ВВЕДЕНИЕ: ВНЕКИШЕЧНЫЕ ПРОЯВЛЕНИЯ ПРИ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЯХ КИШЕЧНИКА – ОБЩИЕ ПОЛОЖЕНИЯ, ЭПИДЕМИОЛОГИЯ, ВОПРОСЫ ПАТОГЕНЕЗА
Одна из доминирующих характеристик воспалительных заболеваний кишечника (ВЗК) – их мультисистемность, т.е. вовлечение в иммуновоспалительный процесс не только кишечника, но зачастую и органов, не относящихся к пищеварительной системе. Внекишечные проявления ВЗК (табл. 1) принято разделять на несколько категорий:
1) непосредственно связанные с активностью заболевания кишечника;
2) не связанные с активностью воспаления в кишке;
3) имеющие неясную взаимосвязь с активностью заболевания кишечника;
4) вторичные, т.е. обусловленные длительным воспалением и/или метаболическими нарушениями и/или побочными эффектами терапии ВЗК (внекишечные осложнения).

На сегодняшний день в Регистре Северо-западного центра лечения воспалительных заболеваний кишечника (Северо-западный регистр пациентов с ВЗК) [2, 3] есть сведения о динамическом наблюдении 1379 больных, среди которых 58,8% (n=811) страдают язвенным колитом и 41,2% (n= 568) болезнью Крона (рис. 1).

Среди когорты наблюдаемых нами пациентов с ВЗК без разделения на нозологии частота выявления каких-либо внекишечных проявлений составляет 22,9%. При болезни Крона они встречаются чаще, чем при язвенном колите: 43,5 против 23,2% (рис.2).
При анализе данных Регистра Северо-западного центра ВЗК у пациентов с язвенным колитом прослеживается тенденция к росту числа внекишечных проявлений при большей протяженности воспалительного процесса. Так, при ограниченном проктите внекишечные проявления выявляются у 13,1% больных, а при левосторонней локализации и тотальном колите в 27 и 35% случаев соответственно (рис. 3). При болезни Крона очевидных различий в частоте внекишечных проявлений в зависимости от локализации воспаления не отмечается. При изолированном терминальном илеите число внекишечных проявлений составило 36,1%, а при остальных вариантах локализаций – колите и илеоколите – по 42,5% (рис. 4). Вероятно, это связано с тем, что Монреальская топическая классификация болезни Крона не учитывает именно протяженность воспалительного процесса в отличие от язвенного колита.

Вместе с тем имеется четкая картина увеличения числа внекишечных проявлений в зависимости от активности ВЗК как при язвенном колите, так и болезни Крона, что продемонстрировано на рисунках 5 и 6.

Частота присутствия внекишечных симптомов у пациентов с ВЗК, по данным литературы, может достигать 50–60% [4–6]. Оценку реальной распространенности усложняет тот факт, что у одного больного возможно наличие более чем одного внекишечного проявления. Так, результаты крупного Швейцарского когортного исследования ВЗК (SIBDCS) с акцентом на внекишечные проявления свидетельствуют, что до 25% пациентов с ВЗК имеют несколько (до 5) внекишечных проявлений [7].
Наибольшая распространенность внекишечных проявлений, по данным авторов различных стран, отмечена при болезни Крона [4, 8, 9], у пациентов женского пола [4, 6, 8–10], у курильщиков [11, 12] и при большей продолжительности заболевания [8, 11].
Опубликованы результаты исследований, в которых установлена большая частота внекишечных проявлений у лиц молодого возраста и при раннем начале ВЗК [13]. Еще в 1970 г. Grossman B.J. и De Benedetti C.D. в своих работах сообщали о значимом числе педиатрических пациентов с ВЗК с внекишечными проявлениями [14], достигающем 68%. В 2006 г. Stawarski А. et al. констатировали, что у пациентов детского возраста внекишечные проявления наблюдаются в 50% случаев при язвенным колите и в 80% при болезни Крона [15]. Напротив, группа исследователей из Швейцарии (SIBDCS) сообщила о всего 16,7% (55/329) пациентов детского возраста с внекишечными проявлениями [16].
Большинство внекишечных симптомов ВЗК возникает уже после установления диагноза язвенного колита или болезни Крона, но некоторые из них могут появиться за несколько лет до дебюта кишечной симптоматики. Например, есть исследования, по результатам которых у увеита, периферического и осевого артрита частота опережения постановки диагноза ВЗК достигала 50, 20 и 40% соответственно [6, 7, 9].
Согласно данным исследователей группы SIBDCS, внекишечные симптомы с частотой до 26% будут проявлять себя еще до диагностирования ВЗК (среднее время – 5 мес до постановки диагноза ВЗК), и в 74% случаев первые симптомы проявятся после диагностирования ВЗК (медиана 92 мес) [7].
При анализе швейцарской когорты пациентов до верифицирования диагноза ВЗК периферический артрит был выявлен у 19,7% пациентов, аксиальная артропатия/анкилозирующий спондилит – у 39,1%, афтозный стоматит – у 27,8%, увеит – у 52,2%, узловатая эритема – у 14,3%, гангренозная пиодермия – у 14,3%, первичный склерозирующий холангит – у 23,8% [7].
Частота обнаружения основных внекишечных проявлений ВЗК отражена в таблице 2.
По современным представлениям, внекишечные проявления считаются результатом антиген-специфического иммунного ответа кишечника на клетки вне кишки либо независимого воспалительного события, которое инициируется в результате наличия генетических факторов риска и/или факторов риска окружающей среды. Особый вклад в патогенез многих внекишечных проявлений при ВЗК вносит нарушение микробиома кишки. Так, у пациентов со спондилоартритом наблюдается снижение микробного разнообразия и увеличение численности Ruminococcus gnavus и рода Dialister, что положительно коррелирует с активностью заболевания [17], у пациентов с псориазом снижается количество фекальных Saccharomyces cerevisiae [18]. Снижение фекального микробного разнообразия рядом авторов выявлено также при первичном склерозирующем холангите (ПСХ) [19] и воспалительных заболеваниях глаз [20]. Ciccia F. et al. (2015) и Cuthbert R.J. et al. (2017) продемонстрировали, что изменение микробиоты кишечника у пациентов с ВЗК и анкилозирующим спондилоартритом вызывает активацию иннантного иммунитета и выступает триггером для дифференцировки лимфоцитов (IL-C3s), чувствительных к интерлейкину 23 (ИЛ-23) [21, 22]. Именно лимфоциты 3-го типа (IL-C3s) обнаруживаются в значимом количестве в периферической крови, синовиальной жидкости и тканях костного мозга пациентов с анкилозирующим спондилоартритом. Большинство из активированных лимфоцитов экспрессирует хоминговый интегрин α4β7, маршрутизирующий их в ткани кишки, а молекула адгезии MAdCAM-1 широко представлена в костном мозге и эндотелии венул кишечника пациентов с анкилозирующим спондилоартритом, что играет центральную роль в привлечении IL-C3 в очаг воспаления синовиальных оболочек суставов и в рециркуляции активированных лимфоцитов между тканями кишки и сустава (рис. 7) [22].
