ВВЕДЕНИЕ
Сахарный диабет (СД) является комплексным хроническим заболеванием, которое ассоциировано с рядом осложнений, вызываемых в первую очередь поражением функции эндотелия во всех сосудистых бассейнах организма. Дисбаланс между функцией β-клеток поджелудочной железы и чувствительностью периферических тканей к инсулину вызывает хроническую гипергликемию, которая, наряду с другим факторами риска, приводит к запуску процессов атерогенеза и развитию микро- и макроангиопатий. Долгое время «классическими» факторами сердечно-сосудистого риска считались повышенное артериальное давление, гиперхолестеринемия и курение. Лишь в последней четверти XX в. пришло понимание, что метаболические нарушения, начиная с самых ранних стадий, являются серьезными заболеваниями в том числе с позиции долгосрочного прогноза пациентов [1, 2].
В анализе сочетанной патологии было показано, что частота смертности у пациентов с СД 2-го типа (СД 2) аналогична таковой у пациентов с анамнезом инсульта или инфаркта миокарда (ИМ), причем сочетание этих заболеваний ассоциировано с мультипликативным эффектом в отношении частоты смертности. Иными словами, связь каждого из этих состояний с долгосрочным риском летального исхода при наличии дополнительной сочетанной патологии кратно возрастала [1, 3].
Более того, совокупность патогенетических процессов выводит СД 2 в отдельную категорию в противовес ранее бытовавшему мнению об эквивалентности диабета заболеваниям исходно кардиоваскулярного генеза [4]. Так, в анализе двух английских регистров (CPRD Англии и регистра Шотландии) было показано, что у пациентов с СД 2, независимо от числа неконтролируемых факторов риска, была выше опасность развития любых сердечно-сосудистых заболеваний (ССЗ). Пациенты с СД 2 и целевым контролем пяти факторов риска имели на 21% большую вероятность наступления любых ССЗ по сравнению с пациентами без диабета. Наибольший риск наблюдался у пациентов с СД 2 и 5 неконтролируемыми факторами риска: угроза развития самого первого сердечно-сосудистого события (ССС) у них была повышена в 2 раза [5].
Наличие добавочного кардиоваскулярного риска при СД также мешает разработке достоверной шкалы долгосрочного сердечно-сосудистого прогноза. Российские руководства по ведению пациентов с СД уже много лет рекомендуют не использовать общепринятые и специально разработанные шкалы рисков ССЗ и смертности при СД [6]. Более того, в последнем обновлении шкалы SCORE, используемой для широкой популяции (SCORE 2), из нее был исключен критерий СД, поскольку зарубежные сообщества также склоняются к тому, что при данном заболевании требуется отдельная стратификация рисков [7]. На настоящий момент как эндокринологические, так и терапевтические и кардиологические профессиональные ассоциации сходятся не на расчетной, а клинической оценке кардиоваскулярного риска в зависимости от наличия/отсутствия у пациентов с СД:
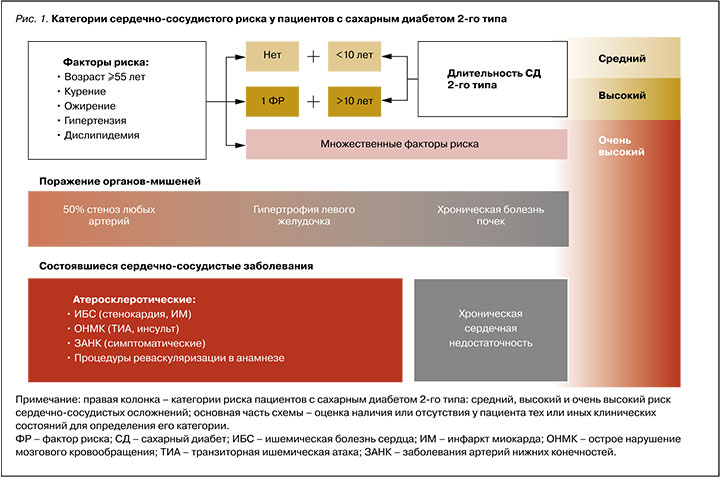
- факторов сердечно-сосудистого риска – артериальной гипертензии, атерогенной дислипидемии, ожирения, длительности СД и возраста;
- поражения органов-мишеней – 50% стеноза коронарных, каротидных или периферических артерий, гипертрофии левого желудочка, хронической болезни почек (т.е. наличия выраженного снижения скорости клубочковой фильтрации и/ или протеинурии);
- уже состоявшихся ССЗ в анамнезе (хронической сердечной недостаточности или атеросклеротических ССЗ – ИМ, стенокардии, инсульта, транзиторной ишемической атаки, заболеваний артерий нижних конечностей с симптоматикой), а также процедур реваскуляризации (стентирования, шунтирования) [6, 8–10].
Схематично градация риска на основе описанных клинических факторов представлена на рисунке 1. Из него следует, что категоризация прогноза пациентов с СД 2 исходно начинается не с низкого, как обычно, а со среднего риска; тем самым выделяется особый исходный модифицирующий вклад этого заболевания в будущий риск осложнений.
Метаанализ данных около 4,5 млн пациентов, а также результаты крупномасштабного проспективного наблюдательного исследования CAPTURE демонстрируют, что в широкой популяции пациентов с СД 2 около одной трети из них уже имеют в анамнезе ССЗ [11, 12]. Наибольшую долю (≈80– 90%) этих заболеваний составляют атеросклеротические ССЗ (АССЗ). Хроническая сердечная недостаточность (ХСН), обычно выступающая более поздним осложнением классической сердечно-сосудистой патологии, при СД может манифестировать и без предшествующих АССЗ, отражая тем самым независимую прогрессию диабетической кардиомиопатии [12, 15]. С другой стороны, даже тут существенную превентивную роль играет управление атеросклеротическими процессами, так как частота развития ХСН при таких заболеваниях, как ишемическая болезнь сердца (ИБС) или хроническая болезнь почек (ХБП), очень высокая. Например, у пациентов после ИМ риск развития ХСН возрастает на 21–89%, а при снижении скорости клубочковой фильтрации (СКФ) на каждые 10 мл/мин/1,73 м2 – на 10% [17].
