ВВЕДЕНИЕ
В современном мире остается актуальным поиск и изучение новых биологических маркеров, способных помогать ранней диагностике сердечно-сосудистых заболеваний (ССЗ), служить лабораторным инструментом оценки эффективности терапии, прогностическим маркером возможных неблагоприятных клинических исходов и значимым критерием стратификации риска [1].
Несмотря на то что были идентифицированы многочисленные биомаркеры, их внедрение в клиническую практику до сих пор остается в значительной степени безуспешным. В то время как кардиоспецифические маркеры, включающие натрийуретические пептиды (НУП) и их предшественники (предсердный и мозговой НУП, ПНУП, ANP и BNP, proANP, proBNP и NT-proBNP) и высокочувствительные тропонины, широко применяются в клинической практике, необходимость использования других маркеров не имеет достаточной доказательной базы. В настоящее время только галектин-3 (Gal-3) и растворимый ST2 рецептор (sST2) – относительно новые биологические маркеры сердечной недостаточности (СН), которые включены в рекомендации Американской коллегии кардиологов (ACC) и Американской ассоциации сердца (AHA), но важность их применения в реальной клинической практике все еще требует подтверждения [2, 3].
Цель представленного обзора – рассмотреть липокалин, связанный с желатиназой нейтрофилов (NGAL), в качестве диагностического и прогностического маркера при сердечно-сосудистой патологии.
МЕТОДОЛОГИЯ ПОИСКА ИСТОЧНИКОВ
В нашей статье представлен обзор актуальных публикаций. Анализ источников литературы проводился в базах данных PubMed, РИНЦ, MedLine, Google Scholar, Science Direct, рассматривались зарубежные и отечественные статьи. Поиск проводился по следующим ключевым словам: «биологические маркеры», «сердечно-сосудистые заболевания», «липокалин, связанный с желатиназой нейтрофилов (NGAL)», biological markers, cardiovascular disease, neutrophil gelatinase associated lipocalin (NGAL). Наш обзор в основном включает описание исследований, выполненных за последние 10 лет. Также мы ссылаемся на отдельные основополагающие источники, написанные в более ранний период времени. Результаты различных исследований показывают, что существует огромный научный интерес к роли NGAL при кардиоваскулярной патологии.
ОСНОВНЫЕ ХАРАКТЕРИСТИКИ NGAL
NGAL, также называемый липокалином-2, – представитель большой группы липокалинов, являющихся внеклеточными белками с множеством функций. Этот белок острой фазы представляет собой гликозилированный мономер простых белковых цепей массой 25 килодальтон (кДа). Первоначально NGAL был идентифицирован как протеин, выделенный из гранул нейтрофилов [4], и впоследствии было доказано, что он ковалентно связан с желатиназой нейтрофилов (ферментом матриксной металлопротеиназной группы – коллагеназой IV, 92 кДа, который содержится в нейтрофилах) [5]. Помимо экскреции активированными нейтрофилами, NGAL высвобождается в небольших концентрациях эпителиальными клетками, клетками почечных канальцев, а в случае воспаления или повреждения – и гепатоцитами [4]. NGAL также обнаружен в эндотелиальных клетках и макрофагах [4, 5]. Его высокие уровни идентифицированы в адипоцитах людей с ожирением [6].
Уровни NGAL зависят от пола, возраста и функции печени и коррелируют с воспалительными параметрами [5]. Было продемонстрировано, что он функционирует как воспалительный модулятор иммунной системы. Это подтверждается тем фактом, что NGAL способен связывать сидерофоры, уменьшая количество связанного с ними железа, доступного для бактерий, ингибируя рост бактерий, истощая внутриклеточные запасы железа [4, 5]. Во время эмбрионального развития NGAL принимает участие в эпителиальной дифференцировке мезенхимальных стволовых клеток и образовании клубочков, проксимальных канальцев, петли Генле, а также дистальных канальцев [7].
Экспрессия NGAL значительно увеличивается при повреждении эпителиальных клеток почек, толстой кишки, печени и легких. Вероятно, это опосредуется группой факторов транскрипции, обозначаемых как NF-κB, которые способны быстро активировать клетки после острого повреждения [8]. Образование NGAL часто индуцируется при ряде опухолей человека, таких как новообразования кишечника, аденокарцинома молочной железы и уротелиальные карциномы [9–11]. Повышенная экспрессия NGAL – предиктор плохого прогноза при раке [9].