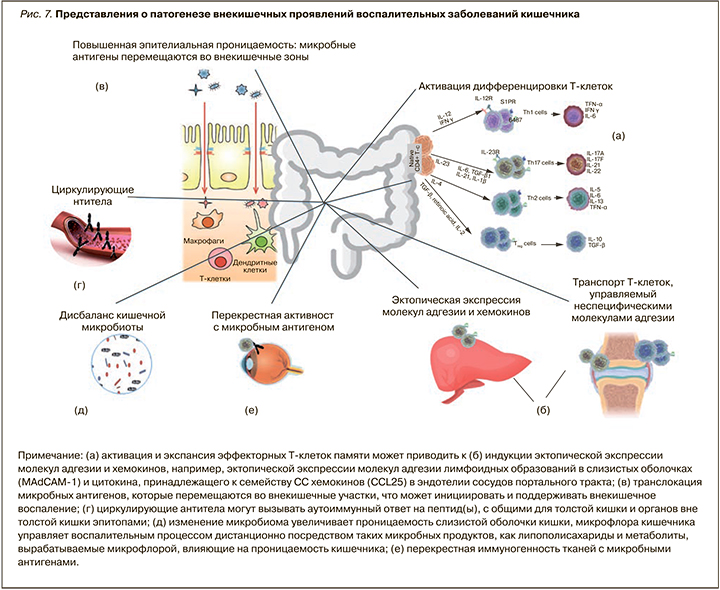
Существует ряд потенциальных механизмов, с помощью которых микробиота кишечника управляет патогенезом внекишечных проявлений ВЗК:
1) молекулярная мимикрия (сходство микробиоты кишечника и немикробных эпитопов, находящихся вне кишечника);
2) бактериальная транслокация вследствие нарушенного кишечного барьера (микробиота или ее компоненты перемещаются из кишечника во внекишечные участки, например, в печень через портальный кровоток);
3) растворимые частицы микробного происхождения, например липополисахариды, могут попадать в кровоток и индуцировать иммунный воспалительный ответ вне кишечника.
Кроме того, дисбиотические изменения, характерные для ВЗК, включая снижение численности Faecalibacterium prausnitzii, являющихся основными продуцентами короткоцепочечных жирных кислот, приводят к нарушению кишечного барьера. Это способствует проникновению антигенов, микробов и их метаболитов в кровоток и взаимодействию их с тканями-мишенями, включая кожу, печень, слизистые оболочки вне кишечника, где они запускают или усиливают иммунные реакции, вызывающие дальнейшее повреждение тканей [23].
ВЗК ассоциированы с повышенной экспрессией провоспалительных цитокинов, в частности фактора некроза опухоли альфа (ФНО-α), ИЛ-1β, ИЛ-12, ИЛ-23 и др. Это влечет за собой модификацию силы и скорости иммунного ответа нейтрофилов («прайминг нейтрофилов») [24], инициирующих воспалительный ответ при контакте с липидными полисахаридами, которые, наряду с другими веществами, попали с системным кровотоком в отличные от кишки органы и ткани.
Что касается генетических аспектов патогенеза внекишечных проявлений, то наблюдается значимый перекрест локусов, ассоциированных с развитием ВЗК и внекишечного воспалительного процесса. Таким образом, генетические локусы, ассоциированные с ПСХ, и связанные с ними гены ВЗК совместно участвуют в регуляции апоптоза Т-клеток (UBASH3A, BCL2L11, FOXO1 и IRF8), а также сигнальных путей и активаторов путей транскрипции (JAK / STAT) (SOCS1, JAK2, STAT3 и TYK2). В случае анкилозирующего спондилоартрита такими генетическими локусами являются TAPBPL, NPEPPS и ERAP1 [25]. Внекишечные проявления с вовлечением опорно-двигательного аппарата связаны с аллелями HLA-A2, HLA-DR1 и HLA-DQw5 у пациентов с БК и аллелями DRB1* 0103, B27 и B58 у пациентов с язвенным колитом [28, 29]. От 25 до 78% пациентов с ВЗК и анкилозирующим спондилоаритритом являются HLA-B27-положительными [26]. Ген NOD2/CARD15, который кодирует рецептор распознавания образов, также связан с сакроилеитом и увеитом [27, 28]. Weizman А. et al. выявили ассоциации между гангренозной пиодермией и известными локусами ВЗК, такими как IL8RA, PRDM1, USP15 и TIMP3 [29]. Для узловатой эритемы были обнаружены значительные перекресты генетических локусов, таких как PTGER4, ITGAL, SOCS5, CD207, ITGB3, а также rs6828740 (4q26). У пациентов с ПСХ также были идентифицированы варианты риска ВЗК, включая UBASH3A, BCL2L11, FOXO1, IRF8, а также SOCS1, JAK2, STAT3 и TYK2 [30]. На сегодняшний день нет информации о специфических генетических локусах, вносящих вклад в возникновение внекишечных проявлений, а также не ясна генетическая предрасположенность очередности появления воспалительных симптомов и их топики, что, вероятно, станет темой будущих исследований.
Среди факторов окружающей среды выявлена взаимосвязь большей частоты внекишечных проявлений ВЗК у курящих: преимущественно это касается болезни Крона, что, возможно, ассоциировано с изменением микробиома кишки под влиянием сигаретного дыма и смол [31, 32]. Severs M. et al. в своей работе отметили, что курение ассоциируется с увеличением на 10% частоты кожных и суставных внекишечных проявлений ВЗК [12].
СКЕЛЕТНО-МЫШЕЧНЫЕ ВНЕКИШЕЧНЫЕ ПРОЯВЛЕНИЯ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЙ КИШЕЧНИКА
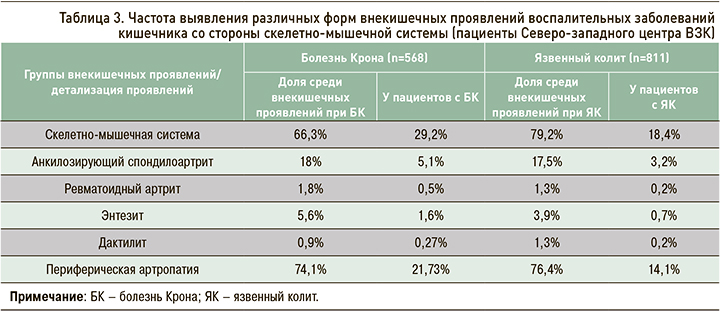
Среди пациентов Северо-западного центра ВЗК скелетно-мышечные симптомы как внекишечные проявления ВЗК отмечаются у 29,2% больных с Болезнью Крона и 18,4% с язвенным колитом и являются лидирующими в структуре всех внекишечных проявлений (табл. 3). Наиболее частый суставной симптом – периферическая полиартропатия (рис. 8). Второе по численности место среди суставных проявлений занимает более серьезная патология – анкилозирующий спондилоартрит, не связанный с активностью ВЗК и способный приводить к дополнительной инвалидизации.