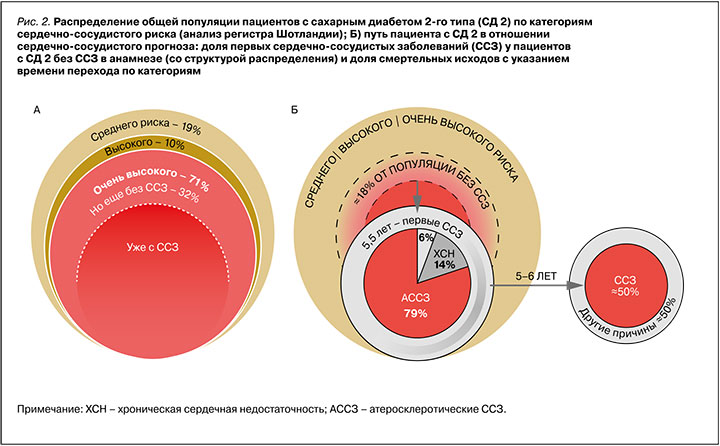
Категории исходного сердечно-сосудистого риска позволяют выделить не только очевидную когорту пациентов с ССЗ, нуждающуюся во всестороннем контроле заболевания, но и обратить внимание на более ранние этапы, когда еще возможно первично предотвратить осложнения. В анализе ранее упомянутого регистра Шотландии на предмет соответствия пациентов с СД 2 вышеописанным категориям риска было показано, что 19% таких пациентов приходится на категорию среднего риска, 10% – высокого, 32% – очень высокого, но без АССЗ. При этом опять же около трети (38%) от популяции составляют пациенты с СД 2 и с ССЗ (рис. 2А) [14]. В другом анализе первых ССЗ было показано, что за время наблюдения (в среднем 5,5 лет) у пациентов с СД 2 без исходного анамнеза ССЗ кардиоваскулярные осложнения развились почти в 18% случаев или, при грубой оценке, ≈10% от всей популяции пациентов с СД 2 (рис. 2Б) [15]. При этом том показано, что за аналогичный период (5–6 лет) ССЗ становятся причиной смерти пациентов с СД 2 в половине случаев (50%), что в контексте всей популяции пациентов с этим заболеванием составляет примерно те же 10% [11]. Это согласуется и с российскими данными, которые свидетельствуют, что ежегодно около 70 000 пациентов с СД 2 погибают от сердечно-сосудистых причин, а средняя длительность их жизни составляет около 11 лет с момента установления диагноза СД 2 [16].
В связи с вышеописанным становится очевидной необходимость использования подходов, направленных на модификацию естественного хода СД 2. В целях профилактики ССЗ (как первичной, так и вторичной) на каждом этапе заболевания у пациентов с СД 2 разного исходного кардиоваскулярного риска необходимо следовать актуальным руководствам, основанным на данных широкомасштабных рандомизированных клинических исследований.
ПРАКТИЧЕСКАЯ ИНТЕРПРЕТАЦИЯ ДАННЫХ КЛИНИЧЕСКИХ ИССЛЕДОВАНИЙ
Для описания влияния интервенции на клинические исходы результаты исследований обычно выражают в терминах снижения относительного риска (∆ОР). Предполагается, что ∆ОР стабильно для каждой включенной группы пациентов, и степень продемонстрированного эффекта одинакова в любой момент времени исследования. Это позволяет косвенно сравнивать между собой исследования одного типа, имеющие разную длительность и включающие популяции разного исходного риска [18].
В то же время оценка влияния интервенции не на относительный, а абсолютный риск (АР) является основополагающей при принятии решения в пользу применения определенного подхода у индивидуального пациента, так как в этом случае первостепенное значение приобретает исходный прогноз и время, в течение которого необходимо находиться на терапии для достижения результата.
Для практического выражения степени снижения АР (∆АР) удобна концепция «числа пациентов, которое необходимо пролечить» (NNT – number needed to treat) для предотвращения одного неблагоприятного события. NNT рассчитывается как обратная величина от разницы между абсолютными рисками события в изучаемой и сравниваемой группах (1/∆АР). Это позволяет перевести статистические данные в фокус клинической пользы и оценить целесообразность интервенции с точки зрения ресурсов, необходимых для реализации эффекта вмешательства [18, 19].
Исходя из формулы расчета более выраженный эффект вмешательства (∆АР) транслируется в меньшее значение NNT, и наоборот. Также NNT – это убывающая степенная функция от времени, т.е. в начале исследования, когда терапия только-только начинает реализовывать свой потенциал, NNT больше, а после оно уменьшается по ходу исследования. NNT может быть математически рассчитан в любой временной точке исследования и гипотетически экстраполирован на более длительный временной горизонт (рис. 3). Однако, как правило, этот параметр рассчитывается по завершении исследования, что соответственно требует указания времени достижения этого результата, так как исследования имеют разную длительность. Также для возможности сравнения этого параметра в различных исследованиях необходимо приводить его для конкретной группы исходного кардиоваскулярного риска.
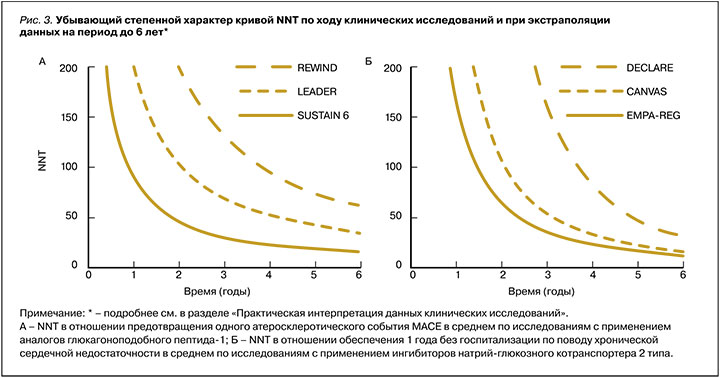
Таким образом, NNT зависит от исходного риска пациента, мощности эффекта и времени на терапии. При прочих равных, чем меньше NNT, время его достижения или оба этих параметра, тем эффективнее одна терапия относительно другой [18, 20].
