ВВЕДЕНИЕ
Вакцинация является одним из величайших практических достижений медицины и одним из главных средств профилактики инфекционных заболеваний. Вакцины – одна из самых экономически эффективных технологий здравоохранения всех времен: наряду с улучшением качества питьевой воды, именно вакцинация оказала наиболее значительное влияния на снижение смертности и рост населения в мире [1]. Ежегодно вакцины спасают примерно от 2 до 3 млн жизней. При этом для достижения коллективного иммунитета посредством вакцинации важно, чтобы охват населения ею был выше определенного, специфичного для заболевания порога [2].
Вакцинация остается ключевым элементом борьбы с продолжающейся пандемией COVID-19 [3, 4]. Вакцинам мы обязаны искоренением оспы и чумы, они играют существенную роль в противостоянии разнообразным болезням, предупреждении пожизненной инвалидности, вносят ощутимый вклад в снижение детской заболеваемости и смертности. Прогнозы свидетельствуют о том, что около 120 млн случаев смертности среди детей, родившихся в период между 2000 и 2030 г., будет предотвращено с помощью вакцинации [5].
В 1997 г. вакцинация против оспы окончательно избавила мир от этой болезни, и данное событие нашло отражение в опубликованной программе Всемирной организации здравоохранения (ВОЗ) по ее ликвидации. Согласно экспертным оценкам, ежегодная глобальная выгода от ликвидации оспы составляет около 1,35 млрд долл. (с использованием 1967 г. в качестве базового года), в то время как общие затраты на ее ликвидацию в эндемичных странах в период с 1967 по 1979 г. составили порядка 300 млн долларов [6].
На сегодняшний день в большинстве стран мира под контролем находится не менее десятка основных инфекционных заболеваний. Однако следует признать, что, несмотря на достижения в сфере создания новых вакцин и усовершенствования старых, до настоящего времени лишь оспа успешно ликвидирована усилиями вакцинации, организованной системой ВОЗ [1, 7]. Доля других инфекционных заболеваний благодаря вакцинации сократилась, в определенных регионах мира они сходят на нет и могут полностью исчезнуть в уже ближайшие годы.
Получение новых вакцин и модернизация старых диктует необходимость понимания и углубления существующих знаний об иммунологических принципах вакцинации. При этом производство новых вакцин представляет огромные трудности с точки зрения изучения целевых патогенных микроорганизмов для будущих кандидатов на вакцины, а также достижения заданных результатов.
ОСНОВНЫЕ ПРИНЦИПЫ ВАКЦИНАЦИИ
Принцип действия вакцин заключается в стимуляции механизмов защиты от патогена путем имитации его естественного взаимодействия с иммунной системой человека, что приводит к формированию иммунной памяти, представленной врожденным и адаптивным иммунитетом. Результаты вакцинации зависят от того, в какой степени сформирована это защита после ее применения [8]. Согласованность между врожденным и адаптивным иммунитетом определяет достижение результатов формирования иммунной памяти. Это взаимодействие, в частности, основано на антигенпрезентирующих клетках (cellules presentatrices d’antigene, CPA), которые образуют связь между двумя компартментами CD4 и хелперными Т-клетками (LTCD4), обеспечивающими формирование иммунной памяти за счет активации В-клеток и способности этих клеток продуцировать защитные иммуноглобулины [9].
