ВВЕДЕНИЕ
Успехи современной противовирусной терапии хронического гепатита С (ХГС) требуют разработки критериев выздоровления от инфекции вируса гепатита С (ВГС) и оптимизации диспансерного наблюдения за пациентами после лечения. Маркером эрадикации вируса на сегодняшний момент служит устойчивый вирусологический ответ (УВО), который доказано ассоциируется со снижением риска развития печеночной недостаточности и улучшением выживаемости по сравнению с нелечеными пациентами и больными с неудачей лечения независимо от схемы противовирусной терапии [1, 2]. УВО определяется как отсутствие РНК ВГС в крови с чувствительностью тест-системы 10 МЕ/мл через 12–24 нед после окончания терапии [3]. Тем не менее риск прогрессирования фиброза печени и развитие гепатоцеллюлярной карциномы (ГЦК) даже после достижения УВО сохраняется. В обширном обзоре Negro F. подчеркивается необходимость разработки более четких рекомендаций для стратификации уровней риска неблагоприятных событий у пациентов после лечения, особенно в условиях упрощения мониторинга пациентов до, во время и после терапии, что оправдано из-за экономических соображений, но может быть преждевременным в отношении сложных пациентов, требующих персонифицированного подхода [4].
В контексте возможности снятия диагноза у пациентов, достигших УВО, и их безопасности в эпидемиологическом отношении (как источников инфекции) актуальным становится вопрос о скрытой инфекции ВГС (СкИ ВГС). Это понятие введено в 2004 г. и определяется как наличие РНК ВГС в гепатоцитах или мононуклеарных клетках периферической крови (PBMC) без обнаруживаемой РНК ВГС в сыворотке [5].
Существование СкИ ВГС среди населения в целом и в отдельных клинических группах освещено в литературе [6], но последствия его в различных клинических ситуациях требуют дальнейшего изучения. Терапевтическая тактика в случае СкИ ВГС также не понятна. Частота СкИ ВГС среди пациентов, достигших УВО после использования препаратов прямого противовирусного действия (ПППД), только начинает изучаться.
Цель исследования – оценить отдаленные исходы ХГС и встречаемость СкИ ВГС у пациентов после успешной противовирусной терапии.
МАТЕРИАЛ И МЕТОДЫ
В исследование было включено 182 пациента от 18 до 87 лет, которые, в соответствии с маршрутизацией пациентов с ХГС, были направлены специалистами первичного звена на обследование и лечение в дневной стационар СПб ГБУЗ «Клиническая инфекционная больница им. Боткина» в 2017–2020 гг.
Всем пациентам определялись генотип ВГС, уровень вирусной нагрузки, активность печеночных трансаминаз, уровни альбумина, общего билирубина, креатинина, протромбинового индекса (ПТИ), альфа-фетопротеина (АФП), выполнялись эзофагогастродуоденоскопия (ЭГДС) для оценки варикозного расширения вен пищевода, ультразвуковое исследование (УЗИ) органов брюшной полости. Плотность печени измерялась в кПа при помощи эластометрии на аппарате «Фиброскан» (Fibroscan). Оценку стадии фиброза печени производили при помощи шкалы METAVIR: F0 – отсутствие фиброза (≤5,8 кПа); F1 – фиброз портальных трактов (слабый фиброз: 5,9–7,2 кПа); F2 – фиброз с немногочисленными септами (умеренный фиброз: 7,3–9,5 кПа); F3 – фиброз с многочисленными септами (выраженный фиброз: 9,6–12,5 кПа); F4 – цирроз печени (>12,5 кПа).
К критериям исключения относился возраст <18 лет.
Исходные характеристики пациентов представлены в таблице 1.