ВЕДЕНИЕ ПАЦИЕНТОВ С СД 2 СО СРЕДНИМ РИСКОМ СЕРДЕЧНО-СОСУДИСТЫХ ОСЛОЖНЕНИЙ
Как было сказано выше, СД ассоциирован с избыточным сердечно-сосудистым риском. При этом в отношении контроля гипергликемии (как независимого фактора риска ССЗ и смертности) имеет значение не только степень превышения целевых показателей углеводного обмена, длительность их нахождения вне контроля, колебания уровня гликемии, но и риск развития гипогликемий.
В недавнем когортном исследовании (The Diabetes & Aging Study) по оценке 10-летней выживаемости 35 000 пациентов с СД 2 было показано, что существенное влияние на прогноз оказывают как каждый шаг превышения уровня референтных значений гликированного гемоглобина (HbA1c=6,5%), так и каждый год недостижения контроля углеводного обмена. Даже первый упущенный год у пациентов с минимальным (6,5–7,0%) превышением референтных значений HbA1c был ассоциирован с возрастанием риска микро-, макрососудистых осложнений и летальности на 39, 28 и 29% соответственно HbA1c [21].
В ранних исследованиях по оценке влияния интенсивного гликемического контроля (UKPDS, ADVANCE, ACCORD, VADT) был предложен термин «гликемическое бремя», описывающий длительное пребывание пациента вне целевых показателей гликемии. Концепция предполагала, что если избегать «гликемического бремени», то можно потенциально предотвратить осложнения СД 2 в будущем. Эта концепция легла в основу необходимости непрерывного контроля гликемии и интенсификации терапии при его потере (поэтапная терапия).
Эти исследования изучали подходы к терапии СД 2 на разных этапах заболевания – от момента установления диагноза до поздних стадий у пациентов, имеющих анамнез ССЗ. В исследованиях использовались доступные на тот момент сахароснижающие препараты, такие как метформин, производные сульфонилмочевины (ПСМ), инсулины, тиазолидиндионы (ТЗД).
В обобщенном метаанализе этих исследований (CONTROL) на репрезентативной популяции из около 27 000 пациентов с разной длительностью СД 2 (30% с ССЗ в анамнезе) было продемонстрировано, что влияние на один лишь гликемический контроль (∆HbA1c на 0,9% пункта) упомянутыми группами препаратов обеспечивает ∆ОР ССЗ на 16% (NNT=98 за 4,4 года), но только у пациентов c менее длительным СД 2 (<5 лет) без ССЗ в анамнезе. В то же время при наличии ССЗ влияние этих интервенций было нейтральным. Возможно, это обусловлено тем, что потенциальные преимущества сахароснижающих средств было нивелировано высокой частотой развития тяжелых гипогликемий [23]. К примеру, исследование ACCORD было даже досрочно прекращено из-за повышения смертности в группе интенсивного контроля [22].
Отдельно стоит отметить, что во многом благодаря данным исследования UKPDS СД 2 стал восприниматься как серьезное заболевание, а адекватный контроль гликемии, несмотря на противоречивые данные в отношении долгосрочных прогнозов, по-прежнему остается основой терапии на всех стадиях заболевания как с позиции прогрессирования собственно СД 2, так и с точки зрения ассоциированных с ним микрососудистых осложнений [24].
Также именно по результатам этого исследования метформин впервые продемонстрировал не только общие положительные метаболические эффекты и безопасность, но и благоприятное влияние на долгосрочный прогноз в отношении ИМ у пациентов с недавно диагностированным СД 2 и избыточной массой тела. В связи с этим метформин и по сей день рекомендован как препарат выбора при инициации терапии и в составе любой комбинированной терапии [6, 24].
Другое исследование – STENO-2 – имело целью определить влияние многофакторного контроля «до терапевтических целей» различных параметров (HbA1c, холестерина, триглицеридов, систолического и диастолического АД) у пациентов с СД 2 со средним кардиоваскулярным риском. Результаты первых 7,8 лет исследования свидетельствовали о ∆ОР на 50% смерти по любой причине (∆АР на 20%; NNT=5 за 7,8 лет) [25]. Наблюдательная часть исследования, продолжавшаяся еще 13 лет, показала дополнительное ∆АР: пациенты, находившиеся в группе интенсивного многофакторного контроля, приобретали дополнительные 8 лет жизни в сравнении с группой традиционного подхода, а ОР развития ХСН снижался на 70% (∆АР=17%, NNT=6 за 21 год терапии) [26, 27].
Исследование STENO-2 стартовало еще в начале XXI в., и с тех пор изменились оценка факторов риска, цели терапии и соответственно подходы к лечению различных заболеваний. Так, в настоящее время при многих нозологиях рекомендована ранняя комбинированная терапия препаратами с комплементарными механизмами действия. К примеру, при артериальной гипертензии рекомендуют уже с момента установлении диагноза инициировать прием блокатора ренин-ангиотензин-альдестероновой системы в комбинации с блокатором кальциевых каналов или диуретиком [9].
Идеи об исходной комбинированной терапии при СД 2 ходили давно. Было очевидно, что прогрессирующая природа патогенетических механизмов оказывает влияние на возможность удержать заболевание под контролем в будущем как с позиции патофизиологии [24, 28], так и терапевтической инерции (усугубляющей проблему «гликемического бремени») [30]. Тем более что метформин, рекомендованный в составе терапии СД 2, одобрен к использованию еще на предиабетических стадиях, что в контексте дальнейшей прогрессии углеводных нарушений, т.е. на старте СД 2, делает монотерапию мало обоснованной.