Итак, клетками, ответственными за презентацию антигенов, выступают макрофаги, В-лимфоциты и особенно дендритные клетки, которые относятся к «профессиональным» CPA. Последние экспонируют на своей поверхности чужеродный антиген в комплексе с молекулами главного комплекса гистосовместимости (major histocompatibility complex) класса II (МНС II). Таким образом, антигенный пептид будет распознаваться Т-лимфоцитами CD4 (LTCD4) [9, 10]. LTCD4 имеют ключевое значение в адаптации иммунного ответа. Распознавание антигена LTCD4 запускает секрецию многочисленных цитокинов, вызывающих пролиферацию хелперов LT и LTCD8, а также дифференцировку B-лимфоцитов в плазматические клетки [10]. При этом В-лимфоциты играют определенную роль в презентации антигена и главную – в секреции иммуноглобулинов (или антител) и их дифференцировке в клетки памяти, индуцированные действием LTCD4 [11]. На начальной стадии, во время первого контакта с антигеном, антитела имеют низкую аффинность (IgM), затем же, после изотипического переключения, сильную аффинность (IgG и IgA).
При втором контакте антиген активирует В-клетки памяти, которые дифференцируются в плазматические клетки, секретирующие высокоаффинные иммуноглобулины (в основном IgG и IgA) в больших количествах. Это производство антител является определяющим для достижения эффективности большинства используемых профилактических вакцин, так как они непосредственно распознают антиген до того, как патоген попадет в клетку. Поэтому они способны ингибировать патогены (вирусы, бактерии, токсины) внеклеточно до их фиксации или проникновения в клетки-мишени [12]. Представленные антитела находятся в свободном состоянии в плазме или жидкостях организма (в основном для IgG и IgM), присутствуют в слизистых оболочках (особенно IgA), могут проникать в большинство тканей, проходить через плаценту (главным образом IgG) и в молоко матери (в основном IgA) [13]. LTCD8 распознают чужеродные антигены, представленные МНС I, на поверхности всех ядросодержащих клеток. После их распознавания LTCD8 уничтожают инфицированные клетки. При этом живые аттенуированные вакцины служат мощными индукторами LTCD8 и в присутствии LTDC4 и LTCD8 трансформируются в ячейки памяти [9].
Существует два типа ответа на вакцинацию: первичный, после первой инъекции, и вторичный, вызванный второй инъекцией (бустерная повышающая доза), которую делают обычно через месяц после первой. Первичный ответ, соответствующий первичной вакцинации, задействует сложную цепь всех составляющих иммунитета, как врожденного, так и адаптивного. Реакция на вакцину начинается с представления CPA как препарата антигена к LTCD4. Таким образом, состав вакцины будет влиять на этот первый шаг и, следовательно, на качество ответа вакцины. Вакцинные антигены должны наилучшим образом воспроизводить исходный сигнал, созданный природным патогеном. Начальный сигнал соответствует обнаружению «сигнала опасности», или патоген-ассоциированных молекулярных паттернов (pathogen-associated molecular patterns, PAMPs), системой рецепторов распознавания паттернов (pattern recognition receptors, PRR), экспрессируемых на поверхности клеток врожденного иммунитета [14]. Следовательно, определенные антигены вакцин, интерпретируемые как PAMP, связываются с CPA через рецепторы семейства PRR, Toll-подобные рецепторы (TLR) и активируют каскад воспалительных реакций. Это приводит к миграции CPA в дренажный узел, синтезу цитокинов (в зависимости от природы антигена), активирующих другие иммунные клетки и, в частности LTCD4, посредством представления пары антиген-пептид – MHC-II. Что касается B-лимфоцитов, также играющими роль CPA, то они активируются при контакте с вакцинным антигеном, который мигрировал в ганглион. В дальнейшем они превращаются в низкоэффективные IgM-продуцирующие плазматические клетки. В присутствии LTCD4 B-лимфоциты будут активированы в плазматических клетках, продуцирующих IgG или IgA с высокой аффинностью и памятью после длинной цепи генетических модификаций. Это отражает важность стимулирования LTDCD4 и CPA, в частности дендритных клеток, и, следовательно, добавления адъювантов [9].