NGAL И АТЕРОСКЛЕРОЗ
Было высказано предположение, что NGAL может быть маркером атеросклероза; это подтверждено рядом исследований, продемонстрировавших чрезмерную экспрессию этого биомаркера в атеросклеротических бляшках на участках с высокой протеолитической активностью [12–14]. Также было высказано предположение, что роль NGAL у пациентов с ишемической болезнью сердца (ИБС) связана с матриксной металлопротеиназой-9 (MMP-9) [15]. В ряде исследований сообщается, что NGAL служит маркером неблагоприятных сердечно-сосудистых событий у пациентов с СН.
Комплекс между NGAL и MMP-9 ингибирует деградацию MMP-9, тем самым увеличивая ее протеолитическую активность (рис.) [13, 15]. Повышенная активность MMP-9 приводит к увеличению деградации базальных мембран и внеклеточного матрикса – процесса, наблюдаемого при прогрессировании нестабильности атеросклеротических бляшек [13,15]. В атеросклеротических бляшках деградация коллагена под действием MMP-9 достаточно высока, что вызывает дестабилизацию фиброзной капсулы и делает бляшку более восприимчивой к эрозии и разрыву [13, 15]. Повышенная активность ММР-9 также вызывает деградацию коллагена [13, 15].
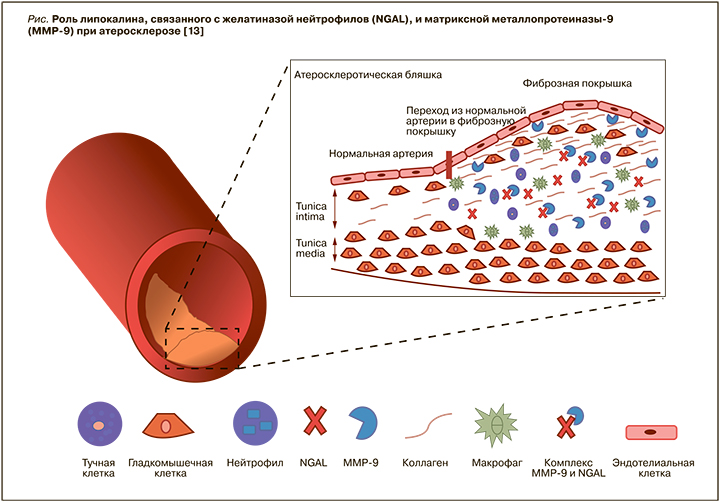
Hemdahl A. et al. [12] проанализировали взаимосвязь между экспрессией NGAL в атеросклеротических бляшках и инфарктом миокарда (ИМ) у мышей, подвергшихся гипоксическому стрессу. У мышей c развившимся ИМ были отмечены более высокие концентрации NGAL и MMP-9 по сравнению с мышами со стабильными бляшками. В образцах атеросклеротических бляшек, полученных при каротидной эндартерэктомии у людей, экспрессия NGAL и MMP-9 наблюдалась и в макрофагах [12].
В 2017 г. австрийские ученые исследовали уровни NGAL и MMP-9/NGAL в образцах крови 136 пациентов со стенозом сонных артерий [14]. Пациенты с уязвимыми бляшками, определенными при ультразвуковом исследовании (бляшки со сниженной эхогенностью) и гистологическом анализе (тип VI по классификации AHA), продемонстрировали самые высокие концентрации NGAL и MMP-9/NGAL. У больных с клинически явным атеросклерозом сонных артерий были выявлены значительно более высокие уровни NGAL по сравнению с бессимптомными больными. В многомерном регрессионном анализе NGAL, но не MMP-9/NGAL, был независимо связан с клинически явным стенозом сонных артерий. Авторы резюмировали, что циркулирующие NGAL и MMP-9/NGAL ассоциированы с уязвимостью бляшек у пациентов со стенозом сонных артерий [14].
