ВВЕДЕНИЕ
Стремительная скорость распространения во всем мире сахарного диабета 2-го типа (СД 2) и ожирения диктует необходимость изучения новых методов профилактики и лечения этих заболеваний. СД 2 характеризуется множественностью патофизиологических компонентов, к которым относятся инсулинорезистентность, нарушение секреции инсулина, ожирение, повышение секреции глюкагона, дислипидемия и др. Иными словами, патогенез СД 2 – нечто большее, чем просто проблема гипергликемии, в связи с чем современные требования к сахароснижающим препаратам значительно расширены. Помимо эффективного контроля гликемии, они должны обладать низким риском гипогликемии, эффектами кардиоренальной протекции и не влиять либо снижать массу тела.
Ретроспективное исследование более миллиона пациентов с СД 2 в США (средний возраст 65 лет, продолжительность СД 4 года, уровень гликированного гемоглобина 6,8%) показало, что 82% из них имели артериальную гипертензию (АГ), 78% – ожирение или избыточный вес, 24% – хроническую болезнь почек (ХБП) и 22% – сердечно-сосудистые заболевания (ССЗ) [1]. Кроме того, данные метаанализа обсервационных исследований в 20 странах свидетельствуют, что глобальная распространенность неалкогольной жировой болезни печени (НАЖБП) среди лиц с СД 2 достигает 56%, а неалкогольного стеатогепатита (НАСГ) – 37%. Самым значимым фактором риска СД 2 выступает ожирение, которое служит основной причиной резистентности к инсулину и вовлечено в этиопатогенез АГ, дислипидемии и НАЖБП [2–4]. Учитывая патофизиологию развития нарушений углеводного обмена, потеря массы тела может оказывать положительное влияние на гликемический контроль, чувствительность к инсулину и сопутствующие заболевания.
Американская диабетическая ассоциация (ADA) рекомендует пациентам с СД 2, имеющим избыточный вес или ожирение, снижать массу тела как минимум на 5% за счет диеты, физической активности и поведенческой терапии [5]. Бóльшая потеря веса способна обратить вспять метаболические нарушения при СД 2, что приведет к улучшению гликемического профиля, вплоть до достижения ремиссии диабета. О положительном эффекте снижения веса у пациентов с СД 2 сообщалось во многих исследованиях, оценивавших различные подходы к лечению этого заболевания (модификация образа жизни, медикаментозное и хирургическое лечение) [6]. Умеренная потеря массы тела не менее 5% от исходной способствует снижению артериального давления (АД), уровня липидов и, как следствие, сердечно-сосудистого риска, а ее уменьшение на ≥7% положительно сказывается еще и на течении НАЖБП. Несмотря на то что для лечения СД 2 в настоящее время доступно множество классов сахароснижающих препаратов, более половины пациентов могут не достигать индивидуальных целевых показателей гликемии. При изменении образа жизни в условиях исследования лишь 2/5 участников достигают умеренной потери веса ≥5 или ≥7% в первый год наблюдения [7–9].
Гормоны кишечного происхождения из класса инкретинов – глюкозозависимый инсулинотропный полипептид (ГИП) и глюкагоноподобный пептид 1 (ГПП-1) – секретируются в ответ на поступление пищи и, стимулируя секрецию инсулина, влияют на постпрандиальный уровень гликемии. Этот эффект снижается у пациентов с СД 2, но может быть частично восстановлен с помощью гипогликемических препаратов. ГИП, так же, как и ГПП-1, снижает аппетит, ингибирует секрецию глюкагона при гипергликемических или эугликемических состояниях и задержку опорожнения желудка у лиц без нарушений углеводного обмена, тогда как у больных с СД 2 этот эффект снижается. В то же время в отличие от ГПП-1 ГИП стимулирует секрецию глюкагона при гипогликемических состояниях [10, 11]. В нормальной физиологии эти два инкретиновых гормона оказывают относительно кратковременное воздействие на соответствующие рецепторы вследствие их быстрой инактивации ферментом дипептидилпептидазой-4 (ДПП-4) и, как следствие, короткого периода полураспада, составляющего лишь несколько минут [12].