Ключевым лимитирующим фактором раннего применения комбинаций сахароснижающих средств были опасения в отношении рисков гипогликемии, которые могут полностью нивелировать потенциальные преимущества интенсивной терапии. К тому же после опубликованных в 2007 г. данных об ухудшении прогноза пациентов на терапии росиглитазоном (ТЗД) во главу угла всех исследований противодиабетических препаратов были поставлены исследования сердечно-сосудистой безопасности (CVOT) [29].
В настоящее время для лечения СД 2 доступны препараты нового поколения, в частности, таблетированные ингибиторы дипептидилпептидазы-4 (иДПП-4) и инъекционные агонисты рецепторов глюкагоноподобного пептида-1 (арГПП-1; семаглутид доступен также в пероральной форме). Они нацелены на компенсацию недостаточности инкретинового эффекта у пациентов с СД 2, тем самым способствуя более адекватному эндокринному ответу как на повышение, так и снижение уровня гликемии. В связи с глюкозозависимым действием прием этих препаратов не сопряжен с риском гипогликемий [63]; кроме того, они как минимум не оказывают негативного влияния на массу тела и безопасны в отношении развития ССЗ в CVOT [31].
Применение препаратов инкретинового ряда также ассоциируется с улучшением функциональных способностей β-клетки [63]. В рекомендациях по ведению пациентов с СД 2 среди свойств препаратов этого ряда отдельно отмечается их потенциальное протективное действие на β-клетку [6], что, по сути, отражает их модифицирующее влияние на течение СД 2 как эндокринологического заболевания.
С учетом указанных свойств и ранее затронутых вопросов ранней комбинированной терапии совместное применение препаратов этого ряда с метформином, особенно на ранних стадиях СД 2, представляется обоснованным.
В 2019 г. завершилось исследование VERIFY, в котором у пациентов с момента установления диагноза СД 2 (исходный HbA1c <7%) оценивалась долгосрочная целесообразность ранней патофизиологически обоснованной комбинированной терапии (сочетание иДПП 4 вилдаглиптина с метформином) по сравнению с традиционной поэтапной тактикой ведения пациентов. Результаты показали, что ранняя комбинированная терапия при безопасной и равной со стандартным подходом степенью снижения HbA1c обеспечивала дополнительные 2 года нахождение пациента в целевых значениях углеводного обмена. При оценке безопасности было обнаружено, что использование ранней комбинированной терапии, в сравнении с поэтапной, демонстрировало тенденцию к численному ∆ОР ССС на треть (∆АР=0,9%, NNT=111 за 5 лет терапии) [32].
Другое долгосрочное сравнительное исследование – GRADE – оценивало влияние на удержание гликемического контроля разных классов сахароснижающих препаратов в дополнение к метформину у пациентов с небольшой длительностью СД 2 (4,7 лет). Согласно предварительным данным (2021), при любом исходном уровне HbA1c (6,8–8,5%) аналог человеческого ГПП-1 лираглутид (1,8 мг) превосходил иДПП-4 ситаглиптин и ПСМ глимепирид и не уступал заместительной терапии инсулином в отношении длительности удержания гликемического контроля. Кроме того, у пациентов, получавших терапию аналогом человеческого ГПП-1, отмечалось дополнительное снижение веса на 4 кг, а также, возможно, благоприятный прогноз в отношении ранней первичной профилактики сердечно-сосудистых исходов. Однако в отношении последнего стоит дождаться публикации расширенных результатов [33, 34]. В то же время лираглутид в дозе 3,0 мг активно применяется по показанию «ожирение», что выводит препарат в этой дозе в разряд первостепенных опций у данной категории пациентов. Более того, в исследовании SCALE лираглутид 3,0 мг продемонстрировал снижение частоты трансформации предиабета в СД 2 по сравнению с плацебо почти на 80%, в связи с чем потенциально ограждал пациентов от избыточного риска, ассоциированного с СД 2 [66].
Недавно зарегистрированная пероральная форма аналога человеческого ГПП-1 семаглутида продемонстрировала некоторые положительные результаты в плане раннего и долгосрочного контроля СД 2. В поданализе исследования PIONEER 1 было установлено, что, хотя монотерапия пероральным семаглутидом в целом приводила к существенному снижению HbA1c (на 1,5% пункта), наибольшее достижение целей терапии отмечалось у пациентов с недавно диагностированным СД 2 (<1 года) [35]. В поданализах исследования PIONEER 2, в котором сравнивалась эффективность перорального семаглутида с ингибитором натрий-глюкозного котранспорта-2 типа (иНГЛТ- 2) эмпаглифлозином в дополнение к метформину, было отмечено, что значительно большая доля пациентов на терапии пероральным семаглутидом находилась в гликемическом контроле на каждом этапе исследования [36]. В самом долгосрочном (2 года) исследовании с применением перорального семаглутида – PIONEER 7 – оценивалась степень достижения целей и долгосрочность эффекта препарата в сравнении с иДПП-4 ситаглиптином в дополнение к любой противодиабетической терапии. Результаты показали, что в среднем пациенты на терапии пероральным семаглутидом удерживали гликемический контроль на протяжении всех 2 лет, тогда как на терапии ситаглиптином контроль в среднем не достигался, и даже появлялись сигналы о прогрессировании гликемических нарушений [37]. Раннее применение семаглутида, который продемонстрировал не только высокую эффективность, безопасность и долгосрочность контроля СД 2, но и другие плейотропные эффекты (контроль пищевого поведения, снижение веса, АД, триглицеридов и маркеров системного воспаления), вкупе с тем, что он единственный среди арГПП-1 доступен в пероральной форме, возможно, создает все предпосылки для применения его в качестве терапевтической опции, всесторонне модифицирующей ход заболевания, и соответственно как препарата для первичной профилактики долгосрочных исходов СД 2 [38, 39]. Недавно стартовавшее исследование ACEND PLUS поставило своей целью оценку долгосрочных исходов на фоне приема перорального семаглутида в популяции ≈20 000 пациентов с СД 2 и средним сердечно-сосудистым риском [40].