Те же ученые при обследовании 83 пациентов с бессимптомным стенозом сонных артерий пришли к выводу, что уровни циркулирующих NGAL и MMP-9/NGAL значительно увеличиваются у бессимптомных пациентов с уязвимыми атеросклеротическими бляшками. Это свидетельствует о том, что сывороточный NGAL может быть предложен в качестве ценного биомаркера для обнаружения нестабильных каротидных бляшек у бессимптомных пациентов, которым в последующем, вероятнее всего, потребуется проведение каротидной эндартерэктомии или стентирования сосудов [16].
NGAL ПРИ СТАБИЛЬНОЙ ИШЕМИЧЕСКОЙ БОЛЕЗНИ СЕРДЦА
Paulsson J. et al. [17] сравнивали уровни нейтрофилов и комплексов MMP-9/NGAL у пациентов со стабильной ИБС и сопоставимых по возрасту и полу здоровых добровольцев. Значения нейтрофилов у пациентов и здоровых людей не различались ни в периферической крови, ни в очаге интенсивного воспаления. Однако уровень интерлейкина-8 (ИЛ-8), который, как известно, стимулирует высвобождение NGAL из нейтрофильных гранул, был выше у пациентов ИБС. Также у больных ИБС была отмечена высокая концентрация комплекса MMP-9/NGAL [17].
Другое исследование, проведенное в 2015 г. на базе сердечно-сосудистого отделения Токийского университета, показало, что больные ИБС с трехсосудистым поражением коронарных артерий по данным коронароангиографии (КАГ) имели более высокие уровни NGAL в сыворотке крови по сравнению с пациентами с однососудистым поражением [18]. Установлена положительная связь между NGAL и анатомической шкалой оценки риска SYNTAX Score (инструмент определения тяжести поражения коронарного русла и прогнозирования исходов эндоваскулярных вмешательств). Больные с высокими концентрациями NGAL (>100 нг/ мл) и BNP (>25 пг/мл) имели более высокий балл SYNTAX по сравнению с пациентами с низкими показателями NGAL (<100 нг/мл) и BNP (<25 пг/ мл) [18].
Woitas R. et al. проанализировали прогностическую роль NGAL у пациентов со стабильной и нестабильной ИБС и сообщили, что уровни NGAL в плазме крови независимо связаны с общей смертностью, а также сердечно-сосудистой смертностью после поправки на общепринятые факторы риска ССЗ [19].
Исследование Li C. et al. было направлено на сравнение уровней NGAL, MMP-9, высокочувствительного С-реактивного белка (вч-СРБ) и ИЛ-1β при разных клинических формах ИБС, а также оценке взаимосвязи между этими биологическими маркерами и тяжестью поражения коронарных артерий у пациентов без заболеваний почек. Согласно клиническому протоколу, было обследовано 365 пациентов, всем им была проведена КАГ. В исследование было включено 124 пациента с ИМ с подъемом сегмента ST (ИМпST), 117 пациентов со стабильной стенокардией и 124 пациента без наличия атеросклеротических бляшек по данным КАГ. Согласно шкале SYNTAX, пациенты с ИМпST и пациенты со стабильной стенокардией были разделены на 2 группы: с высоким (≥33; n=29) и низким (<33; n=212) баллом. Уровни NGAL в плазме, MMP-9 и вч-СРБ у пациентов с ИМпST были выше, чем у пациентов со стабильной стенокардией и контрольной группой (p <0,05); концентрации NGAL и вч-СРБ в плазме у пациентов со стабильной стенокардией значительно превышали таковые в группе контроля (p <0,05). Уровни плазменного ИЛ-1β были аналогичным во всех трех группах (p >0,05). Показатель SYNTAX был положительно связан с NGAL (коэффициент корреляции (r)=0,363; p <0,001), MMP-9 (r=0,377; p <0,001) и вчСРБ (r=0,163; p <0,011). Показатель SYNTAX не был связан с ИЛ-1β (r=- 0,043; p=0,510). Отмечалась положительная корреляция NGAL с MMP-9 (r=0,601; p <0,001) и ИЛ-1β (r=0,159; p=0,014). Площадь под кривой ошибок (AUC ROC) для NGAL, определяющего тяжелый коронарный стеноз, составляла 0,838 (95% доверительный интервал (ДИ): 0,752–0,923; p <0,001), что было больше, чем для MMP-9 (0,818; 95% ДИ: 0,724–0,912; p <0,001), ИЛ-1β (0,485; 95% ДИ: 0,369–0,601; p=0,791) и вчСРБ (0,607; 95% ДИ: 0,492–0,722; p=0,061). Многофакторный регрессионный анализ показал, что уровни NGAL в плазме были независимо связаны с высокими показателями SYNTAX (отношение шансов (OR)=1,109; 95% ДИ: 1,104–1,114; p <0,001). Авторы резюмировали, что NGAL можно рассматривать как маркер стратификации риска пациентов с ИБС [20].