Длительность лечения составляла 8, 12, 24 или 48 нед. Схемы противовирусной терапии были следующими: 17 (9%) пациентов получали даклатасвир + асунапревир, 38 (21%) – софосбувир + даклатасвир ± рибавирин, 3 (1,5%) – софосбувир + ледипасвир, 35 (19%) – паритапревир + ритонавир + омбитасвир + дасабувир, 27 (15%) – софосбувир + велпатасвир ± рибавирин, 25 (14%) – глекапревир + пибрентасвир ± софосбувир, 3 (1,5%) – гразопревир + элбасвир, 2 (1%) – даклатасвир + нарлапревир + ритонавир, 20 (11%) – пэгинтерферон + нарлапревир + ритонавир, 12 (7%) – пэгинтерферон + рибавирин.
Все пациенты достигли УВО, который был определен как отсутствие РНК ВГС в сыворотке крови через 24 нед после окончания терапии. После терапии пациенты со средней периодичностью в 6–12 мес направлялись врачами-инфекционистами поликлиник для прохождения обследования в дневной стационар. Медиана времени от окончания лечения до последнего визита в дневной стационар составила 99 [25-й перцентиль – 56; 75-й – 130] нед.
В последней точке наблюдения 109 пациентам было выполнено тестирование на СкИ ВГС методом ПЦР с предварительной подготовкой клинического материала. Тотальную РНК выделяли из: а) плазмы крови после концентрирования вируса ультрацентрифугированием в течение 1 ч при 24 000g, +40 °С; б) лейкоцитарной фракции крови; в) мононуклеарных лейкоцитов периферической крови, выделенных в градиенте плотности посредством центрифугирования на FicollPaque.
Клинико-лабораторные показатели сравнивались на старте терапии и в конечной точке наблюдения. Для оценки степени влияния УВО на течение хронического гепатита в зависимости от стадии фиброза печени пациенты разделены на 2 группы: с циррозом печени (F4) и без него (F0– F3). Для оценки коморбидной патологии всем пациентам рассчитывался индекс коморбидности Чарлсона [7].
Все этапы исследования были одобрены этическим комитетом при ФБУН «Санкт-Петербургский научно-исследовательский институт эпидемиологии и микробиологии им. Пастера» Роспотребнадзора.
Статистическая обработка данных выполнялась с помощью программ IBM SPSS Statistics (version 21). Нормальность распределения количественных переменных проверялась с использованием критерия Колмогорова–Смирнова. В связи с ненормальным распределением для анализа данных применялись непараметрические методы статистики. Количественные переменные представлены в виде максимального и минимального значения (мин.–макс.), медианы (Ме) и межквартильного интервала [25-й; 75-й перцентили], качественные признаки – в виде абсолютного значения и процента. Изучение динамики показателей проводилось с помощью критерия Вилкоксона. Сравнительный анализ двух независимых групп выполнялся посредством U-критерия Манна–Уитни для количественных переменных, критерия Фишера и χ2 – для качественных переменных. Для выявления и оценки корреляционной связи между двумя рядами сопоставляемых количественных показателей использовался коэффициент ранговой корреляции Спирмена (r). Для всех тестов вероятность (p) считалась незначимой при величине ≥0,05 и значимой при величине <0,05.
РЕЗУЛЬТАТЫ
Особенности пациентов, включенных в исследование
Сопутствующие заболевания присутствовали у 92% пациентов, факт мультиморбидности – у 64%. Рассчитанный индекс Чарлсона коррелировал со стадией фиброза (r=0,6; p <0,05) и варьировал от 0 до 10, медиана 4 [25-й перцентиль – 2; 75-й – 5]. Безинтерфероновые схемы получали пациенты с противопоказаниями к использованию интерферонов. У пациентов с отсутствием коморбидного фона в большем проценте применялись интерфероновые схемы, или пациенты самостоятельно использовали дженерики, не зарегистрированные в РФ. Среди выявленной сопутствующей патологии преобладали болезни сердечно-сосудистой системы (46%). Диагноз «сахарный диабет (СД)», «ожирение», признаки стеатоза печени присутствовали у 16, 25 и 15% наблюдаемых соответственно. У 50 (27%) пациентов при углубленном обследовании был выявлен скрытый гепатит В (определены HBcorAb суммарные при отсутствии HBsAg и HBsAb).
