ВВЕДЕНИЕ
Лечение пациентов с синдромом приобретенного иммунодефицита (СПИД) имеет определенные сложности, связанные с необходимостью применения не только антиретровирусной терапии (АРТ) для подавления ВИЧ и нормализации иммунологических показателей, но и лечения вторичных заболеваний. Схема АРТ у этой категории больных должна иметь максимальную вирусологическую эффективность, способствовать быстрой иммунной реституции и быть метаболически благоприятной.
Подбор АРТ пациентам затруднен из-за необходимости учитывать межлекарственные взаимодействия с препаратами для лечения вторичных заболеваний [1]. В ранее проведенном исследовании показано, что на исход лечения пациентов с ко-инфекцией ВИЧ/туберкулез влияет не столько время начала АРТ или исходный уровень CD4 Т-лимфоцитов, сколько другие факторы, включающие потенциальные лекарственные взаимодействия, побочные эффекты от терапии, большое число одновременно применяемых лекарственных средств и тяжесть сопутствующего заболевания [2]. Применяемые схемы терапии с базовым препаратом из группы ненуклеозидных ингибиторов обратной транскриптазы (ННИОТ) 1-го поколения (эфавиренз) имеют меньшую вирусологическую эффективность, чем схемы терапии с ингибиторами интегразы (ИИ). По сравнению с эфавирензом, через 48 нед терапии долутегравиром или ралтегравиром неопределяемый уровень вирусной нагрузки ВН отмечался чаще: отношение шансов (ОШ) 1,87 (95% доверительный интервал (ДИ): 1,34–2,64) и 1,40 (ДИ: 1,02–1,96) соответственно [3]. При этом у ранее не получавших лечение пациентов, начавших АРТ на основе эфавиренза, чаще регистрировались побочные эффекты средней и тяжелой степени [4].
Применение АРТ на основе ралтегравира у пациентов, переключенных с других схем лечения, снижало уровень триглицеридов (р <0,01), в то время как трансаминазы печени, функция почек и уровень холестерина колебались, оставаясь в пределах нормальных значений, а у пациентов, ранее не получавших лечение, терапия этим препаратом существенно не влияла на функцию почек, печени и липидный профиль сыворотки крови [5] даже при значительном превышении дозы [6]. Лечение схемами АРТ на основе этравирина у ранее не получавших лечение пациентов к концу 48 нед терапии не демонстрировало существенных изменений общего холестерина, липопротеидов низкой плотности, триглицеридов или глюкозы натощак [7].
В настоящем исследовании использовалась схема АРТ, имеющая три мишени воздействия (интегразу ВИЧ, обратную транскриптазу ВИЧ при помощи нуклеозидного и ненуклеозидного ингибиторов этого фермента) и состоящая из препаратов, которые не оказывают значимого воздействия на метаболические показатели.
Цель исследования – определить эффективность и безопасность применения у пациентов с поздней стадией ВИЧ-инфекции схемы АРТ, имеющей три мишени воздействия.
МАТЕРИАЛ И МЕТОДЫ
Проведено открытое проспективное наблюдательное исследование эффективности и безопасности лечения 110 ранее не леченных пациентов с ВИЧ-инфекцией стадией 4В (СПИД). Наблюдение проводилось в течение 24 нед от момента начала АРТ. В зависимости от получаемой терапии пациенты были поделены на две группы:
- группа 1 (n=65): лечение тремя препаратами из трех классов – ИИ (ралтегравир), ННИОТ 2-го поколения (этравирин), нуклеозидным ингибитором обратной транскриптазы (НИОТ – ламивудин);
- группа 2 (n=45): лечение тремя препаратами из двух классов (базовым препаратом и двумя препаратами из класса НИОТ).