Таким образом, у пациентов среднего исходного риска многофакторный контроль факторов кардиоваскулярного риска позволяет потенциально влиять на прогноз пациентов с СД 2. Современные терапевтические опции позволяют быстрее достигать и, что самое главное, безопасно и длительно удерживать целевые значения, начиная с самых ранних стадий заболевания, а также оказывают благоприятные системные плейотропные эффекты на кардиометаболические факторы риска.
На основании вышеперечисленных, новых поступающих данных и данных, которые будут описаны в следующем разделе, последние рекомендации по ведению пациентов с СД 2 предлагают для получения дополнительных долгосрочных преимуществ рассмотреть раннюю комбинированную терапию с применением современных противодиабетических препаратов независимо от исходного HbA1c и в соответствии с исходным кардиоваскулярным риском [6, 41].
ВЕДЕНИЕ ПАЦИЕНТОВ С СД 2 С ВЫСОКИМ И ОЧЕНЬ ВЫСОКИМ РИСКОМ СЕРДЕЧНО-СОСУДИСТЫХ ОСЛОЖНЕНИЙ
Как уже было замечено ранее, преимущества гликемического контроля с позиции прогрессирования СД 2 и долгосрочных исходов были продемонстрированы у пациентов без ССЗ в анамнезе, а интервенции с высоким риском гипогликемий ассоциированы с негативным прогнозом. Более того, при лечении пациентов, уже имеющих ССЗ в анамнезе, у некоторых препаратов были обнаружены негативные эффекты. Так, ТЗД не рекомендованы при ХСН в связи риском отеков, а терапия иДПП-4 (саксаглиптином, алоглиптином) в CVOT демонстрировала тенденцию к усугублению этого заболевания [6]. Поэтому в отношении терапии пациентов высокого и очень высокого кардиоваскулярного риска стоит руководствоваться результатами, полученными в исследованиях CVOT.
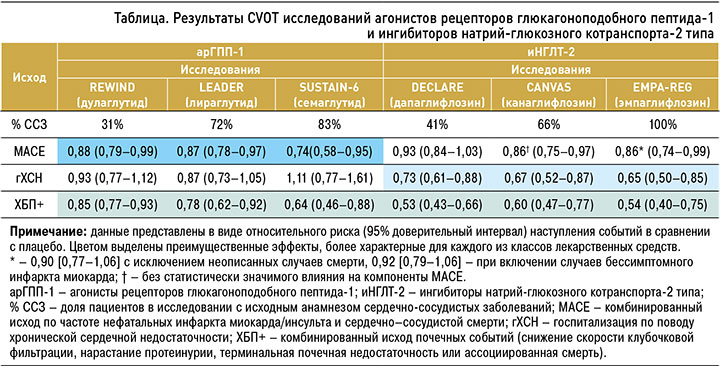
К лекарственным средствам, показавшим свои преимущества в ходе исследований CVOT, относятся иНГЛТ 2 и ранее упомянутый класс арГПП-1 (подкласс аналогов человеческого ГПП-1). Эти препараты в исследованиях оказывали влияние на MACE (комбинированный исход по снижению частоты нефатальных инфарктов, нефатальных инсультов или сердечно-сосудистой смерти), а также на снижение числа госпитализаций по поводу ХСН и комбинированные исходы в отношении почечного прогноза. Основные результаты этих исследований, выраженные в ОР, приведены в таблице, а данные по степени ∆АР, представленные расчетной динамикой NNT, отражены на рисунке 3 [42, 43].
Ингибиторы натрий-глюкозного котранспорта-2 типа
В исследование EMPA-REG (эмпаглифлозин), которое длилось чуть больше 3 лет, были включены пациенты экстремального сердечно-сосудистого риска, имевшие очень отягощенный сердечно-сосудистый анамнез (100% с анамнезом ССЗ). Препарат достиг целей исследования за счет снижения сердечно-сосудистой смертности. При более детальном рассмотрении данных исследования возникали вопросы, так как 40% смертей, зарегистрированных в ходе исследования, были отнесены в категорию сердечно-сосудистых, однако исходно значились как «недоступные к описанию». Более того, в исходную оценку не вошли пациенты с бессимптомным ИМ («перенесенным на ногах»). При поправке исследования на эти данные в дополнительном анализе было выявлено, что, хотя результат по первичному исходу становится незначимым (MACE – 0,90 (0,77–1,06) или 0,92 (0,79–1,06) соответственно), положительный эффект препарата в отношении сердечно-сосудистой смертности сохраняется в первую очередь за счет снижения частоты усугубления ХСН и внезапной смерти. Дополнительно эмпаглифлозин показал позитивное влияние на ХБП [44–46].
В объединенной программе CANVAS с применением канаглифлозина на популяции пациентов с меньшим исходным риском (66% с ССЗ в анамнезе, 34% с множественными факторами риска) также были получены положительные результаты в отношении первичной конечной точки. Однако компонентный анализ не демонстрировал конкретного преимущества препарата с позиции снижения частоты ИМ, инсультов или сердечно-сосудистой смертности; возможно, это было вызвано частичным разослеплением данных по безопасности. В связи с сигналами о повышении уровня липопротеидов низкой плотности при терапии иНГЛТ-2 были раскрыты данные, которые не были включены в анализ, а сам план анализа претерпел существенные изменения. Несмотря на это, канаглифлозин также показал значимый эффект в отношении ХСН и ХБП [47, 48].
В исследовании DECLARE с применением дапаглифлозина на протяжении чуть больше 4 лет у ≈17 000 пациентов, среди которых преобладала группа с множественными факторами риска (59% популяции исследования), не было обнаружено значимого влияния препарата на атеросклеротическую конечную точку MACE, однако опять же имелись указания на протективное действие этого иНГЛТ-2 в отношении прогрессирования ХСН и ХБП [49].