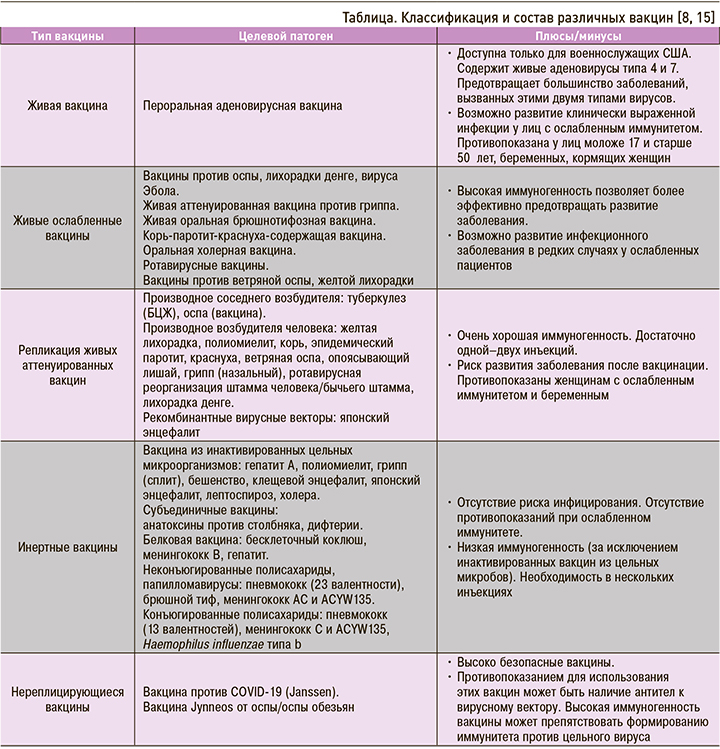
Два основных класса вакцин составляют живые аттенуированные и инертные вакцины (цельные или субъединичные микробы; табл.). Новые вакцины разрабатываются методом генной инженерии (живые и субъединичные вакцины). Конечная цель любой вакцины состоит в том, чтобы вызвать защитный иммунный ответ, специфичный для определенного инфекционного агента, путем продуцирования антител и индукции определенных клеточных компонентов. Вакцина должна отвечать трем основным характеристикам, которые зависят от ее состава [8]:
- быть эффективной, активировать иммунную память и обеспечивать длительную защиту;
- быть безопасной при работе с ней;
- быть простой в управлении с точки зрения модальности и количества администрирования.
Живые аттенуированные вакцины содержат живой инфекционный агент, патогенность которого ослаблена различными процессами. Они вызывают ослабленную, даже бессимптомную форму заболевания, стимулируя весь регистр иммунного ответа с последующим формированием высокой иммунной защиты, как после перенесенной естественной инфекции, но с некоторым риском развития активной формы заболевания после введения вакцины, особенно при ослабленном иммунитете (реальный риск) и беременности (теоретический риск). В случае использования живой вакцины индуцированный иммунный ответ является полным, не требует адъюванта и ограничен небольшим количеством доз (чаще всего одна–две, иногда три). Живые аттенуированные вакцины могут вводиться подкожно (БЦЖ), а также внутрь и через слизистые оболочки при респираторных или пищеварительных инфекциях, индуцируя высокий уровень IgA. Так, перорально в настоящее время применяется ротавирусная вакцина (пероральное введение), интраназально – некоторые вакцины против гриппа [14].
Инертные вакцины, включающие вакцины из цельных микробов и субъединичные вакцины, лишены какой-либо инфекционной силы. Они способны обеспечивать защитный иммунный ответ, но чаще всего требуют использования адъювантов иммунитета и бустерных инъекций в течение всей жизни.
Цельные зародышевые вакцины содержат цельные бактерии или вирусные частицы, которые инактивируются химическими или термическими методами. Такая композиция позволяет получить значительную иммуногенность, близкую к той, которая индуцируется естественной инфекцией, без риска развития заболевания после введения вакцины из-за отсутствия репликативного инфекционного агента, но с возможным развитием выраженной воспалительной реакции. В результате этого, к слову, в свое время была отменена вакцина против микробного коклюша, уступив место бесклеточной вакцине.