Вовлечение суставов при ВЗК, по данным литературы, является наиболее частым внекишечным проявлением с распространенностью 6–46% (20–50% для осевой артропатии и 5–20% для периферического артрита соответственно). В некоторых источниках сообщается, что распространенность суставных внекишечных проявлений ВЗК может уменьшаться с возрастом. В результатах метаанализа, проведенного Karreman M.C. et al., указывается частота суставных проявлений ВЗК 25% для возрастной группы 20–30 лет и 2% для пациентов возраста 50–60 лет [33].
Структура симптоматики со стороны опорно-двигательного аппарата неоднородна: в патологический процесс могут вовлекаться как осевые, так и периферические суставы. ВЗК-ассоциированный энтезит, заключающийся в воспалении места прикрепления сухожилия к кости и приводящий к эрозированию и пролиферации костной ткани, клинически проявляется болевым синдромом и отеком. Частота выявления энтезитов при ВЗК указывается различными авторами в широком диапазоне – от 6 до 54%. Дактилиты, которые представляют собой сосископодобное опухание пальцев рук или ног, характерное для спондилоартрита вне ВЗК, встречается у 2–5% больных ВЗК [34]. Взаимосвязь клинической интенсивности энтезитов и дактилитов с активностью воспалительного процесса в кишке на сегодняшний день изучена недостаточно [35].
Среди аксиальных воспалительных артропатий принято различать анкилозирующий спондилоартрит, характеризующийся воспалительной болью в спине и наличием МРТ-признаков сакроилеита/спондилита (частота выявления при ВЗК – 1–12%), и изолированный сакроилеит (частота выявления при ВЗК – 16–46%) [33, 36, 37].
К основным клиническим проявлениям поражения аксиального скелета относятся воспалительная боль в спине и нарастающее ограничение подвижности всех отделов позвоночника. Международное общество по изучению спондилоартритов (ASAS) разработало критерии диагностики для воспалительных аксиальных и периферических поражений суставов (рис. 9) [36].
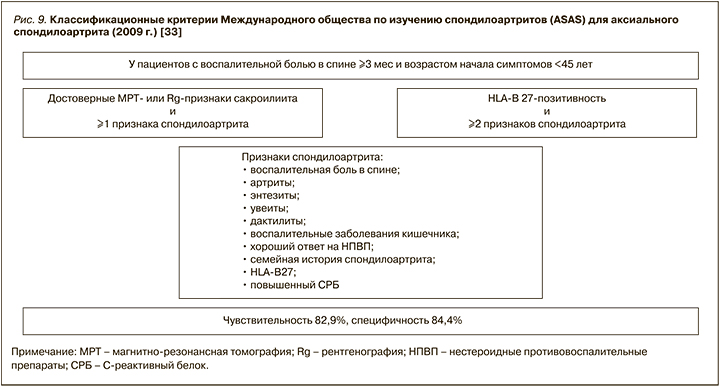
В соответствии с российской версией модифицированных Нью-Йоркских классификационных критериев анкилозирующего спондилита (2016), это заболевание диагностируется, если у пациента имеются:
- клинические признаки: 1) воспалительная боль в спине (согласно критериям экспертов Международного общества по изучению спондилоартритов (ASAS), 2009); 2) ограничение движений в поясничном отделе позвоночника как в сагиттальной, так и фронтальной плоскостях; 3) ограничение дыхательной экскурсии грудной клетки в сравнении с показателями у здоровых лиц, ПЛЮС;
- определяемый методом визуализации сакроилеит (по данным магнитно-резонансной томографии или рентгенографии).
Анкилозирующий спондилоартрит является прогрессирующим состоянием со структурными поражениями и нарушением движения, что, безусловно, негативно влияет на качество жизни пациентов. В настоящее время диагноз анкилозирующего спондилоартрита считается ранним, если он выставлен на «дорентгенологической» стадии заболевания, т.е. если отсутствует достоверный сакроилеит на рентгенограммах (2-я и более стадия по Келлгрену), либо если он выставлен в течение первых двух лет от начала клинической картины, которую можно соотнести с дебютом болезни [38].
На данный момент степень воспалительной активности и функциональных нарушений при анкилозирующем спондилите определяют по визуально-аналоговой шкале (ВАШ) для оценки боли и скованности, а также по ряду суммарных показателей, характеризующих те или иные параметры заболевания. Для оценки боли в позвоночнике используют среднее арифметическое двух показателей по ВАШ или числовой рейтинговой шкале за последнюю неделю: боли в ночное время и боли в течение суток. При помощи этих же шкал определяют такие показатели, как утренняя скованность в позвоночнике и общая оценка активности болезни пациентом.
Общая оценка активности болезни врачом осуществляется с помощью суммарного показателя – индекса BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), представляющего собой среднее значение (от 1 до 10) при оценке ответов пациента на 6 вопросов [39]. Воспалительная активность считается высокой при индексе BASDAI ≥4. Для определения степени активности анкилозирующего спондилоартрита не существует «золотого стандарта». АSAS предложило для этой цели индекс ASDAS (Ankylosing Spondylitis Disease Activity Score), который на сегодняшний день является международным инструментом оценки указанного параметра [40].
Активность и прогрессирование анкилозирующего спондилоартрита не связаны с активностью воспалительного процесса в кишке, а прогноз при осевом поражении ассоциирован не с прогнозом ВЗК, а с прогрессированием суставного поражения.
Периферический артрит у пациентов с ВЗК в основном асимметричный, чаще олигоартикулярный. Диагноз ВЗК-ассоциированной периферической артропатии в основном клинический. Ультразвуковое исследование (УЗИ) и магнитно-резонансная томография (МРТ) могут обнаружить типичные признаки артрита, энтезита, теносиновита и бурсита, при этом специфических лабораторных тестов для оценки индекса активности заболевания не существует. Серологические диагностические тесты (ревматоидный фактор, антициклический цитруллинированный пептид) обычно отрицательны, но положительный результат ни в коем случае не исключает принадлежность периферической артропатии к внекишечным проявлениям ВЗК [41].
Артропатия 1-го типа — это классическая форма артропатии, которая характеризуется олигоартикулярным асимметричным артритом, поражающим менее пяти, преимущественно крупных, суставов (голеностопные, колени, бедра, запястья, локти и плечи). Она связана с активностью ВЗК, обычно сопровождается острыми эпизодами боли продолжительностью менее 10 нед и зачастую сочетается с иными внекишечными проявлениями ВЗК, такими как узловатая эритема и увеит.
Артропатия 2-го типа характеризуется полиартикулярным поражением 5 или более суставов, симметричным, в основном поражающим мелкие суставы обеих рук с болью и отеком, которые обычно сохраняются продолжительное время. Такая форма артропатии обычно не зависит от активности ВЗК.