Все включенные в исследование проходили лечение и наблюдались амбулаторно, поэтому среди пациентов с циррозом печени у 92% был определен класс А этого заболевания по классификации Чайлд–Пью (n=48), только у 8% – класс В (n=4). Случаи цирроза печени класса С зарегистрированы не были. Индекс MELD у пациентов с циррозом печени на старте составил (5,2–20,8), 7,9 [6,9; 9,9]. Факт систематического употребления алкоголя присутствовал только у 5 (3%) пациентов.
Биохимический ответ
Все пациенты в точке УВО достигли биохимического ответа. К концу наблюдения активность аланинаминотрансферазы (АЛТ) снизилась с 59,5 [34; 102] до 16 [12; 24] ед/л (р <0,005), аспартатаминотрансферазы (АСТ) – с 48,5 [32; 84] дo 21 [15; 27] ед/л (р <0,005). У 12 (6,6%) пациентов в конечной точке наблюдения сохранялись повышенные значения АЛТ. Практически у всех были возможные причины этого в виде коморбидных состояний (СД, стеатоз печени, ожирение), тяжелой стадии заболевания печени (цирроз) и реинфекции. Только у 1 пациента 48 лет с F1 и отсутствием коморбидного фона не было выявлено причин повышения АЛТ в конечной точке наблюдения, и у него же фиброз прогрессировал до F2. У 10 (5,5%) пациентов в конечной точке наблюдения были отмечены значения АСТ выше нормы.
Динамика фиброза печени
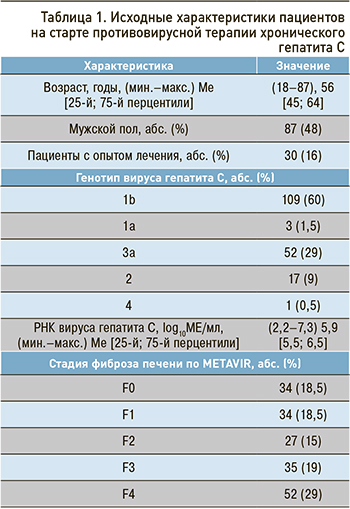
При сравнительной оценке степени фиброза до начала терапии и в конечной точке наблюдения были установлены достоверные различия (p <0,005; рис. 1). Положительная динамика наблюдалась чаще в группе пациентов без цирроза печени изначально (χ2=19; p <0,05).
У 17 (9%) пациентов было зарегистрировано прогрессирование фиброза. У 68 (37%) человек наблюдался регресс фиброза: у 18 обследованных – с F2 до отсутствия или клинически незначимого фиброза (F0–F1), у одной пациентки – с F3 до F0, у 97 (53%) степень фиброза не изменилась. У 12 (24%) пациентов с циррозом печени класса А по Чайлд–Пью к конечной точке наблюдения произошел регресс цирроза. В группах пациентов с прогрессированием фиброза и регрессом выявлены достоверные различия по возрасту (p=0,02). Возраст у лиц с прогрессированием фиброза составил (44–80), 65 [56; 70] лет, а с регрессом (27–82), 54 [45; 63] лет.
Динамика плотности печени, индексов Чайлд–Пью, MELD, FIB4 и APRI
У 7 (13%) пациентов с циррозом печени отмечено снижение индекса ЧП, у двух (4%) – увеличение индекса ЧП, у 29 (56%) – индекс ЧП не изменился. Значимых отличий в индексе MELD у пациентов с ЦП на старте терапии и в конце наблюдения отмечено не было (p=0,5).
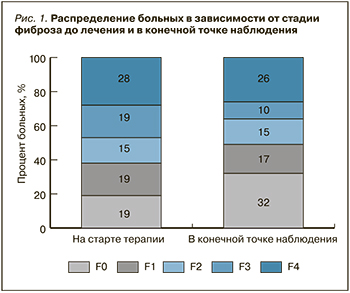
Проведена оценка динамики изменения плотности печени, индексов FIB4 и APRI у пациентов с циррозом и фиброзом F0–F3 (рис. 2).