Субъединичные вакцины состоят из антигенных фракций, которые соответствуют активной фракции возбудителя, индуцирующей достаточную продукцию антител для индукции иммунитета. Параллельно снижается их реактогенность, а также иммуногенность (часто требуется несколько инъекций при первичной вакцинации с последующими пожизненными бустерами и добавлением адъюванта). Существует несколько классов субъединичных вакцин. В зависимости от патогена иммунизирующая субъединица может быть получена различными способами: из самого инфекционного агента (например, капсульного антигена) или при помощи генной инженерии с образованием рекомбинантного белка. Вакцины от генетической рекомбинации (гепатит В, вирус папилломы, менингококк В) обладают очень хорошей иммуногенностью [16]. Иммуногенные белки этих генно-инженерных вакцин реорганизуются в вирусные псевдочастицы (для вируса гепатита В и папилломы), воспроизводя по существу, форму вируса в виде частиц и тем самым вызывая иммунный ответ [17]. Генная инженерия в настоящее время также позволяет производить большое количество субъединичныx вакцин (например, C. difficile, S. aureus) [7]. Чтобы получить эффективную вакцину, используемая субъединица должна вызывать защитный иммунитет; использование ряда вакцинных технологий дает возможность получать субъединичные вакцины, обладающие улучшенной иммуногенностью, с добавлением адъювантов, конъюгированых с иммуногенными белками. Адъюванты при этом необходимы для индукции сильного и длительного иммунного ответа на введенный антиген [18]. Они позволяют мощно активировать первую волну врожденного иммунитета, необходимую для развития эффективного и продолжительного ответа. Существуют адъюванты на основе минеральных солей (алюминия, фосфата кальция) и/или фосфолипидов, которые вводятся одновременно с антигеном [19, 20]. В частности, соли алюминия обеспечивают высокую выработку антител, стимулируя врожденный иммунитет. Они особенно полезны, когда целевые патогены или токсины требуют высокого уровня антител для формирования защиты (при дифтерии, столбняке и коклюше, инфекциях, вызванных гемофильной палочкой типа В, пневмококком, вирусом папилломы человека, при гепатитах А и В) [21].
Конъюгирование с высокоиммуногенными белками обеспечивает эффективность вакцин против Haemophilus influenzae типа b (Hib), менингококками и пневмококками [22]. Действительно, конъюгируя иммунизирующий антиген патогена, на который нацелена вакцинация, с белком, несущим столбнячный токсин (анти-Hib-вакцина) или дифтерию (пневмококковая и менингококковая вакцина), создаются условия для длительной защиты, в частности через стимуляцию LTCD4, дающие возможность реализовать ответ памяти. Эти конъюгатные вакцины представляют большой интерес из-за их иммуногенного эффекта, начиная с 2-месячного возраста.
Формирующаяся индивидуальная защита организма связана с возможностями иммунной системы распознавать, запоминать и оптимизировать иммунный ответ, специфичный для антигена во время повторной встречи с организмом. При должном охвате вакцинацией населения страны или региона это позволяет сформировать у подавляющего числа жителей способность к выработке коллективной защиты, которая делает данный вид профилактики наиболее успешным. Иными словами, в случае высокого уровня охвата населения вакцинацией восприимчивые его части защищены от инфекции присутствием иммунных индивидуумов; в этом суть концепции, получившей название «коллективный иммунитет» [23]. С экономической точки зрения формирование коллективного иммунитета является общественным благом, так как он защищает подавляющее большинство жителей от болезней даже в отсутствие вакцинации [24].