За последние десятилетия в арсенале эндокринологов появились препараты инкретинового ряда – ингибиторы ДПП-4 и устойчивые агонисты ГПП-1. Значимым событием последних лет стало создание молекулы тирзепатида, который на сегодняшний день является единственным твинкретином, одобренным к применению при СД 2. Он представляет собой новый глюкозозависимый агонист рецепторов ГИП и ГПП-1, апробированный в США в качестве дополнения к диете и физическим упражнениям для улучшения гликемического контроля у взрослых пациентов с СД 2, избыточной массой тела или ожирением. Кроме того, тирзепатид исследуется как средство для лечения ССЗ, включая сердечную недостаточность с сохраненной фракцией выброса и ожирением, а также НАСГ.
Клинические исследования SURPASS-1–5 проводились с целью оценки эффективности и безопасности тирзепатида, вводимого 1 раз/нед подкожно (5, 10 и 15 мг) в рамках монотерапии или комбинированной терапии у пациентов с СД 2. Использование тирзепатида в клинических исследованиях было связано с выраженным снижением под его влиянием гликированного гемоглобина (от -1,87 до -2,59%, от -20 до -28 ммоль/ моль) и массы тела (от -6,2 до -12,9 кг) и показателей кардиометаболического риска, таких как АД, висцеральное ожирение и уровень циркулирующих триглицеридов. В SURPASS-2 это снижение было больше, чем при использовании агониста рецептора ГПП-1 семаглутида в дозе 1 мг. Тирзепатид хорошо переносился (низкий риск гипогликемии) при применении без инсулина или препаратов, стимулирующих секрецию инсулина, и демонстрировал в целом профиль безопасности, аналогичный классу агонистов рецепторов ГПП-1. Соответственно, данные этих клинических исследований позволяют предположить, что тирзепатид открывает новую возможность для эффективного снижения гликированного гемоглобина и массы тела у взрослых с СД 2.
ОБЗОР МОЛЕКУЛЯРНЫХ СВОЙСТВ ТИРЗЕПАТИДА
Тирзепатид является инкретином двойного действия, т.е. одновременно агонистом ГИП и рецепторов ГПП-1. Он был разработан для достижения сродства к рецептору ГИП, сопоставимого с аффинностью нативного ГИП, и для связывания с рецептором ГПП-1 с примерно в 5 раз более слабым сродством, чем нативный ГПП-1. Молекула тирзепатида представляет собой линейный пептид из 39 аминокислот и имеет период полураспада примерно 5 дней, что позволяет проводить его подкожные инъекции 1 раз/нед [13]. В доклинических моделях тирзепатид взаимодействовал с рецептором ГПП-1 в качестве агониста, сигнализирующего о выработке циклического аденозинмонофосфата (цАМФ), со сниженным рекрутированием β-аррестина, что потенциально приводит к уменьшению интернализации рецептора ГПП-1. Эти эксперименты позволили предположить, что такой механизм действия может обеспечить устойчивую передачу сигналов тирзепатида к рецептору ГПП-1; это, свою очередь, потенциально способствует большему инсулинотропному эффекту в отношении β-клеток поджелудочной железы. Клинические исследования показали, что влияние тирзепатида на гликемический контроль подкрепляется одновременным улучшением функции β-клеток, чувствительности к инсулину и функции α-клеток [14–16]. Препарата в дозе 15 мг значительно улучшал фазы, а также общую секрецию инсулина и чувствительность тканей к этому гормону. В тестах на толерантность к углеводам тирзепатид также уменьшал секрецию глюкагона натощак и стимулированную приемом пищи [16].