Среди пациентов с отсутствием цирроза печени выраженное увеличение плотности произошло у одного обследуемого 67 лет с тяжелой сердечной недостаточностью на фоне мерцательной аритмии и индексом Чарлсона = 9.
Было выявлено, что у 13 пациентов с плотностью печени менее 12,5 Кпа и отсутствием признаков портальной гипертензии на старте противовирусной терапии показатели FIB4 превышали 3,25. У всех этих пациентов имел место тяжелый фиброз F3 и/или множественные сопутствующие заболевания, а индекс Чарлсона был выше 5 баллов. Установлена высокая положительная связь между индексом Чарлсона и индексом FIB4 (r=0,7; p <0,05). Показатель APRI превышал 1,5 у 12 пациентов без цирроза печени, что было связано с фактом снижения тромбоцитов ниже нормы; в конечной точке наблюдения у всех обследуемых индекс APRI снизился до значения <1,5. Как в группе без цирроза, так и у пациентов с циррозом в конечной точке наблюдения было зафиксировано значимое снижение индексов FIB4 и APRI (p <0,05).
Случаи отрицательной динамики после противовирусной терапии
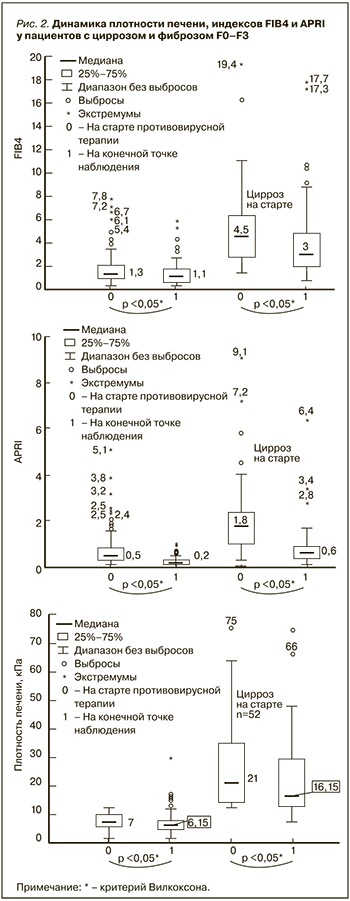
У 27 (14%) пациентов наблюдалась отрицательная динамика заболевания печени (табл. 2). Кроме того, у 7 (4%) исследуемых отмечалась выраженная отрицательная динамика по коморбидному фону: в 5 случаях были выявлены внепеченочные раковые заболевания, у 3 пациенток на 134, 138 и 116-й неделях был обнаружен рак молочной железы, у одной пациентки на 155-й неделе – рак щитовидной железы, еще в одном случае на 58-й неделе – цистаденома. Два пациента погибли на 50-й и 52-й неделях по причине развития острого нарушения мозгового кровообращения и почечной недостаточности на фоне конечной стадии заболевания почек. У двух наблюдаемых на 148-й и 121-й неделях была зарегистрирована реинфекция ВГС.
ОБСУЖДЕНИЕ
Доказано, что процесс фиброгенеза является не линейным и однонаправленным, а обратимым. После устранения этиологического фактора возможно обратное развитие фиброза и даже регресс цирроза [8–10]. В нашем исследовании также показана возможность регресса фиброза после успешной противовирусной терапии. Доля пациентов с выраженным фиброзом и циррозом уменьшилась к концу наблюдения с 47 до 36%. Регресс фиброза (по данным эластометрии печени) наблюдался в 77% случаев. Случаи регресса были ассоциированы с более молодым возрастом и исходно невыраженным фиброзом, что свидетельствует о прямой связи между долгосрочным снижением риска прогрессирования и исходной стадией заболевания и соответствует другим исследованиям о влиянии УВО на течение заболевания печени [1, 11].