Пациенты наблюдались в условиях инфекционного отделения федеральной клиники. В процессе лечения оценивалась динамика клинических (симптомы вторичных заболеваний), вирусологических (ВН), иммунологических (субпопуляции Т-лимфоцитов), метаболических показателей (креатинин, трансаминазы, холестерин, альбумин). Дополнительно изучалась динамика ДНК TREC (кольцевых фрагментов ДНК, образующихся при созревании Т-лимфоцитов; в количественном выражении этот показатель служит маркером активности тимуса, позволяя оценить возможности восстановления количества CD4 Т-лимфоцитов) для выявления пациентов с риском иммунологической неэффективности.
Инструментальная и лабораторная диагностика вторичных инфекций включала следующие обследования (по показаниям):
- инструментальные исследования: компьютерная томография (КТ) органов грудной клетки, ультразвуковое исследование почек и надпочечников, КТ органов брюшной полости с контрастированием, электрокардиография, фибробронхоскопия;
- лабораторные исследования: рутинные клинические и биохимические исследования, выявление генетического материала возбудителей методом полимеразной цепной реакции (ПЦР) в различном биологическом материале (мокроте, бронхоальвеолярном лаваже, крови, моче, кале, ликворе), серологические исследования (выявление антител к токсоплазме, герпес-вирусам 1, 2, 4, 5, 6 типа), микробиологические исследования (посев на микобактерию туберкулеза, нетуберкулезные микобактерии, неспецифическую микрофлору, грибы).
Оценка показателей осуществлялась до начала лечения и через каждые 12 нед АРТ. Первичной конечной точкой служила вирусологическая и иммунологическая эффективность АРТ. Вирусологическая эффективность определялась по среднему уровню ВН и доле пациентов, имевших неопределяемую ВН (РНК ВИЧ менее 50 копий/мл). Иммунологическая эффективность оценивалась по количеству и приросту CD4 Т-лимфоцитов.
Вторичной конечной точкой исследования была динамика дополнительных маркеров: количество ДНК TREC на 105 лейкоцитов, метаболические показатели.
Уровень ВН, количество ДНК TREC исследовались методом ПЦР в «реальном времени», количество CD4, CD8 Т-лимфоцитов – методом проточной цитометрии, общий анализ крови, биохимический анализ крови с использованием рутинных методик.
Статистическая обработка результатов выполнялась с помощью пакета статистических программ Excel, SPSS 23.0, использовались параметрические и непараметрические критерии. Различия считались статистически значимыми при р <0,05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Характеристика групп исследования
Основные демографические, клинические, вирусологические и иммунологические показатели в группах сравнения представлены в таблице 1.
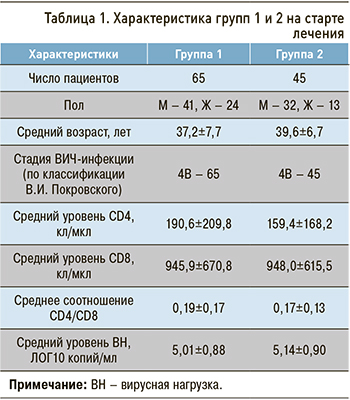
Группы были сопоставимы по возрасту пациентов (р=0,134), полу (р=0,381), стадии ВИЧ-инфекции (все пациенты имели стадию 4В), количеству CD4 Т-лимфоцитов (р=0,471), CD8 Т-лимфоцитов (р=0,988), соотношению CD4/CD8 (р=0,502), уровню ВН (р=0,487), а также по стартовому уровню метаболических показателей: АЛТ (р=0,773), АСТ (р=0,940), креатинина (р=0,955), холестерина (р=0,479), альбумина (р=0,148), общего билирубина (р=0,587), прямого билирубина (р=0,460).
Все пациенты в обеих группах имели клинические проявления ВИЧ-инфекции (рис. 1).