Отдельные анализы возможных медиаторов эффекта в этих исследованиях сходятся на том, что наибольший вклад иНГЛТ 2 в замедление прогрессирование ХСН был обеспечен влиянием этих препаратов на гемоконцентрацию, повышение гематокрита, альбуминурию и метаболизм мочевой кислоты [51–53]. Интересно, что в дополнительном анализе исследования DECLARE у пациентов с предшествовавшим ИМ было показано снижение частоты ИМ 2 типа, который по определению вызван не столько атеросклеротическим поражением, сколько дисбалансом энергетических потребностей [50]. Эти данные свидетельствуют в пользу того, что положительные эффекты, продемонстрированные в исследованиях иНГЛТ-2, обусловлены не столько снижением системных процессов атерогенеза, сколько возможным влиянием препаратов на энергетическое обеспечение миокарда (кетогенез, усиление кислородной емкости крови за счет усиления эритропоэза) и снижение пред- и постнагрузки на него (уменьшение объема циркулирующей крови вследствие натрийуреза) [54].
Как видно, замедление прогрессирования ХСН является класс-специфическим эффектом иНГЛТ- 2. При этом было показано, что чем более выраженно нарушение почечной функции, тем сильнее эффект этих средств в отношении ХСН, но слабее в отношении ХБП, и наоборот. С другой стороны, в CVOT имелись свидетельства и о протективном действии иНГЛТ-2 в отношении этих исходов у пациентов, находящихся только в зоне риска наступления ХСН [57]. В связи с этим, как было рассмотрено в разделе практической интерпретации данных, стоит иметь в виду число пациентов и время (NNT), необходимые для получения пользы у каждой группы исходного риска, чтобы понимать абсолютную пользу подобных интервенций.
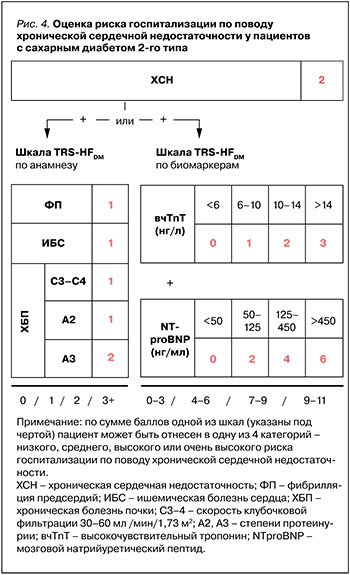
Так, рабочая группа TIMI предлагает две шкалы для определения исходного риска госпитализации по поводу ХСН: по сердечно-сосудистому анамнезу (TRS-HFDM) [59] или по определению кардиомаркеров (TBS-HFDM) (рис.4) [58]. В основе каждой из них лежит статус ХСН в анамнезе и факторы оценки. По количеству баллов одной из шкал пациент может быть отнесен к той или иной степени риска госпитализации по поводу ХСН – низкой, средней, высокой или очень высокой. Основываясь на этих шкалах, можно заметить, что пациенты максимального риска получат большее преимущество от назначения подобной терапии (рис. 5).
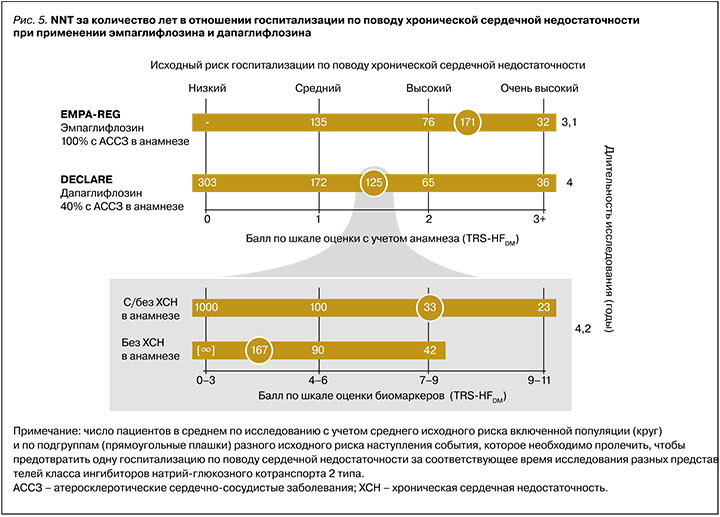
Достоверное доказательство этого эффекта иНГЛТ-2 было показано в исследованиях на популяциях пациентов с экстремальным риском осложнений уже существенно выраженных состояний, связанных с ХСН. Так, в исследованиях EMPEROR Reduced и DAPA-HF у пациентов с ХСН со сниженной фракцией выброса и очень высоким уровнем NTproBNP (≈1500 нг/мл) NNT за 1,3 и 1,5 года составили 20 и 27 соответственно [55, 56]. В то же время пациенты низкого исходного риска, в особенности без ХСН в анамнезе, получат минимальное преимущество или не получат его вовсе (например, NNT за 4 года на терапии дапаглифлозином равнялся 303, см. рис. 5) [58, 59].
Агонисты рецепторов глюкагоноподобного пептида-1
АГПП-1 – это разнородная группа препаратов, которые отличаются по способу применения, молекулярной структуре и степени влияния на факторы риска. К сожалению, многие метаанализы некорректно объединяют данные, полученные, например, на первых представителях этого класса, которые имеют в своей молекулярной основе экзендин-4 (экзенатиде, ликсисенатиде), с результатами, показанными в исследованиях с применением аналогов человеческого ГПП-1 (лираглутида, семаглутида, альбиглутида и дулаглутида). Сравнительные данные [60] и данные реальной практики [62] подтверждают более выраженное влияние аналогов человеческого ГПП-1 на HbA1c, глюкозу плазмы натощак и вес; при этом именно они характеризуются лучшей переносимостью, а также демонстрируют лучший эффект в отношении ССЗ, ассоциированных с атеросклерозом (комбинированный исход MACE, частоту ИМ и госпитализаций по сердечно-сосудистым причинам), по сравнению с экзендинподобными арГПП-1. Поэтому всегда необходимо делать поправку на нивелирование среднего эффекта по данному классу препаратов, так как экзендиновые арГПП-1 смещают результаты в нейтральную сторону.
