоровыми участниками [51]. В настоящее время сразу несколько видов рода Roseburia проходят стадию клинических испытаний с целью создания таргетных высокоактивных пробиотических форм для борьбы с метаболическим синдромом и атеросклеротическим изменением сосудов.
ЗАКЛЮЧЕНИЕ
Управление рисками ССЗ должно быть сосредоточено на персонифицированном подходе к пациенту и своевременном скрининге факторов КВР. Раннее выявление дислипидемии (еще до развития атеросклероза сосудов) и ее коррекция, прежде всего с помощью статинов, имеющих внушительную доказательную базу, обеспечивает профилактику острых сосудистых событий и приводит к улучшению исходов ССЗ [52]. Вместе с тем дислипидемия нередко недооценивается пациентами и врачами и недостаточно контролируется с использованием всех возможностей современной медицины [53].
Доступная пациентам информация о возможных побочных эффектах статинов зачастую становится причиной воздержания от их приема, что указывает на необходимость поиска дополнительных вариантов гиполипидемической терапии. В подобных ситуациях при низком КВР оптимальным представляется нутрицевтический подход к коррекции дислипидемии, имеющий в ряде случаев подтвержденные преимущества по сравнению с отсутствием лечения, а также при недостаточной приверженности медикаментозной терапии [54]; в качестве дополнительной меры возможен перевод пациента на низкие дозы статинов при непереносимости их высоких дозировок [55]. Кроме этого, полученные клинические данные подтверждают положительный эффект при гиперхолестеринемии ряда пробиотиков.