Высказываются предположения, что эти типы суставных проявлений можно рассматривать как континуум, поскольку результаты некоторых динамических исследований демонстрируют меньшую степень поражения суставов в дебюте ВЗК, с олигоартикулярным вовлечением, постепенной прогрессией и полиартикулярностью [42].
Периферические ВЗК-ассоциированные артропатии могут носить «невоспалительный» характер, т.е. протекать без синовита (см. рис. 8). Частота таких «невоспалительных» артропатий, по данным разных наблюдений, составляет от 5 до 16%, но на сегодняшний день остается неясной их взаимосвязь с активностью основного заболевания [9, 41]. Кроме того, ВЗК-ассоциированные «невоспалительные» артропатии необходимо дифференцировать с артралгиями на фоне отмены глюкокортикостероидов (ГКС), парадоксальными артралгиями, вызванными приемом тиопуринов или препаратов анти-ФНО, ведолизумаба, устекинумаба (рис. 10) [43, 44].
ПОРАЖЕНИЯ КОЖИ ПРИ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЯХ КИШЕЧНИКА
По данным Северо-западного регистра ВЗК, кожные проявления занимают 2-е место по частоте выявления среди всех внекишечных проявлений ВЗК (18,4 и 14,5% у пациентов с болезнью Крона и язвенным колитом соответственно); при этом они в 2,5 раза чаще отмечались у пациентов с болезнью Крона (8,1%) по сравнению с язвенным колитом (2,9%). Обращает на себя внимание большая частота язвенного стоматита и узловатой эритемы у пациентов с болезнью Крона и относительно одинаковая для остальных кожных проявлений (табл. 4).
По литературным данным, около 20 % пациентов с ВЗК когда-либо указывают на появление кожных симптомов. Наиболее частый из них – это узловатая эритема с частотой выявления от 1 до 15% у пациентов с ВЗК; несколько чаще она наблюдается при болезни Крона [6, 8].
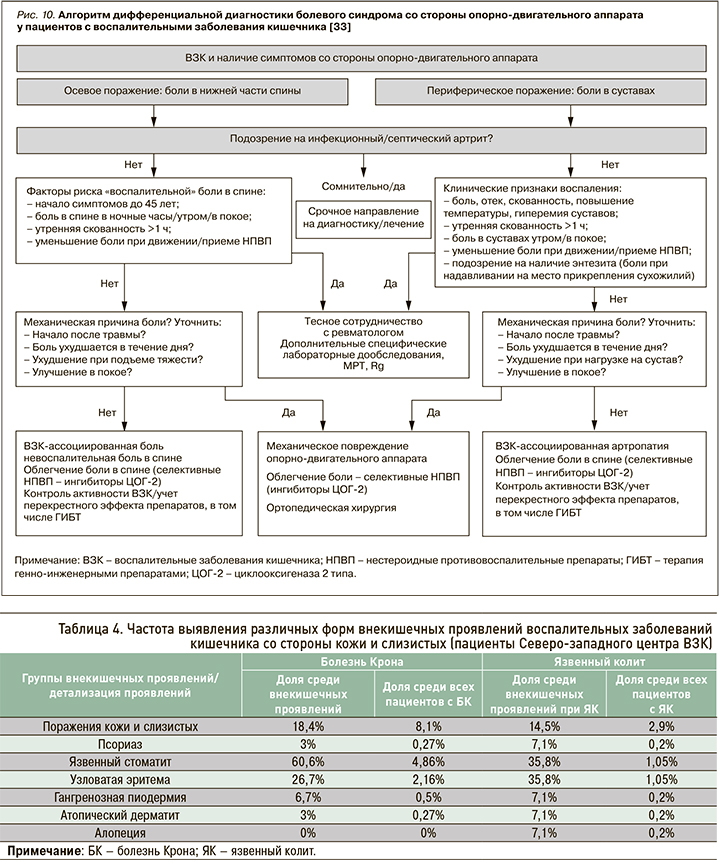
Узловатая эритема характеризуется красно-фиолетовыми поднятыми подкожными болезненными узелками от 1 до 5 см в диаметре, проходящих без образования шрамов. Обычно она возникает на разгибательных поверхностях нижних конечностей, однако у 15% пациентов выявляется на бедрах и предплечьях (рис. 11) [45]. К развитию этого поражения кожи предрасполагает женский пол, у детей оно практически не встречается. Кроме того, узловатая эритема может быть вызвана широким спектром основных состояний, таких как другие воспалительные заболевания (например, саркоидоз), инфекции (стрептококк, туберкулез), злокачественные новообразования, прием некоторых лекарств (сульфаниламидов, комбинированных оральных контрацептивов) или беременность.
Распространенность узловатой эритемы колеблется от 5 до 15% среди пациентов с болезнью Крона и от 2 до 10% у пациентов с язвенным колитом [45]. В одном из исследований Varvicka S.R. et al. наличие узловатой эритемы не было ассоциировано с активностью течения болезни Крона: она выявлялась у 6,8% пациентов в ремиссии и практически в 3 раза реже (2,4%) в стадии обострения [46]. При язвенном колите поражение кожи диагностировалась в 2,5 раза чаще у пациентов с активным воспалительным процессом (4,7%) [46].
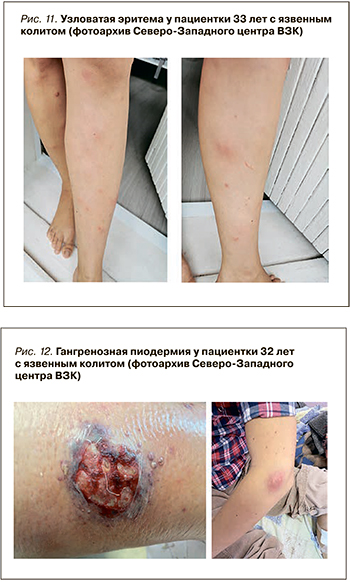
Гангренозная пиодермия при ВЗК возникает реже: по литературным данным, ее частота достигает 0,8–5% случаев. При этом она встречается чаще при язвенном колите (0,9–8%), нежели болезни Крона (0,7–3,5%). Кроме того, это внекишечное проявление в значимой степени влияет на качество жизни и связано с активностью воспалительного процесса в кишке.
Пиодермия часто первоначально представляет из себя эритематозную пустулу или узелок, который в дальнейшем стремительно превращается в глубокую, неправильной формы асептическую язву. Это внекишечное проявление ВЗК возникает преимущественно на нижних конечностях, однако может поражать и другие места, например голову или тело (рис. 12) [47]. Язвы могут быть различных размеров, как одиночными, так и множественными [45]. Так же, как и узловатая эритема, пиодермия с большей частотой выявляется у женщин, однако имеет более тяжелое течение и оставляет после заживления рубцы [47]. К факторам риска развития пиодермии относятся высокая активность ВЗК и поражение толстой кишки (колит) [45].