ДОСТИЖЕНИЯ, ПРОБЛЕМЫ И ПЕРСПЕКТИВЫ ВАКЦИНАЦИИ КАК СРЕДСТВА БОРЬБЫ С ЭПИДЕМИЯМИ И ПАНДЕМИЯМИ
Успехи вакцинации трудно переоценить. На сегодняшний день с помощью вакцин можно предотвратить заболеваемость и смертность по крайней мере от 28 инфекционных болезней, причем для профилактики 19 из этих заболеваний имеются высокодоступные вакцины. Среди них вакцины от ветряной оспы, лихорадки денге, дифтерии, гриппа, гепатитов А и В, гемофильной палочки типа b, вируса папилломы человека, кори, менингококковой инфекции, инфекционного паротита, пневмококка, полиомиелита, ротавируса, краснухи, опоясывающего лишая, столбняка, коклюша, коронавируса. Вакцинация от 10 остальных (из 28) инфекционных заболеваний рекомендуется лицам, путешествующим в эндемические регионы, и ученым, работающих с опасными инфекциями. Речь здесь о вакцинах от аденовирусной инфекции, сибирской язвы, холеры, японского энцефалита, бешенства, оспы, туберкулеза, брюшного тифа и паратифа, желтой лихорадки [25].
Вместе с тем несмотря на значительные успехи в вакцинопрофилактике многие проблемы распространения инфекционных заболеваний еще не решены. Так, в связи с высокой смертностью от инфекционных заболеваний во всем мире ВОЗ в 2018 г. разработала список наиболее социально значимой инфекционной патологии, которая не может быть в настоящее время предотвращена с помощью вакцинации. Разработка вакцин для предотвращения этих инфекционных заболеваний является приоритетной задачей здравоохранения на ближайшие годы. К болезням, попавшим в этот перечень, относятся Конго-крымская геморрагическая лихорадка, болезнь, вызванная вирусом Эбола, вирусная болезнь Марбург, лихорадка Ласса, коронавирус ближневосточного респираторного синдрома (БВРС-КоВ), тяжелый острый респираторный синдром, Нипах и генипавирусные заболевания, лихорадка Рифт–Валли и так называемое заболевание Х — болезнь с неизвестным патогеном [26]. Включение заболевания Х в список приоритетных обусловлено тем, что микробы могут эволюционировать, следствием чего становится возникновение новых неизвестных патогенных для человека штаммов, потенциально способных приводить к развитию эпидемий и пандемий по примеру COVID-19. В настоящее время известно около 260 вирусов из 25 семейств, способных заражать человека. При этом создать вакцины от всех вирусов невозможно, однако задача ученых состоит в том, чтобы сосредоточиться на разработке вакцин против ключевых патогенов, являющимися общими для отдельного семейства вируса. Развившаяся в 2019 г. пандемия новой коронавирусной инфекции показала правильность такого подхода, и вакцина от COVID-19 была создана в рекордные 326 дней. Несмотря на то что разработка новых вакцин стоит достаточно дорого, эти затраты не могут сравниться с прогнозируемым экономическим ущербом различных стран от пандемии коронавируса, оцениваемым в 28 трлн долларов США, и, разумеется, не могут «перевесить» потерей миллионов жизней [27]. Предполагается, что сроки создания вакцин могут быть сокращены до 3 мес.
Также в отдельную категорию инфекционных угроз ВОЗ включило болезни, которые являются эндемичными для некоторых регионов, но могут без надлежащего контроля распространиться на другие места Земли. Примерами таких болезней служат туберкулез, малярия, лихорадка денге и ВИЧ-инфекция [28]. Как отдельную медицинскую проблему ВОЗ рассматривает пандемический грипп, по поводу распространения которого была разработана отдельная система обеспечения готовности [29]. Еще одна угроза, обозначенная ВОЗ, – возрастание резистентности микроорганизмов к использованию противомикробных препаратов, особенно увеличившаяся после начала пандемии коронавируса, в том числе в связи с необоснованным применением таких лекарственных средств [30]. Вероятно, решению обозначенных ВОЗ проблем будут посвящены усилия медицинской науки в уже ближайшие годы.