Chong J. et al. наблюдали 1131 амбулаторного пациента без клинических проявлений ИБС в течение 14,5 лет. Доктора констатировали, что уровень NGAL был ассоциирован с повышенным риском долгосрочных событий ИБС независимо от общепринятых факторов риска и других биомаркеров [21].
Soylu K. et al. оценивали жесткость аорты и уровни NGAL у пациентов ИБС. Контрольную группу составили пациенты, которые имели схожие профили риска и метаболические параметры, а также отсутствие атеросклеротического поражения сосудов по данным КАГ. Уровни NGAL в сыворотке крови были выше в группе ИБС по сравнению с контрольной группой (79,29±38,8 против 48,05±21,4 нг/мл; p <0,001). Концентрации NGAL отрицательно коррелировали с показателями деформации аорты (p <0,01; r=0,57) и ее растяжимости (p <0,001; r=0,62) и положительно – с индексом жесткости аорты (p <0,001; r=0,72) [22].
Китайские врачи измерили сывороточный адипонектин, A-FABP (белок, связывающий жирные кислоты адипоцитов), NGAL, FGF-19 (фактор роста фибробластов 19) и FGF-21, IAP-1 (ингибитор активатора плазминогена-1) и RBP4 (ретинолсвязывающий белок-4) у 1166 пациентов с ИБС. Авторы пришли к заключению, что комбинация трех биомаркеров – NGAL, A-FABP и FGF- 19 – имеет важное значение в прогнозировании неблагоприятных сердечно-сосудистых событий у больных с данной патологией [23].
В исследование Liu H. et al. было включено 633 пациента со стабильной ИБС. Измерения сывороточного NGAL и других лабораторных показателей проводились в течение 24 ч после поступления в стационар. Для оценки взаимосвязи между сывороточным NGAL и смертностью от ССЗ в течение 10-летнего периода наблюдения использовали скорректированный анализ. Кривая Каплана–Мейера показала, что пациенты с высоким уровнем NGAL, как правило, имели более высокий показатель смертности от ССЗ, чем пациенты с низким уровнем этого биомаркера. Многофакторная модель Кокса продемонстрировала, что повышенные уровни NGAL были независимо связаны с высоким риском смерти от ССЗ (отношение рисков (HR)=2,62; 95% ДИ: 1,51–4,96; p <0,001) в течение 10-летнего периода наблюдения после поправки на сопутствующие факторы. ROC-анализ показал, что сывороточный NGAL (AUC=0,917; 95% ДИ: 0,895–0,940; p <0,001) имел идеальную прогностическую ценность в отношении летальности от ССЗ [24].
NGAL И ОСТРЫЙ КОРОНАРНЫЙ СИНДРОМ
Связь между уровнями NGAL и оценками риска (клиническими и ангиографическими) изучалась у пациентов с диагнозом ИМ без подъема сегмента ST (ИМбпST) и сравнивалась со здоровыми людьми [25]. В группе больных наблюдались высокие уровни NGAL, вчСРБ и лейкоцитов. Кроме того, NGAL положительно коррелировал с оценкой риска по шкале GRACE (Global Registry of Acute Coronary Events – шкала, используемая для оценки риска острого коронарного синдрома) [22].