Возможность прогрессирования фиброза уже после достижения УВО подтверждается отечественными и зарубежными исследователями [4, 12, 13]. В качестве факторов риска основная роль при этом отводится коморбидным состояниям, таким как неалкогольная жировая болезнь печени, сахарный диабет (СД), ожирение, злоупотребление алкоголем [4, 13–15].
Вместе с тем у некоторых пациентов не всегда удается выявить причины прогрессирования заболевания печени после УВО. Описаны генетические факторы, связанные с фиброзом печени различной этиологии, что говорит в пользу общих механизмов в прогрессировании заболевания печени независимо от его причины [16]. Выявлены возможные биомаркеры прогрессирования фиброза [17], но в реальной клинической практике пока нет соответствующих тест-систем. В нашем исследовании среди пациентов с прогрессированием фиброза у двух человек (44 и 48 лет) не удалось выявить каких-либо факторов риска. У остальных же изначально были тяжелый фиброз F3 и/или признаки стеатоза печени, сердечная недостаточность, СД, ожирение, систематическое употребление алкоголя. Связи прогрессирования фиброза и наличия скрытого гепатита В не выявлено (χ2=0,06; p=0,8).
Анализ причин смерти больных цирротической стадией хронических гепатитов (ХГ) демонстрирует, что почти у трети пациентов смерть наступает при наличии субкомпенсированной стадии и незаконченной цирротической трансформации ткани печени, что подтверждает отрицательную роль сопутствующей патологии в исходах ХГ [18]. Из трех умерших в нашем наблюдении у одной женщины смерть наступила в результате непосредственно осложнений декомпенсированного цирроза (кровотечение из ВРВП), у двух других – на фоне компенсированного заболевания печени (цирроз класса А, F3) и достижения УВО, при этом причиной смерти стала коморбидная патология.
После эрадикации ВГС риск развития ГЦК у пациентов с циррозом значительно снижается [1, 19]. Результаты некоторых исследований, показавших более высокий риск развития ГЦК после успешного лечения препаратов прямого противовирусного действия (ПППД) по сравнению со схемами с интерфероном [20], опровергнуты ввиду более частой распространенности среди пациентов, пролеченных ПППД, сопутствующих факторов риска, таких как пожилой возраст, декомпенсированный ЦП, СД [1, 21]. По данным некоторых европейских, американских и отечественных исследований, ежегодный риск развития ГЦК после противовирусной терапии у пациентов с циррозом печени составляет около 2,5% в год [10, 22, 23]. Риск выше у пациентов с декомпенсированным циррозом, причем он коррелирует с низким уровнем тромбоцитов, гипоальбуминемией, уровнем билирубина, степенью ВРВП, степенью энцефалопатии [10, 24, 25]. Пять пациентов из нашего исследования были пролечены ПППД и имели компенсированную функцию печени на момент выявления ГЦК (цирроз класса А и F3). Образование в печени было выявлено на сроке наблюдения после противовирусной терапии через 1, 1,5, 2 года и у двух пациентов – через 6 мес. Нельзя исключить, что ГЦК развилась еще до противовирусной терапии, но не была визуализирована. До лечения компьютерная томография не выполнялась. При сравнении больных ЦП с ГЦК и без нее выявлены достоверные различия в уровне АФП и FIB4 в конечной точке наблюдения и тенденция в отношении различий по уровню FIB4 (p=0,05). Все пациенты с ГЦК имели факт мультиморбидности, у двух обследуемых индекс Чарлсона на старте терапии составил 6 баллов, у трех – 7 баллов с прогнозом 10-летней выживаемости 2 и 0% соответственно. Многие исследования по долгосрочному риску развития ГЦК после лечения показали, что он выше не только у пациентов с циррозом, но и у тех, у кого определяется высокий уровень FIB4 в отсутствии признаков цирроза [26, 27]. Именно это послужило поводом для Американской ассоциации по изучению печени (AASLD)/Американского общества инфекционистов (IDSA) расширить группу пациентов, нуждающихся в скрининге на момент ГЦК, от лиц с циррозом до лиц с выраженным фиброзом включительно [28].
