Abstract. Influenza remains to be one of the most common infections, due to its ubiquity, annual outbreaks and epidemics. That is why vaccination against this disease is of a central importance. The article considers most of the influenza vaccines registered in Russia that are relevant in the current season, their features, composition and advantages. Tactics of vaccination of various population groups and the specifics of vaccines use are described, including taking into account ongoing vaccination against COVID-19. Recommendations are given on the use of this group of immunobiological preparations, including aspects of vaccination of pregnant women and persons belonging to other risk groups.
АКТУАЛЬНОСТЬ ПРОБЛЕМЫ
Грипп остается одной из самых распространенных инфекций, что обусловлено его повсеместным распространением, ежегодными вспышками и эпидемиями. Ежегодно этой инфекцией болеют до 10% взрослого населения планеты и до 30% детей. Кроме того, грипп опасен возможными осложнениями, в том числе пневмонией. Сегодня наиболее эффективным средством профилактики гриппа является ежегодная вакцинация, которая позволяет существенно снизить как заболеваемость населения, так и предупредить возникновение тяжелых случаев заболевания и летальных исходов.
Большинство современных противогриппозных вакцины являются мультивалентными, т.е. содержат антигены 3 или 4 актуальных вирусов. Ежегодно, в феврале, Всемирная организация здравоохранения (ВОЗ) рекомендует соответствующие штаммы вируса гриппа для включения в состав вакцин на следующий сезон [1–2].
В состав трехвалентных вакцин сезона 2022/2023 включены:
- A/Victoria/2570/2019 (H1N1)pdm09-подобный вирус;
- A/Darwin/9/2021 (H3N2)-подобный вирус;
- B/Austria/1359417/2021 (линия B/Victoria)-подобный вирус.
Состав четырехвалентных вакцин сезона 2022/2023:
- A/Victoria/2570/2019 (H1N1)pdm09-подобный вирус;
- A/Darwin/9/2021 (H3N2)-подобный вирус;
- B/Austria/1359417/2021 (линия B/Victoria)-подобный вирус;
- B/Phuket/3073/2013 (линия B/Yamagata)-подобный вирус [3].
Наиболее значимая характеристика вакцин – их профилактическая эффективность, которая определяется соответствием вакцинных и циркулирующих штаммов вируса гриппа, особенностями препарата, такими как тип, кратность введения, наличие адъювантов [4, 5]. На эффективность вакцин также влияют сроки начала эпидемии, состояние популяционного иммунитета. Очевидно, что вакцинация наиболее эффективна в случае совпадений циркулирующих вирусов с антигенами, содержащимися в вакцинах в текущем сезоне. Кроме того, существенное значение имеют индивидуальные особенности пациента, например, возраст, наличие острых и/или хронических заболеваний, время вакцинации [6].
Среди здорового населения защита от гриппа обеспечивается даже при неполном соответствии циркулирующих вирусных штаммов составу вакцин. По мнению многих исследователей, люди, относящиеся к группам риска развития осложнений гриппа, как и лица, находящиеся с ними в близком контакте, при неполном соответствии вакцинных и циркулирующих штаммов все равно нуждаются в вакцинации, поскольку риск возникновения осложнений уменьшается даже в том случае, если человек все-таки заболел [7].
ПРИОРИТЕТНЫЕ ГРУППЫ ВАКЦИНАЦИИ
В 2006 г. вакцинация против гриппа была введена в Национальный календарь прививок. Это привело к существенному снижению заболеваемости гриппозной инфекцией в нашей стране [8]. Прививочная кампания против гриппа в осенний период 2022 г. предполагает охват не менее 60% от численности населения субъекта Российской Федерации и не менее 75% от численности лиц из групп риска, предусмотренных Национальным календарем, сотрудников и получателей социальных услуг стационарных организаций социального обслуживания.