Встречаемость гангренозной пиодермии в когорте пациентов ВЗК Регистра Северо-западного центра ВЗК составляет от 0,4 до 2,6% [48]. В отличие от узловатой эритемы она чаще выявляется при активном течении ВЗК: у 2,4% пациентов с активной БК против 1,4% в стадии ремиссии и у 3% больных с обострением язвенного колита против 1,5% в ремиссии [46].
Тем не менее требуется проведение широкого спектра дифференциальной диагностики, в том числе с забором морфологического материала, если это необходимо, для исключения сосудистых причин появления кожных язв (венозных, артериальных, окклюзионных или васкулитных), гематологических заболеваний (истинной полицитемии), злокачественных новообразований, инфекций и лекарственных поражений тканей (рис. 13, 14) [49, 50]. При истинной гангренозной пиодермии забор биопсии может ухудшить клиническую ситуации вследствие паттергии.
Важное кожное ВЗК-ассоциированное внекишечное проявление – гнойный гидраденит (инверсные угри), имеющий распространенность до 23% у пациентов с ВЗК (0,4–15% при болезни Крона и 0,1–6,1% при язвенном колите) по сравнению с 0,1–4% в общей популяции [6, 51, 52].
По результатам исследования греческой когорты пациентов с ВЗК (n=1260) Janse I.C. et al. (2016) выделили независимые факторы, ассоциированные с большей частотой гидраденита (женский пол, курение, более высокий индекс массы тела, молодой возраст), а также ассоциацию риска этого внекишечного проявления с генами SULT1B1 и SULT1E1 [52].
По данным Lukach A.J. et al. (2018), наиболее излюбленные участки для появления гнойного гидраденита – паховая, перианальная и подмышечная области. Клинические факторы, увеличивающие риск его появления у пациента с ВЗК, очень схожи с результатами, полученными в работе Janse I.C. et al.: курение (при сочетании ВЗК с гидраденитом этот факт выявлялся в 6 раз чаще; p <0,01), ожирение (в 11 раз чаще при гидрадените у пациентов с ВЗК; p <0,01). У пациентов с гнойным гидраденитом и болезнью Крона чаще выявлялось распространенное воспаление – илеоколит (OR 8,31; 95% доверительный интервал (ДИ): 2,90–23,80; p <0,01) и перианальное поражение (OR 2,85; 95% ДИ: 1,19–6,81; p <0,01) [53].
Псориаз также рассматривается как заболевание, ассоциированное с ВЗК, и встречается у 2,7–8,3% пациентов с ВЗК. Он имеет более высокую распространенность при болезни Крона (2,8–3,3%), чем при язвенном колите (2,1–2,9%).
Из различных форм псориаза при ВЗК наиболее распространенным подтипом является вульгарный псориаз или хронический бляшечный псориаз [54]. Вульгарный псориаз характеризуется наличием четко выраженных мономорфных эритематозных бляшек с серебристыми жаберными чешуйками и локализуется обычно в разгибательных областях локтей и коленей, волосистой части головы, околопупочной и перианальной областях [55]. Поражения могут затрагивать кожные складки на изгибах, ногти, скальп и суставы (псориатический артрит); последний развивается у 30% пациентов с псориазом средней и тяжелой степени [54, 55]. На сегодняшний день для оценки активности заболевания используются такие шкалы, как Psoriasis Area и Severity Index [56].
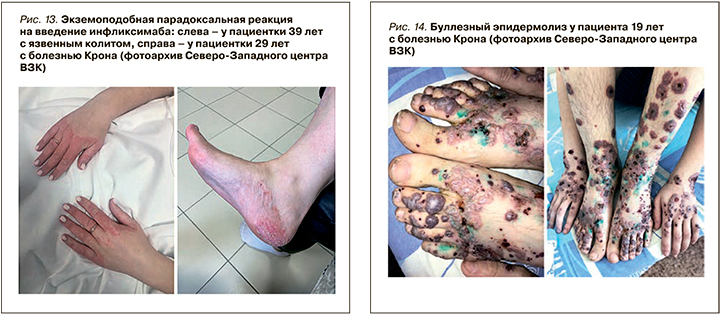
Еще одна возможная форма псориаза у пациентов с ВЗК – парадоксальный псориаз, который может быть вызван применением анти-ФНО агентов, особенно инфликсимаба и адалимумаба [54, 57, 58]. Клинически парадоксальный псориаз очень похож на классический псориаз, но его воспалительный путь отличается – в нем преобладает интерферон типа 1 [54]. Терапия этой формы заболевания без отмены препарата – индуктора псориаза крайне затруднена.
К более редким ВЗК-ассоциированным кожным проявлениям относятся синдром Свита, метастатическая болезнь Крона, орофасциальный гранулематоз с хейлитом Мишера, эритема Гаммеля и буллезный эпидермолиз (рис. 14). Большинство из них связано с активностью воспалительного процесса в кишке (за исключением «метастатической» болезни Крона).
Ассоциированный с активностью ВЗК синдром Свита (Sweet’s syndrome, «острый фебрильный нейтрофильный дерматоз») описан как появление разноразмерных болезненных или папулосквамозных экзантем или узелков на руках, ногах, туловище или лице [47]. В целом он входит в группу острых нейтрофильных дерматозов, которая включает гангренозную пиодермию, но может отличаться по внешнему виду, распространению и гистологическим особенностям. Дерматоз, как правило, связан с активным ВЗК (в 67–80% случаев), но может предшествовать появлению кишечных симптомов у 21% пациента и был зарегистрирован через 3 мес после проктоколэктомии по поводу язвенного колита [59].
Синдром Свита с большей частотой встречается у пациентов женского пола (>80%) и связан с наличием иных внекишечных проявлений (артритом, лихорадкой или глазными симптомами). Этот синдром в большей степени ассоциирован со злокачественными новообразованиями, инфекциями и реже с ВЗК [47]. Кроме того, есть сообщения о синдроме Свита как побочном эффекте терапии тиопуринами [60].
При «метастатических» поражениях кожи у пациентов с болезнью Крона наблюдается появление очаговых экзантем, гистологически демонстрирующих наличие гранулем саркоидного типа [61]. «Метастазы болезни Крона» могут появляться и на слизистых оболочках ротовой полости и гениталий [61]. «Метастатическая» болезнь Крона обычно не ассоциирована с активностью кишечного заболевания [47, 62], однако при терапии таких пациентов анти-ФНО препаратами кожные симптомы редуцируются.
Очень редким внекишечным поражением выступает орофасциальный гранулематоз, или синдром Мелькерссона–Розенталя (синдром Мишера) [63, 64]. Под ним подразумевают полиэтиологическое заболевание аутоиммунной природы, при котором развивается характерная триада: паралич или неврит лицевого нерва, отек губ или нижней части лица и складчатый язык с возможным развитием язвенных поражений рта. Синдром Мишера развивается преимущественно у мужчин 14–20 лет. При гистологическом исследовании выявляются лимфедема и гранулемы, а также агрегаты эпителиоидных гистиоцитов [63].