Данные исследований на мышах дают основания для предположений об улучшении резистентности к инсулину при применении тирзепатида вне зависимости от массы тела [17]. Исследования с участием пациентов с СД 2 демонстрируют, что потеря массы тела может лишь частично объяснять улучшение чувствительности к инсулину и что тирзепатид способен обеспечить более высокую чувствительность к инсулину, чем селективный агонист рецептора ГПП-1. Дополнительные результаты исследований с использованием визуальной аналоговой шкалы указывают на снижение аппетита, а соответственно и потребляемой пищи на фоне терапии тирзепатидом [18, 19]. Правда, значения этих показателей не отличались от таковых при использовании агониста рецептора ГПП-1 семаглутида.
ПРОГРАММА КЛИНИЧЕСКИХ ИССЛЕДОВАНИЙ SURPASS-1–5
Клиническая программа фазы 3 SURPASS-1–5 была разработана для охвата пациентов с разной длительностью СД 2 и различными вариантами лечения, применяемыми в клинической практике при этом заболевании [20–24]. В дополнение к измерению гликированного гемоглобина (HbA1c), исследование SURPASS-3 включало субисследования с непрерывным мониторингом уровня глюкозы в течение 24 ч, а также с применением магнитно-резонансной томографии (МРТ) для изучения влияния лечения на содержание жира в печени и другие показатели, такие как висцеральный жир [25, 26]. Критериями повышенного сердечно-сосудистого риска в SURPASS-4 служили известные ССЗ атеросклеротического генеза, либо возраст ≥50 лет и ХБП в анамнезе со скоростью клубочковой фильтрации (СКФ) <60 мл/мин/1,73 м2 или наличие в анамнезе сердечной недостаточности (класс II или III по NYHA) [23].
Исследования различались между собой, поскольку для изучения течения СД 2 были привлечены различные популяции пациентов с различными исходными данными (например, продолжительностью СД и средним уровнем HbA1c). Представленные в статье данные основаны на определении эффективности каждого исследования, которая совпадает с результатами оценки эффективности определенной схемы лечения независимо от приверженности пациентов исследуемому препарату или начала терапии неотложных состояний [20–24].
Продолжительность исследований
Длительность исследований, составлявшая 40 (SURPASS-1, -2 и -5) и 52 нед (SURPASS-3), позволяла постепенно (в течение периода до 20 нед) увеличивать дозу терзипатида до 15 мг и поддерживать эту дозировку для оценки ее терапевтической эффективности на протяжении срока до 32 нед. В исследовании SURPASS-4 аналогичные периоды равнялись 52 и 104 нед соответственно.
Группы сравнения
В группах сравнения в исследовании SURPASS-1 и -5 использовалось плацебо, в SURPASS-2 – агонист рецептора ГПП-1 семаглутид 1 мг (наивысшая доступная доза этого препарата на момент начала исследования), в SURPASS-3 – инсулин деглюдек 100 Ед/мл, в SURPASS-4 – инсулин гларгин 100 Ед/мл.
Применение инсулина в SURPASS-3,-4 и -5
В SURPASS-3 и -4 базальные инсулины титровались с помощью установленных алгоритмов лечения до достижения предварительно установленного значения глюкозы натощак. Результаты изменения HbA1c с момента начала введения инсулина были сравнимы с результатами фазы 3 клинических исследований. Среднее суточное использование на неделе 52 для инсулина деглюдека равнялось 48,8 ЕД, для инсулина гларгин 100 ЕД/мл – 43,5 ЕД. В SURPASS-5 доза базального инсулина гларгин 100 ЕД/мл (среднее начальное значение 0,4 ЕД/кг/сут) могла быть скорректирована для поддержания целевого значения глюкозы натощак (<100 мг/дл) с использованием самостоятельного ее мониторинга в крови. Если участники, получавшие только инсулин гларгин, на 40-й неделе увеличили дозы инсулина по сравнению с исходным значением на 25,1 ЕД (75%), то пациенты, принимавшие тирзепатид, напротив, значительно снизили его дозировки. Важно отметить, что участники программы исследований могли уменьшить дозу инсулина, однако в соответствии с протоколом исследования не имели права полностью прекращать инсулинотерапию.