В России и в мире обозначены группы риска развития тяжелых форм гриппа (по возрасту, наличию сопутствующих заболеваний и т.п.). Кроме того, определен ряд лиц, для которых вакцинация рекомендована по эпидемическим показаниям [9, 10]. В нашей стране к указанным категориям относят:
- детей в возрасте с 6 мес, учащихся 1–11 классов;
- обучающихся в профессиональных образовательных организациях и вузах;
- работников медицинских и образовательных организаций, транспорта, коммунальной сферы и сферы предоставления услуг;
- лиц, работающих вахтовым методом, сотрудников правоохранительных органов и государственных контрольных органов в пунктах пропуска через государственную границу;
- работников организаций социального обслуживания и многофункциональных центров, государственных гражданских и муниципальных служащих;
- беременных женщин;
- взрослых старше 60 лет;
- лиц, подлежащих призыву на военную службу;
- лиц с хроническими патологиями, в том числе с легочными, сердечно-сосудистыми заболеваниями, метаболическими нарушениями и ожирением [11].
Циркуляция вирусов гриппа во время продолжающейся пандемии COVID-19 может иметь серьезные последствия для уязвимых групп населения и привести к дополнительной нагрузке на систему здравоохранения. В связи с этим ВОЗ предлагает временно определить приоритетные группы вакцинации в следующем порядке.
1. Медицинские работники.
2. Лица старшего возраста, к которым должны быть отнесены лица старше 50 лет, подверженные более высокому риску тяжелого течения COVID- 19 и гриппа.
3. Дополнительные группы риска:
3.1. Беременные женщины остаются наиболее приоритетной группой для вакцинации против гриппа. Существующие данные об инфицировании COVID-19 во время беременности свидетельствуют о потенциальном повышении риска тяжелого течения COVID-19 у этой категории женщин.
3.2. Лица с сопутствующими заболеваниями, например пациенты с диабетом, гипертонической болезнью, ВИЧ-инфекцией, астмой и хроническими заболеваниями сердца и легких.
3.3. Дети, особенно в возрасте от 6 месяцев до 2 лет, также остаются одной из приоритетных групп для вакцинации против гриппа в связи с риском протекания этого заболевания в тяжелой форме [12–14].
ТИПЫ ВАКЦИН ОТ ГРИППА и ИХ ПРЕДСТАВЛЕННОСТЬ В РОССИИ
Противогриппозные вакцины различаются по технологии производства.
Живые вакцины содержат ослабленный вирус.
В состав цельновирионных вакцины входят целые обезвреженные вирусы (фактически не применяются в настоящее время в России).
Субъединичные вакцины включают поверхностные антигены вируса гриппа, которые наиболее значимы в развитии иммунитета. Внутренние белки в таких вакцинах отсутствуют, что обеспечивает меньшее число побочных реакций.
Сплит-вакцины (расщепленные) содержат поверхностные и внутренние антигены вируса гриппа.
Наиболее широко для иммунизации населения используются субъединичные и сплит-вакцины, поскольку они характеризуются высокой эффективностью и безопасностью (низким риском развития побочных явлений) [15, 16].
Использование адъювантов направлено на усиление иммунного ответа и снижение антигенной нагрузки вакцин. В настоящее время ведется поиск новых, более эффективных адъювантов [17–19].
Отечественные и зарубежные противогриппозные вакцины, зарегистрированные в России, приведены в таблицах 1–5.