ПОРАЖЕНИЯ ПОЛОСТИ РТА
До сих пор продолжаются споры, относится ли поражение полости рта к внекишечным проявлениям ВЗК, или это следует расценивать как самостоятельное заболевание верхних отделов ЖКТ (рис. 15). Пациенты с ВЗК страдают не только афтозным стоматитом, но и пародонтитом. Распространенность поражений слизистой рта у этих пациентов составляет от 5 до 50% [65]; чаще они выявляются у при болезни Крона, чем при язвенном колите – в 10 и 4% случаев соответственно. В педиатрической практике поражения слизистой рта встречаются реже (до 23% пациентов) [66].
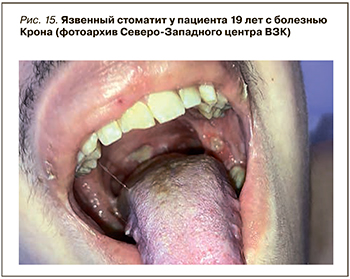
Афтозный стоматит проявляется округлыми болезненными дефектами с желтым псевдомембранозным дном и гиперемированными краями на слизистой оболочке губ и щек, подобными афтозным поражениям в кишке.
Пародонтит является хроническим воспалением десен, ведущим к разрушению мягких тканей, кости и потере зубов. Гингивит и пародонтит чаще обнаруживаются в когорте пациентов с ВЗК по сравнению со здоровыми людьми [67] и, по всей вероятности, имеют ассоциацию с изменением микрофлоры полости рта [68]. У курильщиков риск развития заболевания выше [67]. Афтозный стоматит и пародонтит обычно связаны с активностью ВЗК и ассоциированы с перианальным поражением [45].
ПОРАЖЕНИЕ ГЛАЗ
Орган зрения находится на 3-м месте после суставов и кожи по предрасположенности к аутоиммунному воспалению в сочетании с ВЗК. По мировым литературным данным, до 7% пациентов с ВЗК когда-либо имели поражения глаз, самыми частыми из которых были эписклериты, склериты и передние увеиты [69]. Намного реже отмечаются васкулит сетчатки, папиллит, инфильтраты роговицы, миозиты и поражение зрительного нерва [69]. В педиатрической когорте внекишечные глазные проявления встречаются значительно чаще, чем у взрослых. Более высокий риск развития этих внекишечных проявлений имеет место при болезни Крона, чем при язвенном колите. Также поражение глаз обычно ассоциировано с наличием кожных или суставных внекишечных проявлений (рис. 16) [69].
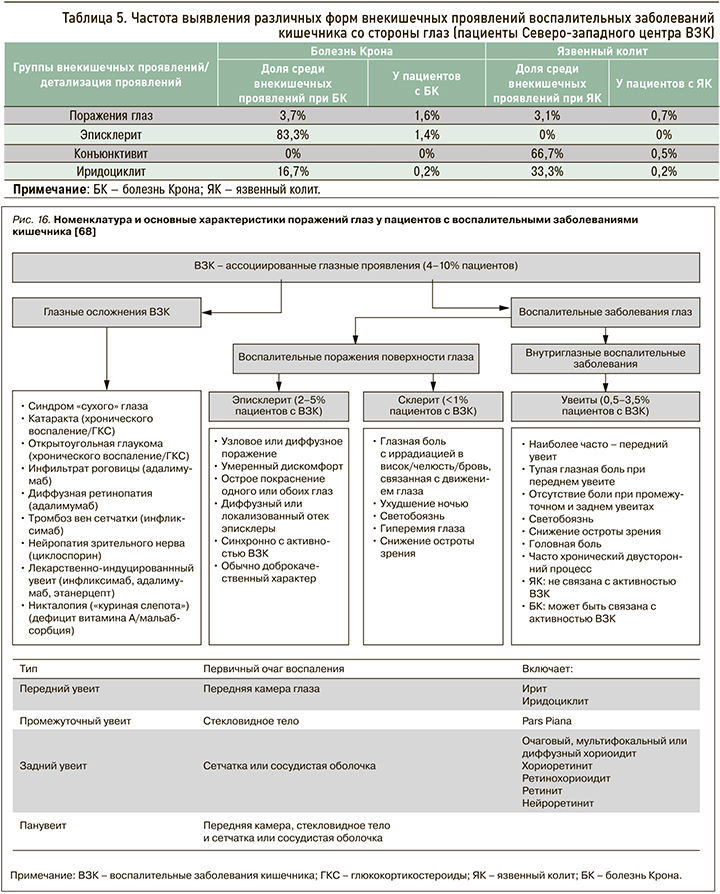
По данным регистра Северо-западного центра ВЗК, глазные внекишечные проявления отмечаются не так часто, как суставные и кожные симптомы: у 0,7% больных с язвенным колитом и вдвое чаще при болезни Крона (1,6%) (табл. 5).
Эписклерит – это воспаление соединительной ткани между склерой и конъюнктивой, которое выступает самым частым глазным проявлением ВЗК и напрямую связано с активностью заболевания [70]. Склерит, наоборот, редкое проявление ВЗК, встречающееся менее чем в 1% случаев [70]. Опасность его заключается в том, что при отсутствии адекватной терапии заболевание может привести к пожизненной потере зрения.
Под увеитом подразумевают воспаление пигментированной оболочки глаза, которое, в отличие от поражения склеры и эписклеры, слабо ассоциировано с активностью заболевания (рис. 17). В исследовании швейцарской когорты пациентов с ВЗК это осложнение выявлялось в 2 раза чаще при активной болезни Крона по сравнению с ремиссией (12,2 и 5,2% соответственно), однако при язвенном колите такой корреляции не было (4,1 и 3,5% соответственно) [46].
ВНЕКИШЕЧНЫЕ ПРОЯВЛЕНИЯ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЙ КИШЕЧНИКА, СВЯЗАННЫЕ С ГЕПАТОБИЛИАРНОЙ СИСТЕМОЙ
Эта группа внекишечных проявлений ВЗК включает ПСХ, аутоиммунный гепатит (АИГ), IgG4-ассоциированный холангит, гранулематозный гепатит и др. Более того, различные препараты, применяемые при лечении ВЗК, могут самостоятельно вызывать токсический или лекарственно-индуцированный АИГ. Помимо прочего, при введении пациентов в иммуносупрессию повышается риск реактивации вирусного гепатита В или развития гепатита, вызванного другими вирусами (вирусом Эпштейн–Барр, цитомегаловирусом) [71].