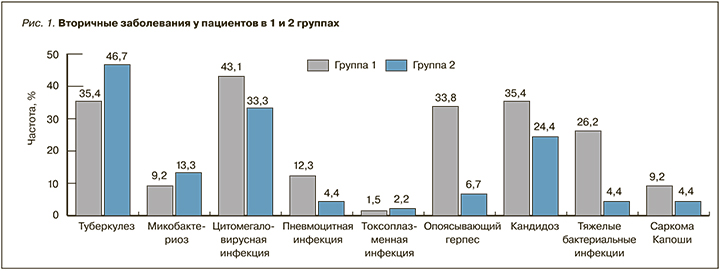
Наиболее частым вторичным заболеванием в группе 1 была активная цитомегаловирусная инфекция (у 28 пациентов из 65), а в группе 2 – туберкулез (21 из 45). В группе 1 у 21,5% (14/65) пациентов имело место одно ассоциированное с ВИЧ-инфекцией заболевание, у 78,5% (51/65) – два и более. В группе 2 аналогичные показатели составляли 71,1% (32/45) и 28,9% (13/45). Вирусный гепатит С наблюдался у 16,9% (11/65) и 20% (9/45) пациентов, вирусный гепатит В – у 3,1% (2/65) и 2,2% (1/45) пациентов в группах 1 и 2 соответственно. В группе 2 был один случай гепатитов В+С.
Во всех случаях лечение пациентов начиналось с терапии вторичных заболеваний, АРТ присоединяли к концу второй недели лечения в соответствии с рекомендациями ВОЗ [8]. Большинство пациентов получало 5–6 препаратов и более.
В группе 1 52,3% (34/65) пациентов получали сульфаметоксазол + триметоприм (как с лечебной, так и профилактической целью), 38,5% (25/65) – ганцикловир, 4,6% (3/65) – валганцикловир, 36,9% (24/65) – противотуберкулезные препараты (ПТП), 12,3% (8/65) – ацикловир, 35,4% (23/65) – противогрибковые средства, 26,1% (17/65) – антибиотики широкого спектра действия, 9,2% (6/65) – средства терапии микобактериоза (кларитромицин, рифабутин, левофлоксацин или моксифлоксацин, этамбутол), 1,5% (1/65) – липосомальный доксорубицин, 13,8% (9/65) – средство химиопрофилактики туберкулеза (изониазид). В группе 2 пациенты в 46,7% (21/45) случаев получали ПТП, в 31,1% (14/45) – ганцикловир, в 4,4% (2/45) – валганцикловир, в 24,4% (11/45) – сульфаметоксазол+триметоприм, в 24,4% (11/45) – противогрибковые средства, в 6,7% (3/45) – ацикловир, в 4,4% (2/45) – антибиотики широкого спектра действия, в 13,3% (6/45) – средства терапии микобактериоза (кларитромицин, рифабутин, левофлоксацин или моксифлоксацин, этамбутол), в 2,2% (1/45) – средство химиопрофилактики туберкулеза (изониазид).
В группе 1 все пациенты получали единую схему АРТ. В группе 2 базовым препаратом в схеме у 13,3% (6/45) пациентов служил ННИОТ 1-го поколения, у 37,8% (17/45) – ингибитор протеазы (в двух случаях – дарунавир, бустированный ритонавиром, в остальных – лопинавир, бустированный ритонавиром), у 48,9% (24/45) – ИИ. У 93,3% случаев (42/45) в состав нуклеозидной основы входил тенофивир, в 6,7% (3/45) вместо него использовался абакавир.
Динамика клинических проявлений ВИЧ-инфекции
За период наблюдения летальных исходов у пациентов в группах исследования не было. В 38,5% (25/65) случаев в группе 1 и в 33,3% (15/45) в группе 2 (р=0,583) отмечался полный регресс симптомов вторичных заболеваний, в 56,9% (37/65) и 62,2% (28/45) случаев (р=0,579) – уменьшение симптомов вторичных заболеваний, в 4,6% (3/65) и 4,4% (2/45) случаев (р =0,967) – отсутствие выраженной динамики (в обеих группах все случаи микобактериальной инфекции). Синдром восстановления иммунной системы (СВИС) регистрировался у 3,1% (2/65) и 2,2% (1/65) пациентов 1-й и 2-й групп соответственно (р=0,787). На фоне коррекции терапии вторичных заболевания проявления СВИС у пациентов обеих групп были купированы. Различий в клинических исходах по окончании 24 нед терапии между группами не было.