Гипогликемический эффект тирзепатида
Первичным показателем для всех пяти исследований SURPASS служили изменения относительно «базовой линии» уровня HbA1c через 40 или 52 нед при начальных значениях этого маркера в диапазоне 7,94–8,52% [15, 20, 21, 22]. Во всех пяти исследованиях применение тирзепатида ассоциировалось со снижением HbA1c от -1,87 до -2,59% в среднем. Степень уменьшения уровня HbA1c зависела от дозы препарата и была значительно больше при введении тирзепатида в дозах 5, 10 и 15 мг по сравнению с плацебо (0,04%, SURPASS-1), семаглутидом 1 мг (-1,86%, SURPASS-2), инсулином деглюдек (-1,34%, SURPASS-3), инсулином гларгином 100 ЕД/мл (-1,44%, SURPASS-4) и плацебо с фоновым инсулином (-0,93%, SURPASS-5). Результаты исследования SURPASS-4 демонстрируют, что снижение HbA1c сохранялось также на 78-й и 104-й неделях; это свидетельствует о поддержании гликемического контроля при лечении тирзепатидом более 1 года [26]. Наибольшей величина снижения HbA1c оказалась в SURPASS -4 и -5, что, вероятно, было обусловлено более высоким его исходным уровнем в этих исследованиях. В SURPASS-1 менее выраженная дозозависимость эффекта исследуемого препарата могла быть связана с относительно небольшим стажем СД 2 у включенных пациентов и более высокой функцией β-клеток у них, так как все три дозы тирзепатида привели к почти нормогликемии через 40 нед и достижению потенциально минимального значения HbA1c (средний уровень 5,9–6,1%).
Значительная доля участников в каждом исследовании, получавших тирзепатид, достигла HbA1c <7,0 и ≤6,5%, что, согласно американским и европейским рекомендациям, соответствует целевым его значениям для большинства пациентов [27, 28]. Во всех пяти исследованиях SURPASS у 81–97% участников, применявших тирзепатид, уровень HbA1c снизился <7%, а у 66–95% он составил ≤6,5%; в этом отношении исследуемый препарат существенно превзошел плацебо, семаглутид 1 мг, инсулин деглюдек, инсулин гларгин 100 ЕД/мл и плацебо с фоновым инсулином, применявшиеся в группах сравнения. Кроме того, к концу исследований в группе тирзепатида по сравнению со всеми другими препаратами оказалась наибольшей доля пациентов с уровнем HbA1c <5,7%. В исследовании SURPASS-2 составной показатель, который оценивает долю участников с HbA1c ≤6,5% без клинически значимой или тяжелой гипогликемии и с ≥10% потерей массы тела, был достигнут у 32–60% участников, получавших тирзепатид, тогда как в группе семаглутида 1 мг аналогичная доля пациентов равнялась 22% [15].
Уровень глюкозы в сыворотке крови натощак был значительно снижен при применении любой дозировки тирзепатида в исследованиях SURPASS-1, -2 и -5 на 40-й или 52-й неделе по сравнению с плацебо, семаглутидом 1 мг и плацебо с фоновым инсулином соответственно. В SURPASS-3 показатели гликемии натощак в конечной точке при использовании доз терзипатида 10 и 15 мг не отличались от таковых при введении инсулина деглюдека. В свою очередь, в SURPASS-4 полученные уровни глюкозы натощак не различались при инъекциях терзипатида 5 и 10 мг и инсулина гларгина 100 Ед/мл, но были значительно ниже в группе исследуемого препарата в дозе 15 мг.