В 2016 г. сотрудниками НИИ комплексных проблем сердечно-сосудистых заболеваний и Кемеровской государственной медицинской академией была выполнена работа по изучению клинической и прогностической значимости NGAL у больных ИМпST. Было обследованы 85 больных, госпитализированных по поводу ИМпST давностью менее 24 ч. Пациентам определяли концентрации сывороточного NGAL в 1-е и 12-е сутки после поступления в стационар. В течение 36 мес наблюдения оценивали развитие повторных ИМ и летальность. Медиана концентрации NGAL в 1-е и 12-е сутки заболевания составила 1,33 (0,36–1,90) и 1,63 (1,25–2,61) нг/мл соответственно, что в 3,32 и 4,07 раза превышало референтные значения. Таким образом, на 12-е сутки заболевания концентрация NGAL увеличилась на 22,55% (р=0,0009). Обнаружена связь более высоких уровней NGAL, определяемого на 12-е сутки ИМ, с наличием у больных структурного поражения почек, трехсосудистого поражения коронарных артерий и с передней локализацией ИМ. У лиц с уровнем NGAL выше 2,6 нг/мл частота смертельных исходов увеличивалась с 9,52 до 31,83% (OR 4,42; 95% ДИ: 1,30–15,16; р=0,012). Авторы пришли к выводу, что повышенные концентрации NGAL в крови пациентов ИМпST ассоциируются с тяжелым клиническим статусом. У больных, подвергшихся чрескожному коронарному вмешательству (ЧКВ), повышение уровня NGAL к 12-м суткам наблюдения было незначимым, тогда как у больных без ЧКВ этот показатель увеличился более чем в 3 раза. Концентрация NGAL более 2,6 нг/мл на 12-е сутки госпитализации была связана с 4-кратным увеличением общей смертности в течение 36 мес наблюдения [25].
Avci A. et al. включили в исследование 235 пациентов с ИМпST, которые были разделены на группы в соответствии с показателями фракции выброса левого желудочка (ФВ ЛЖ). Измеряли NGAL в плазме, тропонин I, креатинкиназу MB (КФК-МБ, CK-MB) и СРБ. Согласно клиническому протоколу, было обследовано 34 пациента, страдающих СН со сниженной ФВ ЛЖ (СНнФВ), и 34 пациента с СН с сохранной ФВ ЛЖ (СНсФВ). Все больные проспективно наблюдались в течение 6 мес. Далее пациентов разделили на две подгруппы: умерших (n=14) и выживших (n=34). Концентрации NGAL были выше у пациентов СНнФВ, чем у СНсФВ, но различия оказались статистически незначимыми (p=0,07). Уровни NGAL в плазме были значительно выше у умерших пациентов, чем у выживших (p <0,001). Анализ ROC-кривой показал предикторное значение маркера NGAL в отношении сердечно-сосудистой смертности – 190 нг/мл (чувствительность 86% и специфичность 77%) [26].
Отечественными врачами М.А. Шаленковой с соавт. было проведено исследование, целью которого служила оценка роли NGAL при внепочечных осложнениях у больных с острым коронарным синдромом (ОКС). У 110 пациентов с ОКС в 1–3-й день госпитализации определяли содержание NGAL в сыворотке крови (s-NGAL) и мочевого (u-NGAL), NT-proBNP в крови; также участникам исследования выполняли эхокардиографию (ЭхоКГ). В госпитальном периоде определяли частоту развития неблагоприятных сердечно-сосудистых событий и гемодинамические параметры при поступлении (систолическое, диастолическое артериальное давление, частоту сердечных сокращений). Концентрация u-NGAL при острой СН (10,4 (2,7; 51,2) нг/мл) была статистически достоверно выше, чем у больных с ОКС без острой СН (3,8 (1,7; 8,6) нг/мл; р=0,03). Обнаружены более высокие значения u-NGAL и NT-proBNP у больных ОКС, имеющих признаки повышения давления в легочной артерии (10,17 (4,87; 51,2 нг/ мл) и 744,6 (368,7; 2034,9) пг/мл), по сравнению с больными без таких изменений (3,41 (1,72; 7,39) нг/ мл; р=0,004 и 431,8 (99,6; 780,1) пг/мл; р=0,012). Установлена предикторная способность величины u-NGAL >9,96 нг/мл в отношении развития острой СН, а при значении u-NGAL >5,81 нг/мл – легочной гипертензии. Получена значимая положительная связь показателей u-NGAL с индексами конечно-диастолического размера (КДР), конечно-систолический размера (КСР); отрицательная с индексом конечно-диастолического объема (КДО) и ФВ ЛЖ; концентрации s-NGAL положительно коррелировали с минутным объемом (МО) и сердечным индексом (СИ). Установлена статистически достоверная прямая взаимосвязь уровней u-NGAL и NT-proBNP [27].