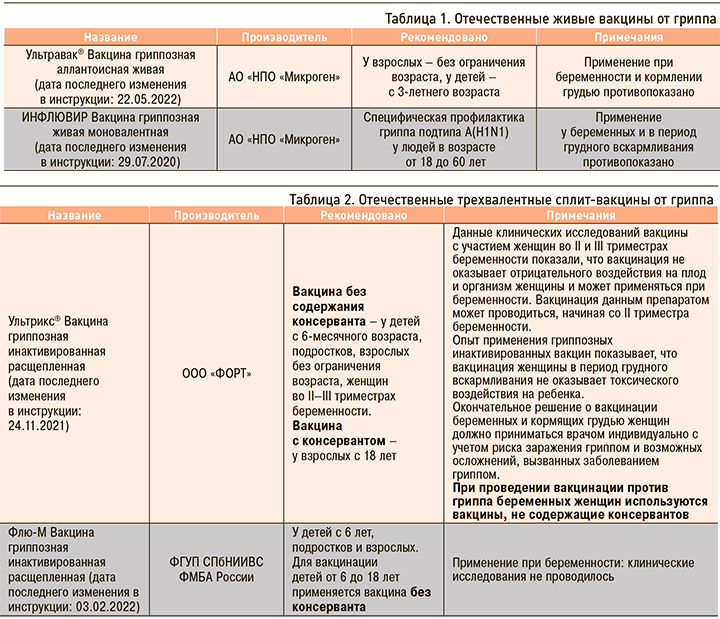
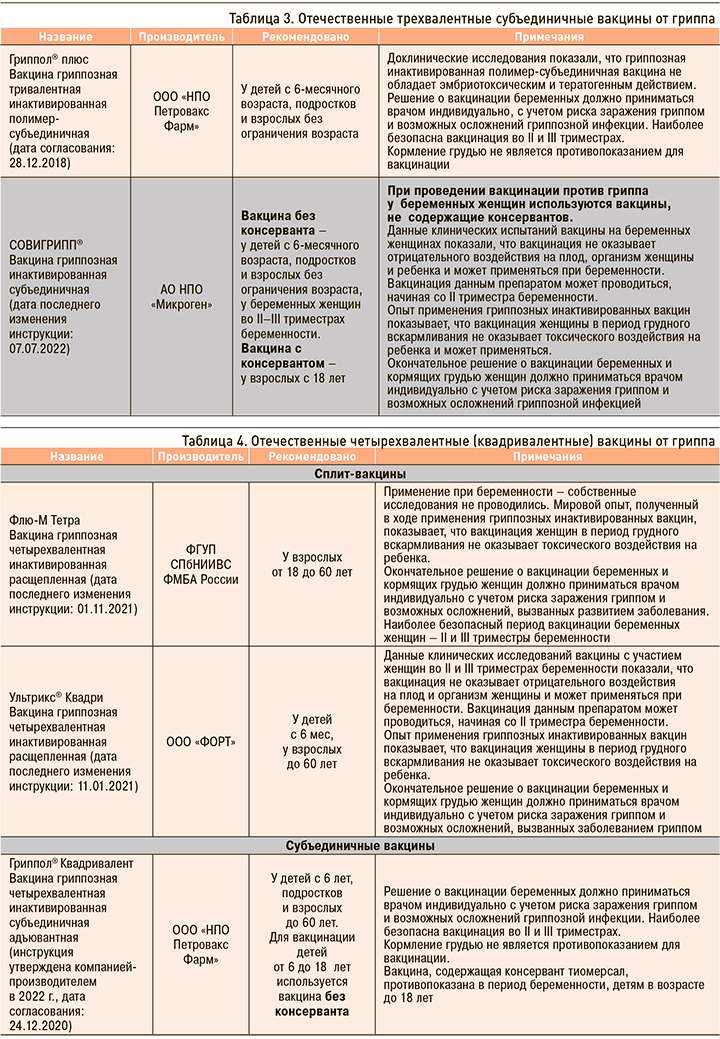
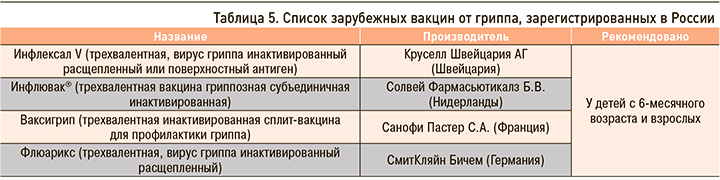
Несомненно, что на фоне сохраняющейся циркуляции SARS-CoV-2 остается актуальной вакцинопрофилактика как гриппа, так и COVID-19. В текущем сезоне-2022/2023 планируется широкое применение отечественных вакцин Ультрикс, Ультрикс Квадри, Гриппол плюс, Гриппол квадривалент, Совигрипп, ФЛЮ-М, ФЛЮ-М Тетра. Все эти вакцины прошли многочисленные исследования безопасности и эффективности [20, 21, 22, 23].