Добавим, что в SURPASS-3 и -4 значительное снижение гликемии натощак отмечалось уже через 2 нед (самое раннее измерение) после начала терапии, когда все участники получали 2,5 мг терзипатида [18, 24]. По двум исследованиям и трем группам доз изменение этого показателя через 2 нед варьировало от -1,7 до -1,9 ммоль/л (-18 до -20%). В небольшом исследовании фазы 1, проведенном через 24 ч после введения начальной дозы тирзепатида 2,5 мг, уменьшение гликемии натощак относительно базового уровня составило -1 ммоль/л, хотя разница с плацебо не была статистически значимой. Однако на 8-й день (перед введением препарата) наблюдалось уже статистически значимое по сравнению с плацебо снижение глюкозы натощак на -2,2 ммоль/л, что указывает на ранний потенциал уменьшения уровня гликемии после начала лечения тирзепатидом [24].
Общее среднесуточное значение глюкозы в крови перед приемом пищи и средний ее показатель через 2 ч после приема пищи, измеренные в профилях самоконтроля гликемии в 7 точках, показывают, что тирзепатид позволял поддерживать значительно более низкий уровень глюкозы в крови на 40-й или 52-й неделе в течение всего дня во всех пяти исследованиях. Помимо значительного снижения HbA1c и глюкозы натощак, лечение тирзепатидом значительно увеличило (до 73%) долю времени нахождения пациентов в целевом диапазоне гликемии (3,9–7,8 ммоль/л) по сравнению с инсулином деглюдеком (48%) на 52-й неделе [24]. Это время, проведенное в целевом диапазоне, сопровождалось также меньшей, чем в группе инсулина деглюдека, вариабельностью гликемии.
ТИРЗЕПАТИД И ОЖИРЕНИЕ: ИССЛЕДОВАНИЕ SURMOUNT-1
Ожирение ассоциировано с многочисленными осложнениями, включая АГ, дислипидемию, СД 2 и др. В частности, известно, что чем выше индекс массы тела (ИМТ) и объем талии (ОТ), тем выше риски развития СД 2 и ССЗ [28–32]. Наличие ожирения повышает вероятность сердечно-сосудистой смертности независимо от других факторов риска [24].
В исследованиях SURPASS-1–5 было продемонстрировано значительное снижение массы тела на фоне лечения тирзепатидом, что очень благоприятно отражалось на метаболическом профиле пациентов. Эти результаты вызвали большой интерес к возможностям использования тирзепатида при ожирении независимо от наличия СД 2 и послужили предпосылкой для проведения двойного слепого, рандомизированного плацебо-контролируемого клинического исследования SURMOUNT-1. В нем было показано, что у участников с подтвержденным диагнозом ожирения без СД 2 препарат в течение 72 нед обеспечивал существенное и устойчивое снижение массы тела, а также улучшал кардиометаболические показатели [31].
В исследование SURMOUNT-1 вошли 2539 человек с ИМТ >27 кг/м2 без СД 2, имевшие хотя бы одно ассоциированное с ожирением состояние/заболевание. После рандомизации им вводили подкожно тризепатид в дозировках 5 (n=630), 10 (n=636) или 15 мг (n=636) либо плацебо (n=643) 1 раз/нед в течение 72 нед в дополнение к изменению образа жизни. Для достижения значимого эффекта с точки зрения улучшения метаболического здоровья снижение массы тела должно было составлять ≥5% [32]. Совместными основными критериями эффективности служили процентное изменение веса от исходного и снижение веса на ≥5%.
К концу исследования средние процентные достоверные изменения массы тела составили -15,0, -19,5 и -20,9% для тирзепатида в дозировках 5, 10 и 15 мг соответственно и только -3,1% для плацебо. Доля участников со снижением веса на ≥5% равнялась 85, 89 и 91% в группах, получавших тирзепатид 5, 10 и 15 мг соответственно, и 35% в группе плацебо. У 50% пациентов на тирзепатиде 10 мг и 57% на 15 мг снижение массы тела достигло ≥20%, тогда на плацебо – лишь у 3% (табл.) [33–35].