Динамика вирусологических показателей
У пациентов обеих групп ВН критически снижалась в течение первых 12 нед АРТ до уровня 1,38 ЛОГ 10 копий/мл и 2,02 ЛОГ 10 копий в группах 1 и 2 соответственно (р=0,004), затем темпы снижения замедлялись. Через 24 нед АРТ средний уровень ВН составил 1,04 и 1,37 ЛОГ10 копий/мл в группах 1 и 2 соответственно (р=0,049), неопределяемый уровень ВН отмечался у 55 пациентов (84,6%) в группе 1 и у 30 (66,7%) в группе 2 (р=0,028). У остальных пациентов в группе 1 ВН колебалась в пределах 1,7–2,0 ЛОГ 10 копий/мл (50–100 копий/мл), в группе 2 – 1,7–2,95 ЛОГ 10 копий/мл (50–890 копий/мл; рис. 2).
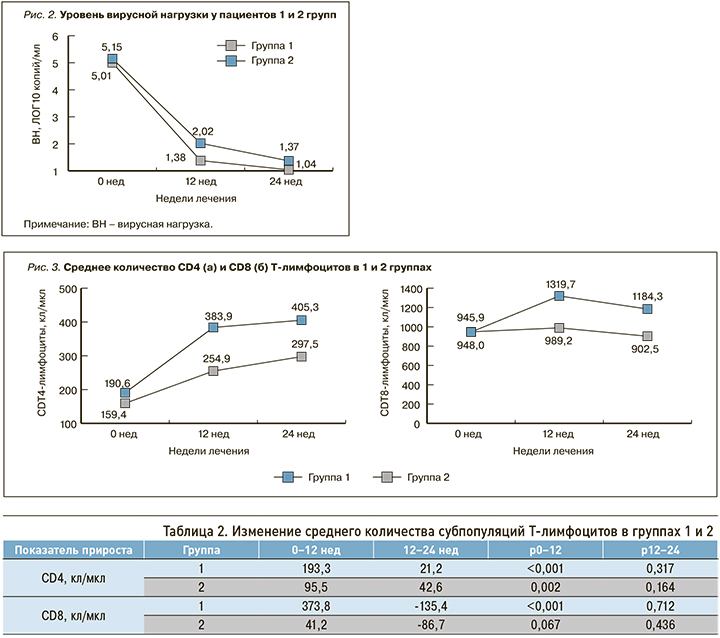
Через 24 нед АРТ в 1-й группе исследования средний уровень ВН был значимо ниже, а доля пациентов с неопределяемой ВН – выше. Подавление репликации вируса достигалось быстрее и в большем числе случаев при лечении схемой с тремя точками приложения.
Динамика иммунологических показателей
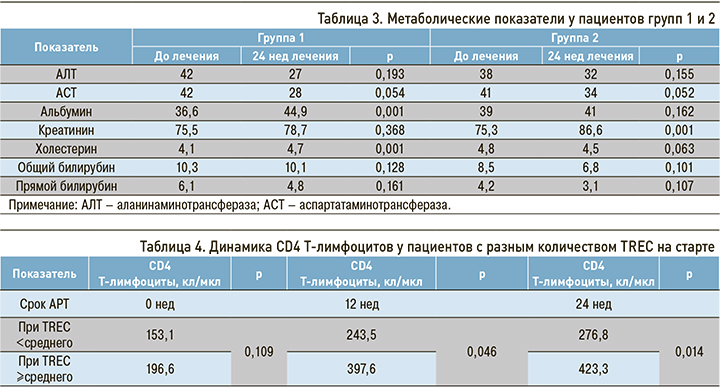
После начала АРТ в обеих группах наблюдалось повышение среднего количества CD4 Т-лимфоцитов. Среднее количество CD8 Т-лимфоцитов повышалось в течение первых 12 нед АРТ и снижалось к концу 24-й недели АРТ (рис. 3).