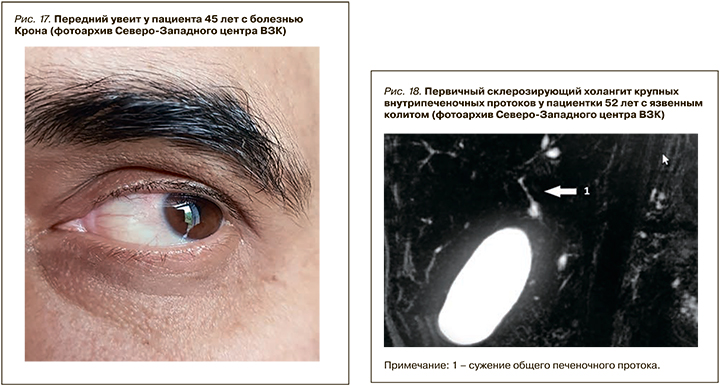
В нашей когорте пациентов с ВЗК вовлечение печени было диагностировано у 8,2% человек – с большей частотой при язвенном колите (5,3%) и меньшей при болезни Крона (2,9%). В структуре всех поражений печени преобладала неалкогольная жировая болезнь печени (НАЖБП) – 54,7% (4,5% среди пациентов с ВЗК), в 5,2% случаев был верифицирован АИГ (0,43% среди пациентов с ВЗК), в 12,6 % (1,1% всей когорты) – ПСХ (рис. 18, 19).
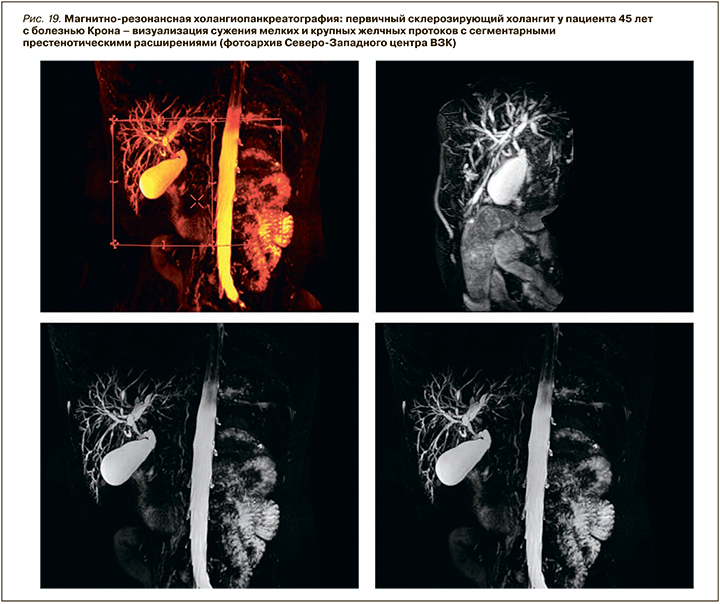
По литературным данным, ПСХ – классическое внекишечное проявление, ассоциированное с ВЗК, но не связанное с активностью воспалительного процесса в кишке. Это хроническое аутоиммунное холестатическое заболевание печени с диффузным воспалением и фиброзом внутри- и внепеченочных желчных протоков, их облитерацией с формированием мультифокальных билиарных стриктур. ПСХ характеризуется прогрессирующим течением, приводящим к развитию цирроза печени и печеночной недостаточности. У пациентов с ВЗК он обнаруживается в 2–8% случаев при язвенном колите и в 1–3% при болезни Крона. При этом у 60–80% пациентов с ПСХ отмечается его ассоциация с ВЗК, причем чаще с язвенным колитом – более чем в 75–80% случаев. Оставшиеся 20–25% приходятся на долю болезни Крона или неклассифицируемого колита (около 5%) [72 ,73].
Существует несколько гипотез, связывающих патогенез ПСХ и ВЗК. Среди них – гипотеза генетической предрасположенности. Однако один из самых последних генетических анализов показал ограниченное совпадение между локусами ПСХ и ВЗК, что может говорить в пользу перекрывающихся, но различных генетических механизмов. Вероятно, генетическая предрасположенность к аутоиммунному повреждению желчных протоков, реализующаяся посредством токсических или инфекционных агентов, проникающих сквозь поврежденную кишечную стенку, потенциально является одним из основных механизмов, ведущим к ПСХ у пациентов с ВЗК [74].
Другие предположения, свидетельствующие в пользу взаимосвязи ПСХ и ВЗК, включают гипотезу хоминга кишечных лимфоцитов и гипотезу «протекающей кишки». Согласно первой, активированные лимфоциты из воспаленной, обладающей повышенной проницаемостью кишки могут проникать в энтерогепатическую циркуляцию и персистировать как клетки памяти, способствуя развитию воспалительных изменений в печени. Т-клетки, активированные в кишечнике во время обострения ВЗК, могут дифференцироваться в эффекторные клетки, обладающие способностью связываться с печеночным эндотелием и кишечным эпителием. Активация и экспансия этих клеток памяти в печени может в конечном итоге привести к индукции в ней MAdCAM-1 и CCL25, способствуя рекрутированию CCR9+ и α4β7+ Т-клеток слизистой и активации воспаления. Развитие ПСХ после колэктомии у пациентов с ВЗК или развитие ВЗК после трансплантации печени по поводу ПСХ побудили некоторых исследователей предположить, что аберрантное самонаведение лимфоцитов между кишечником и печенью может быть связано с патогенезом фенотипа ПСХ–ВЗК [75].
Гипотеза «протекающей кишки» говорит о взаимосвязи прогрессирующего печеночного и билиарного повреждения и повышенной кишечной проницаемости с транслокацией бактериальных метаболитов из кишечника. Для ВЗК характерно нарушение кишечного микробиома, отличающееся более низким биологическим разнообразием и снижением уровня представителей Firmicutes [1]. У ПСХ–ВЗК-пациентов имеются нарушения кишечной микробиоты, но, по-видимому, они отличаются от таковых у пациентов с изолированным ВЗК. Современные данные несколько противоречивы, но свидетельствуют о заметном увеличении у этой когорты пациентов бактерий родов Veillonella, Escherichia и Megasphaera, в то время как количество представителей родов Prevotella, Roseburia и Bacteroides, у них, напротив, существенно снижено. Взаимодействие между микробиотой и метаболизмом желчных кислот также может играть важную роль в фенотипе ПСХ–ВЗК. Получается, что специфические изменения микробиоты кишечника у пациентов с ПСХ–ВЗК могут значимо влиять на метаболизм желчных кислот, и наоборот, нарушенный метаболизм желчных кислот при ПСХ может специфически модулировать микробиоту. Результатом подобных процессов, возможно, становится формирование порочного круга воспаления в системе (оси) «кишечник–печень» [75].
Таким образом, в настоящее время многофакторный патогенез фенотипа ПСХ–ВЗК, ассоциированный с генетической предрасположенностью, иммунными механизмами, измененной кишечной микробиотой и нарушенным метаболизмом желчных кислот, объяснен не полностью и требует дальнейшего изучения для понимания взаимосвязи между этими заболеваниями.
Несмотря на возможную общность патогенетических механизмов, эти два заболевания могут возникать в разное время. По данным большинства исследований, диагноз ВЗК устанавливается раньше ПСХ. ПСХ может быть диагностирован через много лет после колэктомии, но и, напротив, ВЗК может быть выявлен у пациента спустя годы после первоначального диагноза ПСХ или даже после трансплантации печени [72, 73].
На сегодняшний день остается дискутабельным вопрос об ассоциации АИГ с наличием ВЗК. В литературе есть отдельные данные о распространенности АИГ у детей с ВЗК, согласно которым его частота равняется 1,6% [76].