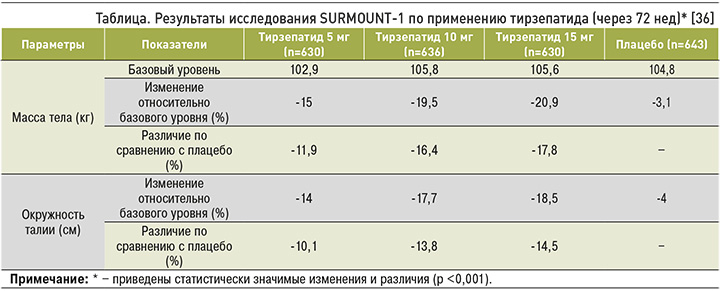
Среднее уменьшение ОТ составило -14 см при введении тирзепатида в дозе 5 мг, -17,7 см – при применении 10 мг и -18,5 см при использовании 15 мг, в то время как в группе плацебо данный показатель был равен -4 см. Оценочные различия между группами, получавшими тирзепатид в дозировках 5, 10 и 15 мг и плацебо, составили -10,1, -13,8 и -14,5 см соответственно.
КАРДИОМЕТАБОЛИЧЕСКИЙ ЭФФЕКТ ТЕРЗИПАТИДА
Значительное снижение массы тела, достигнутое на фоне применения тирзепатида, сопровождалось существенным уменьшением ОТ по сравнению с плацебо в каждом исследовании [37]. Например, в исследовании SURPASS‐2 этот показатель снизился 6,9–9,9 см при введении тирзепатида и на 5,6 см при приеме семаглутида в дозе 1 мг на 40-й неделе исследования по сравнению со средним исходным значением, равнявшимся 109,3 см. Точно так же в SURPASS‐4 наблюдалось уменьшение ОТ в течение 52 нед в группах, получавших тирзепатид, и это эффект сохранялся вплоть до 104 нед, хотя количество участников постепенно уменьшалось после 1 года исследования [38].
Что касается изменений в липидном профиле, то на 40-й неделе исследования SURPASS‐2 тирзепатид превосходил семаглутид 1 мг по степени снижения уровня триглицеридов (от -19,0 до -24,8% против -11,5%) и повышения липопротеидов высокой плотности (от 6,8 до 7,9% против 4,4%) [15]. Изменения концентраций липопротеидов низкой плотности (от -5,2 до -7,7% против -6,4%) и общего холестерина (от -5,5 до -6,3% против -4,8%) оказались схожими в группах тирзепатида и семаглутида 1 мг. В популяции с высоким сердечно-сосудистым риском в исследовании SURPASS‐4 все дозы тирзепатида при сравнении с инсулином гларгином 100 ЕД/мл более выраженно снижали относительно исходных показателей уровни триглицеридов (от -16,3 до -22,5% против -6,4%), липопротеидов низкой плотности (от -6,8 до -8,3% против 1,4%) и общего холестерина (от -5,1 до -5,6% против 0%) и повышали уровень липопротеидов высокой плотности (от 6,7 до 10,8% против 2,9%). Такие улучшения наблюдались на фоне применения 82% пациентов в начале исследования фоновой гиполипидемической терапии, которая мало изменялась на протяжении всего периода наблюдения [39].