Целью работы Sabanovic-Bajramovic N. et al. была оценка ассоциаций NGAL с риском возникновения осложнений у пациентов с ИМпST, получивших фибринолитическую терапию до выполнения ЧКВ. В исследование вошли 54 пациента с диагнозом ИМпST, получавших фибринолитическую терапию (альтеплаза) до ЧКВ. Всем больным были выполнены КАГ и ЧКВ в течение максимум 48 ч после фибринолиза. Образцы крови брали сразу после поступления перед введением альтеплазы. Пациенты были разделены на две группы в соответствии со значениями NGAL (меньше или больше 134,05 нг/мл). Уровни NGAL продемонстрировали положительную связь со средним систолическим и диастолическим артериальным давлением (p=0,001 и p=0,003 соответственно), концентрациями BNP (p=0,0001) и высокочувствительным тропонином I (p=0,002). В группе больных с высоким уровнем NGAL относительный риск летального исхода был выше в 6,4 раза (p=0,002), развития СН – в 2,88 раза (p=0,0002), ранней постинфарктной стенокардии – в 2,24 раза (p=0,0158), желудочковых нарушений ритма (желудочковой тахикардии, фибрилляции желудочков) – в 1,96 раза (p=0,0108) [28].
Профессор медицинского факультета Университета Осло Nymo S. et al. изучили связь между показателями NGAL и прогнозом у пациентов ИМбпST и ИМпST. Концентрация NGAL была измерена в крови 1121 пациента с ОКС (30% женщин, средний возраст 65 лет) в первое утро после поступления в стационар. После коррекций по 14 переменным концентрация NGAL предсказывала долгосрочную (медиана 167 мес) смертность (HR 1,33; 95% ДИ: 1,10–1,61; p=0,003) для четвертого квартиля (q) концентрации NGAL. Уровни NGAL также предсказывали долгосрочную смертность (HR 1,63; 95% ДИ: 1,31–2,03; p <0,001; n=741) при коррекции баллов по шкале GRACE, а также ФВ ЛЖ и концентрации BNP и CRP. После такой коррекции концентрация NGAL предсказывала долгосрочную смертность у пациентов с ИМбпST (HR 2,02; 95% ДИ: 1,50–2,72; p <0,001), но не у пациентов с ИМпST (HR 1,32; 95% ДИ: 0,95–1,83; p=0,100). У всех пациентов комбинация концентрации NGAL и оценки GRACE дала HR 5,56 (95% ДИ: 4,37–7,06; p <0,001) для q4/q4 в отношении обеих переменных. Ученые резюмировали, что уровень NGAL при ОКС связан с долгосрочным прогнозом после поправки на клинические факторы, а измерение уровней циркулирующего NGAL может помочь выявить пациентов, особенно с ИМбпST, нуждающихся в более тщательном наблюдении после ОКС [29].
Небольшое исследование Lahiri A. et al. показало, что NGAL в плазме у пациентов ОКС обладает прогностической ценностью в отношении госпитальной летальности [30].
В 2021 г. доктора отделения кардиологии Университетской больницы Копенгагена определяли концентрации NGAL у 1626 пациентов с ИМпST при поступлении в стационар. Больные были стратифицированы в соответствии с квартилями NGAL (q1–4). Повышение концентрации NGAL было связано с более старшим возрастом, большим количеством сопутствующих заболеваний и худшими показателями, включая низкое артериальное давление и ФВ ЛЖ. С поправкой на факторы, связанные с неблагоприятным исходом, NGAL оставался независимо связанным как с поздним развитием кардиогенного шока (q4 по сравнению с q1–3; OR 3,64; 95% ДИ: 1,79–7,41), так и с 30-дневной смертностью (HR 3,18; 95% ДИ: 1,73–5,84) [31].
NGAL У ПАЦИЕНТОВ С СЕРДЕЧНОЙ НЕДОСТАТОЧНОСТЬЮ
Yndestad A. et al. показали, что у пациентов с хронической СН (ХСН) III функционального класса (ФК) по NYHA (Нью-Йоркская классификация ФК СН) уровень NGAL в плазме был значительно выше, чем у пациентов с I или II ФК по NYHA и контрольной группой. Кроме того, высокие исходные уровни NGAL в плазме были связаны с увеличением частоты комбинированной конечной точки (нефатального ИМ, инсульта, сердечно-сосудистой смерти и общей летальности) через 27 мес (медиана) наблюдения. Авторы также сообщили о значительной связи между NGAL в плазме и NT-proBNP [32].