ЗАКЛЮЧЕНИЕ
Таким образом, ежегодная вакцинация – наиболее эффективный и безопасный способ защиты населения от гриппа. Многолетний опыт использования вакцин доказал их пользу в профилактике заболеваемости, а эффективность и безопасность вакцинации подтверждается доклиническими и клиническими исследованиями.
1. Gessner B.D., Shindo N., Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: A systematic review. Lancet Infect Dis. 2011; 11(3): 223–35. https://dx.doi.org/10.1016/S1473-3099(11)70008-1.
2. Centers for Disease Control and Prevention (CDC). Influenza vaccination: A summary for clinicians. URL: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm#:~:text=Everyone%206%20months%20of%20age,risk%20for%20serious%20influenza%20complications (date of access – 01.11.2022).
3. Медицинский вестник. ВОЗ объявила состав вакцин от гриппа сезона 2022–2023 годов. 28.02.2022. Доступ: https://medvestnik.ru/content/news/VOZ-obyavila-sostav-vakcin-ot-grippa-sezona-2022-2023-godov.html (дата обращения – 01.11.2022). [Medical Bulletin. WHO announces composition of influenza vaccines for 2022–2023 season. URL: https://medvestnik.ru/content/news/VOZ-obyavila-sostav-vakcin-ot-grippa-sezona-2022-2023-godov.html (date of access – 01.11.2022) (In Russ.)].
4. Vaccines against influenza WHO position paper. Wkly Epidemiol Rec. 2012; 87(47): 461–76.
5. World Health Organization. Virological surveillance updates.URL: http://www.who.int/influenza/gisrs_laboratory/updates/summaryreport (date of access – 01.11.2022).
6. Ерофеева М.К., Максакова В.Л., Шахланская Е.В. с соавт. Профилактическая эффективность гриппозных вакцин в современных условиях (обзор литературы). Фарматека. 2020; 27(1): 7–13. [Erofeeva M.K., Maksakova V.L., Shakhlanskaya E.V. et al. Preventive efficacy of influenza vaccines in modern conditions (literature review). Farmateka. 2020; 27(1): 7–13 (In Russ.)].https://dx.doi.org/10.18565/pharmateca.2020.1.7-13. EDN: UESDUI.
7. Ghendon Y. Vaccination against influenza viruses: Current status. Adv Biotechnol Processes. 1990; 14: 159–201.
8. Nichol K. Efficacy/clinical effectiveness of inactivated influenza virus vaccines in adults. Textbook of Influenza. In: Nicholson K.G., Webster R.G., Hay A.J. (eds). Textbook of Influenza. Oxford: Blackwell Science. 1998: pp. 358–372. ISBN: 978-0632-04803-8.
9. Wong K.K., Jain S., Blanton L. et al. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics 2013; 132(5): 796–804. https://dx.doi.org/10.1542/peds.2013-1493.
10. Millman J., Reed C., Kirley P.D. et al. Chaves improving accuracy of influenza-associated hospitalization rate estimates. Emerg Infect Dis. 2015; 21(9): 1595–601. https://dx.doi.org/10.3201/eid2109.141665.
11. Приказ Министерства здравоохранения Российской Федерации от 14 сентября 2020 г. № 967н «О внесении изменения в приложение n 1 к приказу Министерства Здравоохранения Российской Федерации от 21 марта 2014 г. n 125н «Об утверждении Национального календаря профилактических прививок и календаря профилактических прививок по эпидемическим показаниям»». [Order of the Ministry of Healthcare of the Russian Federation dated September 14, 2020 No. 967n «On Amendments to Appendix n 1 to Order of the Ministry of Healthcare of the Russian Federation dated March 21, 2014 n 125n “On Approval of the National Calendar of Preventive Immunizations and the Calendar of Preventive Immunizations for Epidemic Indications”» (In Russ.)].
12. World Health Organization. Strategic Advisory Group of Experts on Immunization (SAGE).URL: https://www.who.int/immunization/policy/sage/en/ (date of access – 01.11.2022).