Известно, что допустимое содержание жира в печени составляет менее 5%, хотя оценка этого параметра зависит от используемого метода измерения. Один из самых точных методов оценки висцерального жира – магнитно-резонансная томография (МРТ) с измерением доли жира. Уменьшение на треть и более избыточного содержания жира в печени благоприятно влияет на течение НАСГ и может способствовать его разрешению. В подгруппе исследования SURPASS‐3 с использованием МРТ было отмечено, что тирзепатид снижал содержание жира в печени значительно более выраженно, чем инсулин деглюдек (-8,09 против -3,38%) на 52-й неделе. Наряду с этим, к концу исследования в группе тирзепатида отмечалась более высокая доля пациентов с уровнем жира в печени ≤10% (60–78 против 35% в группе инсулина деглюдека) и снижением его содержания относительно исходного значения ≥30% (67–81 против 32%). Более того, до 48% участников, получавших терзипатид, достигли содержания жира в печени менее 6%. Наконец, применение этого препарата ассоциировалось с клинически значимым снижением таких параметров, как объем висцерального жира (от -1,10 до -1,65 л) и объем подкожных жировых отложений (от -1,40 до -2,25 л), которые увеличивались у пациентов, применявших инсулин деглюдек (+0,38 и +0,63 л соответственно).
ТИРЗЕПАТИД И АРТЕРИАЛЬНАЯ ГИПЕРТЕНЗИЯ
Известно, что АГ связана с высоким риском ССЗ и смертности, однако контроль АД у пациентов зачастую остается недостаточно эффективным. Тирзепатид благоприятно влияет на этот показатель [15], что последовательно подтверждалось во всех упоминавшихся исследованиях.
Так, в SURPASS-1 на фоне применения препарата среднее снижение систолического артериального давления (САД) варьировало от -4,7 до -5,2 мм рт.ст. по сравнению с -2,0 мм рт.ст. при приеме плацебо. В то же время по влиянию на диастолическое артериальное давление (ДАД) значимых различий между тирзепатидом и плацебо не наблюдалось.
В SURPASS-2 САД и ДАД уменьшились на -4,8 и -1,9 мм рт.ст. соответственно при введении тирзепатида в дозе 5 мг, на -5,3 и -2,5 мм рт.ст. – при применении 10 мг, на -6,5 и -2,9 мм рт.ст. при использовании 15 мг; для семаглутида 1 мг аналогичные показатели составили -3,6 мм и -1,0 мм рт.ст.
В SURPASS-3 на фоне использования тирзепатида отмечались значительные снижения как среднего САД (от -4,9 до -6,6 мм рт.ст.), так и среднего ДАД (от -1,9 до -2,5 мм рт.ст.), в то время как при введении инсулина деглюдека значительных изменений этих параметров выявлено не было.
В SURPASS-4 у пациентов на тирзепатиде уменьшение средних САД и ДАД достигало от -2,8 до -4,8 мм рт.ст. и от -0,80 до -1,0 мм рт.ст. соответственно, а вот на фоне инъекций инсулина гларгина эти показатели увеличились на +1,3 и +0,7 мм рт.ст.
В SURPASS-5 средние изменения САД и ДАД составили от -6,1 до -12,6 мм рт.ст. и от -2,0 до -4,5 мм рт.ст. соответственно в группах, получавших тирзепатид, против -1,7 и -2,1 мм рт.ст. в группе плацебо. Аналогично в исследовании SURMOUNT-1 у участников, которым вводился тирзепатид, было зарегистрировано снижение САД на -7,2 и ДАД на -4,8 мм рт.ст. в среднем. В группе же, применявшей плацебо, аналогичные изменения равнялись -1 и -0,8 мм рт.ст.
Благоприятное влияние тирзепатида на АД может быть обусловлено улучшением эндотелиальной функции и/или снижением воспаления [24], хотя для точного определения механизмов, лежащих в основе этих результатов, требуются специализированные исследования. В то же время анализ, выполненный после завершения фазы IIb исследования, показал, что применение тирзепатида было связано с дозозависимым снижением уровней высокочувствительного С-реактивного белка и молекулы межклеточной адгезии 1 типа (ICAM1/CD54) через 26 нед в сравнении с исходными данными [25].