Дополнительно в метаанализе, включившем данные по иДПП-4, иНГЛТ-2 и арГПП-1, было подтверждено нейтральное влияние иДПП-4 и экзентиднподобных арГПП-1 на сердечно-сосудистый прогноз, тогда как иНГТЛ-2 демонстрировали преимущество только в аспекте ХСН, а аналоги человеческого ГПП-1 только в аспекте АССЗ [61].
Аналоги человеческого ГПП-1 влияют на атеросклеротические исходы, в первую очередь благодаря снижению риска наступления ИМ и инсульта, а также проявляют нефропротективные эффекты. Механизмы влияния этих препаратов на аспекты патогенеза продолжают изучаться, однако накоплено много данных о том, что они, помимо основного противодиабетического действия, оказывают прямое благоприятное влияние на процессы системного воспаления, атерогенеза и формирования атеросклеротических бляшек, а также на дополнительные компоненты метаболического синдрома [63].
В первом исследовании CVOT в этом ряду, проведенном с использованием лираглутида 1,8 мг в популяции пациентов с СД 2 и высоким сердечно-сосудистым риском (LEADER), было получено достоверное преимущество препарата в отношении MACE (∆ОР=13%, NNT=53 за 3,8 года) за счет снижения общей и сердечно-сосудистой смертности, а также уменьшения частоты инсультов и ИМ. Однако дополнительный анализ подгруппы пациентов только с множественными факторами риска не выявил положительной динамики у этой категории пациентов [64, 65]. Данные по применению лираглутида у пациентов среднего кардиоваскулярного риска описаны в разделе выше.
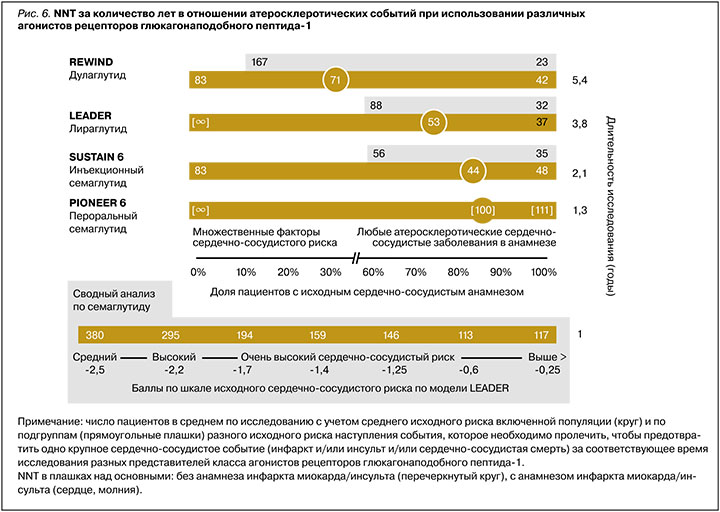
В исследовании REWIND с использованием дулаглутида в популяции ≈10 000 пациентов с СД 2 (31% ССЗ в анамнезе) было достоверно подтверждено ∆ОР MACE на 12% у пациентов как с множественными факторами риска, так и ССЗ в анамнезе (NNT=83 за 5,4 года для пациентов только с факторами риска, NNT=71 за 5,4 года в среднем). На основании этих данных дулаглутид был одобрен для применения у пациентов с СД 2 и множественными факторами сердечно-сосудистого риска с целью снижения риска MACE. В то же время при детальном рассмотрении протокола исследования было установлено, что группа пациентов с множественными факторами риска включала, помимо прочего, пациентов с выраженным снижением СКФ, пациентов с ХСН и анамнезом транзиторной ишемической атаки и геморрагического инсульта, что, возможно, несколько нивелирует показанное преимущество препарата в данной когорте [67, 69].
Другой представитель класса аналогов человеческого ГПП-1 – семаглутид – доступен как в инъекционной, так и в пероральной форме. В CVOT исследовании SUSTAIN 6 с использованием парентеральной формы у пациентов с высоким сердечно-сосудистым риском (83% с ССЗ в анамнезе) препарат показал снижение MACE (∆ОР=26%, NNT=44 за 2,1 года исследования). При грубом сравнении с ранее описанными исследованиями степень влияния семаглутида на MACE была самой выраженной как численно, так и по скорости достижения эффекта (см. рис. 4) [68].
Второе исследование со сходным дизайном, но уже в отношении пероральной формы семаглутида (PIONEER 6) имело короткую продолжительность (<16 мес) и было направлено не на демонстрацию сердечно-сосудистых преимуществ препарата, а преследовало регистрационные цели. Но даже в этом исследовании было продемонстрировано положительное влияние препарата на MACE (∆ОР=21%), однонаправленное с инъекционной формой. Несмотря на то что этот показатель не достигал статистической значимости из-за отсутствия достаточного времени для накопления необходимого эффекта, в то же время стоит отметить, что в исследовании были получены данные о снижении как общей, так и сердечно-сосудистой смертности (∆ОР≈50%) на терапии пероральным семаглутидом [70]. Однородность данных, полученных на двух формах семаглутида, была подтверждена в сводном анализе SUSTAIN 6 и PIONEER 6 [71].