Рост обеих субпопуляций Т-лимфоцитов в течение первых 12 нед АРТ был связан с восстановлением угнетенного Т-клеточного ростка. Средний прирост как CD4 Т-лимфоцитов, так и CD8 Т-лимфоцитов был более значительным в группе 1 (табл. 2).
Среднее соотношение CD4/CD8 повышалось с 0,19 до 0,38 (+0,19; р <0,001) в группе 1 и с 0,17 до 0,35 (+0,18; р <0,001) в группе 2.
Схема АРТ с тремя точками приложения по сравнению со стандартной схемой с двумя точками приложения обеспечивала более быстрый и интенсивный рост субпопуляций Т-лимфоцитов.
Динамика метаболических показателей
Уровень АЛТ, АСТ, креатинина, холестерина, общего и прямого билирубина в группах оценивался на старте лечения и через 24 нед лечения (табл. 3). До начала терапии группы были сопоставимы по уровню всех вышеуказанных показателей.
За 24 нед АРТ в 1-й группе средние уровни АЛТ, АСТ, креатинина, общего и прямого билирубина, несмотря на сложные межлекарственные взаимодействия и большое количество применяемых препаратов, значимо не изменялись. Отмечался прирост холестерина, однако средний уровень этого показателя за пределы нормальных значений не выходил. К концу 24 нед терапии 8 пациентов (12,3%) имели уровень холестерина выше 5,5 ммоль/л, при этом в 4 случаях его уровень был выше нормы и до начала лечения. Наблюдалось возрастание среднего уровня альбумина. Таким образом, прирост холестерина был связан с улучшением белково-синтетической функции печени.
После аналогичного срока АРТ во 2-й группе уровни АЛТ, АСТ, общего и прямого билирубина, альбумина, холестерина значимо не изменялись. В то же время отмечалось повышение среднего уровня креатинина, что было обусловлено включением в стандартную схему лечения у практически 100% пациентов препарата тенофовир, который может оказывать негативное воздействие на почки. Применение схемы с тремя точками приложения, не включающей тенофовир, продемонстрировало хорошую переносимость, сопоставимую с препаратами для лечения вторичных заболеваний.
Динамика ДНК TREC в ходе антиретровирусной терапии
На старте лечения у пациентов отмечались значительные колебания ДНК TREC – от 0 до 139 копий/105 лейкоцитов. Медиана ДНК TREC находилась на уровне 27,6 копий/105 лейкоцитов, что было на порядок ниже показателей у здоровых взрослых. Среднее количество ДНК TREC составило 51,1 копий/105 лейкоцитов.
Количество CD4 Т-лимфоцитов через 12 и 24 нед было вариабельным и определялось количеством ДНК TREC до начала лечения (R xy=0,583, р=0,002 и R xy=0,637, р=0,008). Кроме того, прирост количества CD4 Т-лимфоцитов по окончании первых 12 нед АРТ также определялся этим показателем (R xy=0,523, р=0,007).
У пациентов при стартовом количестве TREC менее среднего (51,1 копий/105 лейкоцитов) среднее количество CD4 Т-лимфоцитов через 12 нед АРТ было ниже по сравнению с пациентами, которые имели на старте количество TREC, равное среднему уровню или больше. Аналогичная тенденция отмечалась и через 24 нед АРТ (табл. 4).
Количество ДНК TREC до начала лечения позволяло прогнозировать количество CD4 Т-лимфоцитов через 12 и 24 нед АРТ, а также их прирост за первые 12 нед терапии. Таким образом, ДНК TREC может быть использовано для прогноза динамики CD4 Т-лимфоцитов.