13. Рекомендации СКГЭ ВОЗ по вакцинации против сезонного гриппа в период пандемии COVID-19. Временные рекомендации. 21 сентября 2020 г. Доступ: https://www.who.int/ru/publications/m/item/who-sage-seasonal-influenza-vaccination-recommendations-during-the-covid-19-pandemic (дата обращения – 01.11.2022). [Recommendations of the Strategic WHO Expert Advisory Group on seasonal influenza vaccination during the COVID-19 pandemic. Interim recommendations. September 21, 2020. URL: https://www.who.int/ru/publications/m/item/who-sage-seasonal-influenza-vaccination-recommendations-during-the-covid-19-pandemic (date of access – 01.11.2022) (In Russ.)].
14. Яппаров Р.Г., Карнаухова Е.Ю., Антонова Т.В., Лиознов Д.А. Иммунный ответ на вакцинацию против гриппа у больных ВИЧ-инфекцией. ВИЧ-инфекция и иммуносупрессии. 2020; 12(1): 75–82. [Yapparov R.G., Karnaukhova E.Yu., Antonova T.V., Lioznov D.A. Immune response to influenza vaccination in HIV patients. 2020; 12(1): 75–82 (In Russ.)]. https://dx.doi.org/10.22328/2077-9828-2020-12-1-75-82. EDN: JQJUQZ.
15. Talbot H.K., Coleman L.A., Zhu Y. et al. Factors associated with maintenance of antibody responses to influenza vaccine in older, community-dwelling adults. 2015; 15: 195. https://dx.doi.org/10.1186/s12879-015-0926-8.
16. Gillard P., Chu D.W., Hwang S.-J. et al. Long-term booster schedules with AS03A-adjuvanted heterologous H5N1 vaccines induces rapid and broad immune responses in Asian adults. BMC Infect Dis. 2014; 14: 142. https://dx.doi.org/10.1186/1471-2334-14-142.
17. ГОСТ Р 52379-2005 «Национальный стандарт российской федерации надлежащая клиническая практика Good Clinical Practice (GCP)» (утвержден Приказом Федерального агентства по техническому регулированию и метрологии от 27 сентября 2005 г. № 232-ст). [GOST R 52379-2005 «National Standard of the Russian Federation Good Clinical Practice (GCP)» (approved by the Order of the Federal Agency for Technical Regulation and Metrology dated September 27, 2005 No. 232-st) (In Russ.)].
18. Федеральный закон от 12.04.2010 № 61-ФЗ «Об обращении лекарственных средств» (в ред. Федеральных законов от 27.07.2010 № 192-ФЗ, от 11.10.2010 N 271-ФЗ, от 29.11.2010 № 313-ФЗ, от 06.12.2011 № 409-ФЗ, от 25.06.2012 № 93-ФЗ, от 25.12.2012 № 262-ФЗ, от 02.07.2013 № 185-ФЗ, от 25.11.2013 № 317-ФЗ, от 12.03.2014 № 33-ФЗ, от 22.10.2014 № 313-ФЗ). [Federal Law No. 61-FZ of April 12, 2010 «On the Circulation of Medicines» (as amended by Federal Laws No. 192-FZ of July 27, 2010, No. 271-FZ of October 11, 2010, No. 313-FZ of November 29, 2010, of December 6, 2010) .2011 No. 409-FZ, dated 25.06.2012 No. 93-FZ, dated 25.12.2012 No. 262-FZ, dated 02.07.2013 No. 185-FZ, dated 25.11.2013 No. 317-FZ, dated 12.03.2014 No. 33- FZ, dated October 22, 2014 No. 313-FZ) (In Russ.)].
19. Руководство по экспертизе лекарственных средств. Том I. М.: Гриф и К. 2013; 328 с. [Guidelines for the examination of medicines. Volume I. Moscow: Grif i K. 2013; 328 pp. (In Russ.)]. ISBN: 978-8125-1858-5.