Дополнительно в обоих этих исследованиях NNT, по сути, пропорционально снижалось от времени экспозиции препаратом в исследованиях как в среднем, так и у пациентов разного исходного сердечно-сосудистого риска. Так, за 1,3 года терапии пероральным семаглутидом и за 2,1 год инъекционным NNT для разных групп пациентов составили 111 и 48 (для группы со 100% ССЗ в анамнезе), 100 и 48 в среднем (83% с ССЗ), 67 и 33 (0% с ССЗ) соответственно. В отличие от исследования REWIND, в исследованиях с семаглутидом наблюдалась обратная зависимость: чем меньше был исходный сердечно-сосудистый риск пациентов, тем более выражен был эффект семаглутида в плане предотвращения кардиоваскулярных событий. Подобная находка была обнаружена и в обобщенном анализе всей клинической программы SUSTAIN и PIONEER, включая исследования гликемического контроля. В нем было показано, что пациенты с минимальным риском получают даже большее относительное преимущество (∆ОР на 55%) в отношении риска MACE, чем пациенты с очень высоким риском в среднем по исследованиям (∆ОР на 26%). Авторы предлагают гипотезу, согласно которой это обусловлено тем, что ранние патофизиологические нарушения еще более склонны к модифицирующему действию семаглутида в сравнении с более запущенными состояниями [72].
Влияние семаглутида на MACE при меньшем исходном кардиоваскулярном риске у пациентов подтверждается и в другом анализе. В нем сравнили эффект семаглутида, продемонстрированный только у тех пациентов в SUSTAIN 6 и PIONEER 6, которые соответствовали профилю исходного кардиоваскулярного риска пациентов из исследования REWIND (т.е. 69% без ССЗ и 31% с ССЗ с аналогичным распределением по индивидуальным нозологиям). Было установлено, что эффект семаглутида у этой категории пациентов был как минимум не хуже, чем дулаглутида, или даже превосходил его (∆ОР MACE на 24% на терапии семаглутидом по сравнению с дулаглутидом) [73]. В настоящий момент инъекционный семаглутид имеет дополнительное к основному показание по применению у пациентов с СД 2 и высоким риском ССЗ, а в отношении перорального семаглутида идет дополнительное исследование SOUL, которое планируется завершить к 2025 г. [74]. Для достоверного подтверждения улучшения долгосрочного прогноза на терапии пероральным семаглутидом у пациентов с СД 2 и средним кардиоваскулярным риском начато уже упоминавшесся исследование ASCEND PLUS [40].
Сводные данные в отношении NNT, необходимого для предотвращения комбинированного события MACE, у разных представителей аГПП-1 для категорий пациентов разного исходного риска представлены на рисунке 6.
ЗАКЛЮЧЕНИЕ
СД 2 является важной клинической проблемой как с точки зрения эпидемиологического характера распространения заболевания, так и с позиции бремени, которое оно накладывает на сообщество и прогноз каждого индивидуального пациента. Контроль факторов сердечно-сосудистого риска и управление прогнозом с помощью современных препаратов имеет решающее значение у пациентов с установленным диагнозом СД 2.
Во всем мире наблюдается недостаточная частота назначения противодиабетических препаратов с доказанными сердечно-сосудистыми преимуществами. Так, по зарубежным данным, только каждый 6–9 пациент с СД 2 (≈10–20%) имеет в составе терапии препараты из группы иНГЛТ-2 или арГПП-1 [75, 76]. По российским данным, этот показатель еще более удручающий: лишь каждый 38-й пациент (2,7%) получает лечение этими препаратами [16]. При этом, по оценке данных Шотландского регистра и с позиций современных представлений о категориях сердечно-сосудистого риска, этот показатель должен быть в 1,4–4 раза больше [14]. Еще больше ситуация усугубляется тем, что отсутствует практическая дифференциация назначений с позиции исходного кардиоваскулярного риска. Хотя правильное распределение имеющихся терапевтических ресурсов позволит получить максимум пользы у пациентов, которым это требуется в большей степени.
Особое значение кардиопротективная терапия СД 2 приобретает в ситуациях дополнительного риска, например, в условиях постоянно нарастающей пандемии COVID-19, поскольку пациенты с СД 2, особенно с отягощенным анамнезом, вносят существенный вклад как в российскую, так и в глобальную частоту госпитализаций и смертности при этой инфекции [77, 78]. Исходная настороженность насчет применения иНГЛТ-2 при коронавирусной инфекции была опровергнута в недавно опубликованном исследовании DARE-19 с использованием дапаглифлозина. И, хотя в нем дапаглифлозин не продемонстрировал улучшения прогноза пациентов, его прием у пациентов, госпитализированных по поводу коронавирусной инфекции, было безопасным [79]. Недавний же метаанализ исходов 20 000 пациентов с СД 2 с COVID-19 свидетельствует о том, что пациенты, получавшие терапию арГПП-1 до госпитализации, имели на 47% меньший шанс летального исхода [80].
Необходимо стремиться к полному и корректному назначению противодиабетической терапии в соответствии с клиническими потребностями каждого пациента. Клинические рекомендации последних лет стремятся сделать акцент на раннем назначении противодиабетических препаратов с доказанными долгосрочными преимуществами как с позиции модификации хода заболевания, так и с учетом основного прогноза пациента. Следует иметь в виду возможность применения комбинированной терапии у пациентов с СД 2 со старта заболевания как с позиции его контроля, так и для обеспечения коррекции нарастающих рисков.
Наряду с этим необходимо учитывать те категории пациентов, у которых то или иное преимущество терапии современными противодиабетическими препаратами было продемонстрировано в большей степени. Так, у пациентов с СД 2 и высоким или очень высоким сердечно-сосудистым риском атеросклеротических заболеваний в первую очередь стоит рассмотреть назначение в составе терапии препаратов группы аналогов человеческого ГПП-1, тогда как у пациентов с очень высоким риском госпитализации по поводу ХСН – иНГЛТ 2 [6, 8, 9, 42, 43, 61]. При противопоказаниях или непереносимости возможна замена одного из этих классов лекарственных средств другим. Кроме того, в отдельных ситуациях возможно и комбинированное назначение препаратов этих групп для обеспечения всесторонней протекции в отношении будущих исходов [81].