Сохранение РНК ВГС в PBMC после успешной этиотропной терапии показано при использовании схем с интерфероном. При этом полученные данные противоречивы и, вероятно, связаны со временем, прошедшем после окончания лечения, и обследованием (от 35 до 0%), что ставит вопрос о длительности возможной персистенции инфекции ВГС после УВО [29–31]. Частота и значимость СкИ ВГС после этиотропной терапии с использованием ПППД сейчас активно изучаются. В египетском исследовании с большой выборкой из 1280 пациентов, достигших УВО, СкИ ВГС была выявлена у 50 (3,9%) [32]. В исследованиях с меньшими выборками частота СкИ ВГС была выше – до 12% [33, 34]. В исследование 27 пациентов, достигших УВО, которое было проведено в Санкт-Петербурге, показатель распространенности СкИ ВГС равнялся 14,8% (4 случая) [35].
В нашем исследовании (n=109) СкИ ВГС обнаружена у 6 (5,5%) пациентов на сроке наблюдения (50–174), 146 [100, 170] нед. Все пациенты с выявленной СкИ ВГС получали лечение по безинтерфероновым схемам.
Влияние СкИ ВГС после УВО на прогрессирование заболевания печени обсуждается. Есть сведения о таких ее возможных серьезных последствиях, как рецидив, внепеченочные проявления (криоглобулинемия, неходжкинская лимфома), прогрессирование фиброза, декомпенсация цирроза, развитие ГЦК даже при отсутствии цирроза [35–39]. Выборка из шести пациентов со СкИ ВГС недостаточна для оценки ее влияния на течение заболевания печени. При сравнении пациентов со СкИ ВГС и без нее значимых различий в биохимических показателях выявлено не было (p <0,05). У двух пациентов до лечения был установлен выраженный фиброз (F3), у двух – цирроз класса А (в одном случае – F0, в другом – F2). Регресс цирроза на 50-й неделе наблюдения до F3 зафиксирован у одного пациента 38 лет c индексом Чарлсона = 1. У одной пациентки 65 лет с циррозом и индексом Чарлсона до терапии = 6 на 100-й неделе наблюдения была обнаружена ГЦК, и у нее же отмечались повышенные до 2 верхних границ нормы показатели АЛТ и АСТ после лечения; у остальных пяти пациентов трансаминазы были в пределах референсных значений. Еще в одном наблюдении у женщины 62 лет с индексом Чарлсона = 4 на 175-й неделе наблюдения было зафиксировано прогрессирование фиброза F3 до цирроза печени.
ЗАКЛЮЧЕНИЕ
Частота случаев регресса фиброза после эрадикации ВГС коррелирует с более молодым возрастом, отсутствием коморбидности и исходно невыраженным фиброзом. У пациентов с выраженным фиброзом и циррозом сохраняется риск прогрессирования заболевания печени и развития ГЦК после достижения УВО.
Наше наблюдение, отечественные и зарубежные исследования указывают на возможные клинические и биохимические маркеры, прогнозирующие повышенный риск прогрессирования заболевания печени и развития ГЦК после противовирусной терапии: уровень АФП, индекс FIB4, индекс Чайлд–Пью, индекс коморбидности и др.
С целью более точной стратификации рисков и оптимизации диспансерного наблюдения за пролеченными пациентами требуются дальнейшие исследования, направленные на разработку многофакторных шкал и включение их в рекомендации для практикующих врачей.
Встречаемость СкИ ВГС в нашем исследовании составила 5,5%. Значимость СкИ ВГС в прогрессировании заболевания печени и необходимость включения ее в прогностическое шкалы риска предстоит еще доказать или опровергнуть.