20. Лиознов Д.А., Харит С.М., Ерофеева М.К. с соавт. Оценка реактогенности и иммуногенности вакцины гриппозной четырехвалентной инактивированной субъединичной. Эпидемиология и вакцинопрофилактика. 2018; 17(3): 57–62. [Lioznov D.A., Kharit S.M., Erofeeva M.K. et al. Assessment of reactogenicity and immunogenicity of the quadrivalent live attenuated influenza vaccine. Epidemiologiya i vaktsinoprofilaktika = Epidemiology and Vaccinal Prevention. 2018; 17(3): 57–62 (In Russ.)].https://dx.doi.org/10.31631/2073-3046-2018-17-3-57-62. EDN: URQFMQ.
21. Ерофеева М.К., Стукова М.А., Шахланская Е.В. с соавт. Оценка профилактической эффективности гриппозных вакцин. Эпидемиология и вакцинопрофилактика. 2021; 20(5): 52–60. [Erofeeva M.K., Stukova M.A., Shakhlanskaya E.V. et al. Evaluation of the preventive effectiveness of influenza vaccines in the epidemic season 2019–2020 in St. Petersburg. Epidemiologiya i vaktsinoprofilaktika = Epidemiology and Vaccinal Prevention. 2021; 20(5): 52–60 (In Russ.)].https://dx.doi.org/10.31631/2073-3046-2021-20-5-52-60. EDN: ZCBUZK.
22. Никифорова А.Н., Исакова-Сивак И.Н., Ерофеева М.К. с соавт. Результаты изучения безопасности и иммуногенности отечественной субъединичной адъювантной вакцины Совигрипп у добровольцев 18–60 лет. Эпидемиология и вакцинопрофилактика. 2014; 2: 72–78. [Nikiforova A.N., Isakova-Sivak I.N., Erofeeva M.K. et al. The results of studying the safety and immugenicity of domestic subunit adjuvanted vaccine Sovigripp in volunteers from 18 to 60 years old. Epidemiologiya i vaktsinoprofilaktika = Epidemiology and Vaccinal Prevention. 2014; 2: 72–78 (In Russ.)]. EDN: SBEUNX.
23. Фельдблюм И.В., Субботина К.А., Новгородова С.Д. с соавт. Реактогенность, безопасность и иммуногенность отечественной гриппозной инактивированной расщепленной вакцины Флю-М при иммунизации взрослых 18–60 лет. Журнал микробиологии, эпидемиологии и иммунобиологии. 2018; 5: 31–37. [Feldblyum I.V., Subbotina K.A., Novgorodova S.D. et al. Reactogenicity, safety and immunogenicity of domestic Flu-M inactivated split influenza vaccine for the immunization of adults aged between 18 and 60. Zhurnal mikrobiologii, epidemiologii i immunobiologii = Journal of Microbiology, Epidemiology and Immunobiology. 2018; 5: 31–37 (In Russ.)]. https://dx.doi.org/10.36233/0372-9311-2018-5-31-37. EDN: FKNUUT.
Ivan I. Tokin, PhD in Medicine, head of the Department of experimental and clinical research, A.A. Smorodintsev Influenza Research Institute of the Ministry of Healthcare of Russia, associate professor of the Department of infectious diseases, I.I. Mechnikov North-Western State Medical University of the Ministry of Healthcare of Russia. Address: 197376, Saint Petersburg, 15/17 Professora Popova Str. E-mail:
. ORCID: https://orcid.org/0000-0002-9824-3945
Rafael G. Yapparov, PhD in Medicine, associate professor of the Department of infectious diseases with IFVE course, Bashkir State Medical University of the Ministry of Healthcare of Russia, chief physician of Republican Center for the Prevention and Control of AIDS and Infectious Diseases. Address: 450005, Ufa, 18 Kustarnaya Str. E-mail:
Dmitry A. Lioznov, Dr. med. habil., professor, director of A A. Smorodintsev Influenza Research Institute of the Ministry of Healthcare of Russia, head of the Department of infectious diseases and epidemiology, Academician I.P. Pavlov First Saint Petersburg State Medical University of the Ministry of Healthcare of Russia. Address: 197376, Saint Petersburg, 15/1€ Professora Popova Str. ORCID: https://orcid.org/0000-0003-3643-7354