ОБСУЖДЕНИЕ
Лечение пациентов с ВИЧ-инфекцией в поздней стадии схемой АРТ препаратами из трех разных классов (группа 1) демонстрировало более высокую вирусологическую эффективность по сравнению со стандартной схемой АРТ из двух разных классов (группа 2): через 24 нед терапии доля пациентов с неопределяемым уровнем ВН составила 84,6 и 66,7% соответственно (р=0,028). Увеличение количества СD4 Т-лимфоцитов происходило в течение первых 12 нед АРТ в обеих группах, более значимый рост отмечался в группе 1. Значимое увеличение CD8 Т-лимфоцитов за этот же период АРТ отмечалось только в группе 1, колебания количества CD8 Т-лимфоцитов на указанный период значимыми не были. Установлена совместимость терапии препаратами из трех различных классов с препаратами для лечения туберкулеза основного и резервного ряда (при условии замены рифампицина на рифабутин), равно как и со средствами для лечения МАК-инфекции, пнемоцистной пневмонии и цитомегаловирусной инфекции.
Наши данные сопоставимы с наблюдениями некоторых зарубежных авторов.
Терапевтические концентрации этравирина и ралтегравира (при их совместном приеме) регистрировались в крови практически во всех случаях, что способствовало достижению вирусологической эффективности более, чем у 90% пациентов. Длительный период полувыведения этравирина и более высокая несвязанная фракция ралтегравира (57%) обеспечивали адекватные концентрации препаратов в половых органах. Ралтегравир и этравирин обладают хорошими взаимодополняющими фармакокинетическими профилями и могут одновременно использоваться в схемах лечения [9].
В последнее время для лечения ВИЧ-инфекции стали применяться схемы, содержащие один НИОТ (не тенофовир) вместо двух или вообще не содержащие нуклеозидных ингибиторов обратной транскриптазы [10–13]. Традиционно схемы АРТ имеют две точки приложения при воздействии на вирус: ингибирование одного из ферментов ВИЧ базовым препаратом в схеме и ингибирование обратной транскриптазы средствами, входящими в «нуклеозидную» основу. Материалы проведенного исследования показали возможности воздействия на вирус путем применения схемы АРТ с тремя точками воздействия: ингибирование двух ферментов ВИЧ двумя базовыми препаратами и ингибирование обратной транскриптазы препаратом из группы НИОТ.
Оценка количества ДНК TREC в ранее выполненных исследованиях продемонстрировала, что этот показатель может служить ранним маркером последующей динамики СD4 Т-лимфоцитов у пациентов с иммунодефицитом. В нашем исследовании также показано, что ДНК TREC до начала АРТ определяет прирост количества CD4 Т-лимфоцитов после начала лечения.
ЗАКЛЮЧЕНИЕ
1. Пациенты с поздней стадией ВИЧ-инфекции, направленные из различных регионов России на госпитализацию в стационар федеральной клиники, имели от 1 до 4 сопутствующих заболеваний, получали лечение 5–6 препаратами.
2. Терапия ВИЧ-инфекции препаратами из трех классов (ИИ, ННИОТ 2-го поколения, НИОТ) продемонстрировала большую вирусологическую, иммунологическую эффективность по сравнению со стандартной схемой АРТ, состоящей из препаратов двух классов (ИП и два НИОТ, ННИОТ 1-го поколения и два НИОТ, ИИ и два НИОТ).
3. Поражение иммунной системы, сопровождающееся снижением количества CD4 Т-лимфоцитов, ведет к истощению Т-клеточного неогенеза, которое проявляется снижением пролиферативной активности костного мозга и тимуса.
4. Количество ДНК TREC позволяет прогнозировать темпы восстановления уровня CD4 Т-лимфоцитов на проводимой терапии.