В исследовании Villacorta H. et al. прогностическая ценность исходного уровня NGAL была оценена у 61 пациента ХСН. Первичной конечной точкой была комбинация смерти от ССЗ и госпитализаций по поводу СН в течение 10,6 мес (медиана) наблюдения. У 15 пациентов, достигших конечной точки, наблюдались более высокие исходные концентрации NGAL. Исследователи пришли к выводу, что NGAL в плазме может быть предиктором неблагоприятного исхода у пациентов с СН [33].
У 2130 пациентов с ХСН, участвовавших в исследовании GISSI-HF, измеряли отношение альбумина к креатинину в моче (UACR), скорость клубочковой фильтрации (СКФ) и три маркера повреждения канальцев в моче – N-ацетил-бета-D-глюкозаминидазу (NAG), молекулу повреждения почек 1 (KIM-1) и NGAL. Было выявлено, что повышенный уровень NGAL в моче был ассоциирован с общей смертностью и частотой госпитализаций по поводу декомпенсации СН у этой категории больных [34].
Maisel et al. [35] исследовали прогностическое значение NGAL в плазме у пациентов с острой СН. Пациенты наблюдались в течение 30 дней, первичной комбинированной конечной точкой служила повторная госпитализация по поводу СН или смертность от всех причин. Уровни NGAL были значительно выше у пациентов с неблагоприятными сердечно-сосудистыми событиями. Пациенты с неблагоприятными сердечно-сосудистыми событиями также имели более высокие уровни BNP. AUC при использовании анализа ROC (площадь под кривой ошибок) была выше для NGAL (0,73), чем для BNP (0,65), что указывает на то, что NGAL в плазме является хорошим предиктором комбинированной конечной точки через 30 дней у пациентов с острой СН. Кроме того, сочетание высокого NGAL и высокого BNP или высокого NGAL и низкого BNP была связана с более высоким риском повторной госпитализации, связанной с СН, или смертности от всех причин, чем низкий NGAL и низкий BNP или низкий NGAL и высокий BNP.
Oikonomou E. et al. при обследовании пациентов, страдающих хронической СН, показали, что уровни NGAL прямо коррелировали с Gal-3 (rho=0,26; p = 0,04) и NT-proBNP (rho=0,30; p=0,005) и обратно коррелировали с ФВ ЛЖ (rho=-0,31; p=0,02) и значением клиренса креатинина [36].
Результаты исследования Labr K. et al. продемонстрировали, что NGAL, связанный с NT-proBNP, был более сильным предиктором неблагоприятных сердечно-сосудистых событий (смертность от всех причин, госпитализация по поводу острой СН, имплантация вспомогательного устройства ЛЖ и ортотопическая трансплантация сердца), чем значения только NGAL или только NT-proBNP [37].
ЗАКЛЮЧЕНИЕ
Поиск новых биологических маркеров, изучение их патофизиологической роли и изменения их уровня под действием различных вариантов лечения позволяют глубже понять патогенетические аспекты развития и течения ССЗ [2]. Новые биомаркеры, такие как фактор роста фибробластов-23, адреномедуллин, маркер фиброза Gal-3, стимулирующий фактор роста ST2, хемокин-CX3CL1, суррогатный маркер вазопрессина и другие, все больше находят свое место в реальной клинической практике [38, 39]. По данным современной литературы, уровень описанного в нашем обзоре биологического маркера NGAL повышается при различных вариантах ИБС, а также может выступать медиатором ремоделирования сердца у пациентов, перенесших ИМ, и выступать предиктором неблагоприятных сердечно-сосудистых событий как при хронической, так и острой СН.
В настоящее время мы имеем современные технологии для идентификации новых биологических маркеров. Следующим закономерным шагом, вероятнее всего, станет создание мультимаркерной модели. Конечно, для этого потребуется совершенствование биоинформационных технологий, необходимых для анализа большой базы данных. Возможности этой области огромны не только для обнаружения новых биомаркеров, но и возможного прогресса в лечении СН. Необходимо дальнейшее более глубинное понимание роли NGAL, а также будущие клинические исследования для определения диагностической, прогностической и, возможно, терапевтической значимости этого маркера.