ЗАКЛЮЧЕНИЕ
Тирзепатид – первый в своем классе двойной агонист рецепторов ГПП-1 и ГИП. Исследования SURPASS-1–5 и SURPASS J-mono продемонстрировали его эффективность и безопасность при использовании у пациентов с СД 2 по сравнению с плацебо и другими гипогликемическими препаратами. В частности, в SURPASS-4, где этот препарат сравнивался с инсулином гларгином, тирзепатид в дозе 15 мг снижал у пациентов с СД 2 уровень HbA1c на 2,58% от исходных значений. В SURPASS-1 до 52% пациентов с СД 2 достигли на тирзепатиде уровня HbA1c <5,7%. При этом в плане безопасности препарат был аналогичен агонистам рецепторов ГПП-1 – он не вызывал значительных эпизодов гипогликемии (<3 ммоль/л).
Применение тирзепатида приводило к дозозависимому снижению массы тела. В SURMOUNT-1, где оценивалась эффективность тирзепатида у пациентов с избыточным весом и ожирением без СД 2, доля участников, достигших снижения массы тела на ≥5%, составила 85, 89 и 91% при применении этого препарата в дозировкахах 5, 10 и 15 мг соответственно; при этом введение дозе 15 мг обеспечивало значительное снижение массы – на 20,9%. Во всех исследованиях также было установлено снижение ИМТ и ОТ с улучшением показателей АД, липопротеидов низкой плотности и триглицеридов на фоне применения тирзепатида. В частности, в исследовании SURMOUNT-1 наблюдалось среднее снижение ОТ на 18,5 см при введении тирзепатида в дозе 15 мг. В SURPASS-5 у пациентов, получавших тирзепатид, среднее изменение САД варьировало от -6,1 до -12,6 мм рт.ст..
В мае 2022 г. Управление по контролю качества пищевых продуктов и лекарственных средств (FDA) одобрило применение тирзепатида в форме раствора для подкожных инъекций 1 раз/нед (в дозировке, корректируемой в зависимости от переносимости препарата для достижения целевого уровня глюкозы в крови) в рамках монотерапии или комбинированной терапии в сочетании с диетой и физической активностью для достижения лучших гликемических показателей крови у пациентов, страдающих СД 2. В октябре 2022 г. в рамках процедуры ускоренной регистрации тирзепатид был одобрен к применению для пациентов с избыточной массой тела и ожирением без СД 2. Препарат применяется в дозах 5, 10 и 15 мг, возможность использования других дозировок еще предстоит изучить. Нежелательные явления, связанные с введением тирзепатида, включают тошноту, рвоту, понос, запор, дискомфорт в верхней части живота, снижение аппетита и боль в животе. Эти побочные эффекты часто наблюдаются при синдроме раздраженного кишечника и могут свидетельствовать о потенциальном воздействии тирзепатида на микрофлору кишечника.
Тирзепатид продемонстрировал поразительную эффективность в аспекте лечения СД 2 и ожирения. Отсроченный профиль безопасности препарата требует более продолжительных исследований с оценкой долгосрочных результатов, однако уже сегодня тирзепатид можно с полным основанием отнести к передовым безопасным средствам для лечения указанных заболеваний, а текущие исследования препарата сулят его применение будущем при сердечной недостаточности и НАЖБП.
Добавим, что в дополнение к исследованиям двойного агониста ГПП-1 и ГИП тирзепатида в настоящее время на животных моделях активно изучаются триагонисты ГПП-1, ГИП и глюкагона. Предклинические исследования свидетельствуют, что триагонисты, вызывая одновременную активацию рецепторов ГПП-1, ГИП и глюкагона, нормализуют массу тела у ожиревших мышей и увеличивают энергозатраты в большей степени, чем моноагонисты рецептора ГПП-1 и двойные агонисты рецепторов ГПП-1 и ГИП. Можно предположить, что мономолекулярная тройная активация рецепторов ГПП-1, ГИП и глюкагона также может стать новым стандартом для фармацевтических вмешательств. Оценить перспективы такой терапии призваны новые исследования.