Группа испанских исследователей – Rocha H.C. et al (2021) – опубликовали данные динамического исследования 54 пациентов с аутоиммунными заболеваниями печени и ВЗК, большинство из которых составляют пациенты с язвенным колитом и ПСХ (64,5%). Ретроспективный анализ, проведенный исследователями, продемонстрировал ассоциацию предшествующего диагноза АИГ и дебюта ВЗК de novo после трансплантации печени у этих больных (p=0,012; OR: 7,1; 95% ДИ: 1,215–42,43) [77].
Кроме того, на сегодняшний день есть сведения о более частом синдроме перекреста АИГ/ПСХ у пациентов с ВЗК, который также чаще наблюдается при язвенном колите по сравнению с болезнью Крона [78, 79].
В литературе описано еще одно редкое, выявляемое при болезни Крона, внекишечное проявление – гранулематозный гепатит. Его предполагаемая распространенность составляет менее 1% [80]. Заболевание может проявляться лихорадкой, повышением активности щелочной фосфатазы, гепатоспленомегалией. Однако необходимо учитывать, что гранулематозный гепатит потенциально может быть осложнением терапии месалазином и сульфасалазином [81].
IgG4-ассоциированный холангит – заболевание желчных путей с неизвестным иммунопатогенезом, описанное у пациентов с язвенным колитом [82], но частота ассоциации этой патологии с ВЗК и его активностью в настоящее время изучена недостаточно. Идентификация плазматических клеток, которые продуцируют IgG4, инфильтрирующие желчный проток и другие органы, имеет решающее значение для постановки диагноза [83]. Механическая желтуха может быть первым симптомом, тогда как при ПСХ она встречается редко [84].
Несмотря на то что ВЗК обычно считается заболеванием, вызывающим снижение массы тела с частым развитием мальабсорбции (особенно при болезни Крона), ряд работ указывает на высокую, по сравнению с общей популяцией, распространенность при ВЗК неалкогольной жировой болезни печени (НАЖБП). Тем не менее основные причины и предрасполагающие факторы к НАЖБП у пациентов с ВЗК остаются малоизученными; повышенный риск развития этого заболевания печени предположительно связан в первую очередь с длительным применением различных лекарственных средств (стероидов, иммунодепрессантов и биопрепаратов), а также перенесенными хирургическими вмешательствами на кишечнике, активностью кишечного воспаления, продолжительностью заболевания [85–87].
В то же время в метаанализе 7 обсервационных исследований с участием 1610 пациентов с ВЗК, выполненном Lapumnuaypol K. et al. (2018), значимой ассоциации между приемом лекарств для лечения ВЗК и распространенностью НАЖБП получено не было. Это указывало на отсутствие взаимосвязи между стеатозом печени и только с лекарственным воздействием при терапии ВЗК. Объединенные отношения шансов НАЖБП у пациентов, принимающих биологические агенты, иммуномодуляторы, метотрексат и стероиды, составили 0,85 [95% ДИ: 0,49–1,46], 1,19 (95% ДИ: 0,70–2,01), 3,62 (95%). ДИ: 0,48–27,39) и 1,24 (95% ДИ: 0,85–1,82) соответственно [88].
С другой стороны, Magrì S. et al. в 2019 г. опубликовали результаты своего исследования по определению факторов риска НАЖБП при ВЗК. НАЖБП была статистически значимо связана с наличием метаболического синдрома (OR=4,13; p=0,001) и ожирением (OR=9,21; p=0,0002). У пациентов с ВЗК и НАЖБП отмечалось более высокое потребление калорий и липидов независимо от активности заболевания. При многомерном анализе наличие метаболического синдрома оказалось единственным значимым фактором, который был связан со стеатозом печени, оцененным инструментально (OR=3,40; p=0,01) [89].
Первичный билиарный холангит (ПБХ) часто сопровождает различные аутоиммунные заболевания, включая синдром Шегрена, хронический тиреоидит и ревматоидный артрит, но его ассоциация только с ВЗК – ситуация крайне редкая [90]. В литературе имеются единичные публикации о случаях перекреста этих заболеваний [91, 92]. Его клиническая картина отличается от типичного ПБХ, с большей вероятностью присутствует у мужчин, диагностируется в более молодом возрасте и ассоциируется с ранее диагностированным легким левосторонним язвенным колитом.
Вторичный амилоидоз печени – редкое осложнение ВЗК; оно чаще встречается при болезни Крона, чем при язвенном колите (0,9 против 0,07%) [93]. Хроническое воспаление в кишечнике способствует отложению амилоида в сосудистых сетях и синусоидах практически любого органа, включая печень. По некоторым данным, клинические проявления этого заболевания могут ограничиваться бессимптомной гепатомегалией, и оно чаще встречается у мужчин при болезни Крона в виде колита [94].
Ассоциация абсцессов печени и ВЗК встречается редко [95, 96], однако, по отдельным данным, они могут быть первыми проявлениями болезни Крона [97]. Механизмы такого явления связаны либо с прямым распространением внутрибрюшных абсцессов, либо со вторичной портальной пиемией, возникающей из-за повышенной кишечной проницаемости. Факторами риска развития абсцессов печени выступают внутрибрюшные гнойные процессы, свищевая форма болезни Крона, недостаточность питание, лечение ГКС [26].
Есть сведения, что пациенты с болезнью Крона имеют в 2 раза больший риск образования камней в желчном пузыре по сравнению с контрольной группой без ВЗК, тогда как язвенный колит не связан с повышенным риском желчнокаменной болезни (ЖКБ) [98]. Частота образования камней в желчном пузыре повышается у пациентов с илеитом при болезни Крона или резекцией подвздошной кишки и варьирует от 13 до 34% [99]. Факторами риска, ассоциированными с развитием ЖКБ, являются илеоцекальная локализация болезни Крона и хирургические вмешательства, связанные с резекцией подвздошной кишки. Другие факторы включают возраст пациента, частоту клинических рецидивов, продолжительность пребывания в стационаре и использование полного парентерального питания. Высказывается предположение о связи рисков развития ЖКБ с нарушением всасывания желчных кислот и их энтерогепатической циркуляцией. Более того, при БК было описано снижение моторики желчного пузыря, а у пациентов с илеоанальным анастомозом выявлены повышенные концентрации холестерина в желчных камнях [100].
Острый идиопатический панкреатит – очень редкое внекишечное проявление ВЗК, преимущественно поражающее пациентов с болезнью Крона. Распространенность панкреатита составляет 0,06% среди взрослых пациентов, тогда как в педиатрической практике он встречается намного чаще – в 2,2% случаев [101]. Учитывая частое назначение иммуносупрессоров, которые могут вызывать поражение поджелудочной железы, требуется дифференциация для разделения токсического и идиопатического генеза ее поражения. Вовлечение двенадцатиперстной кишки в воспаление при болезни Крона также может быть ассоциировано с острым панкреатитом [102]. Кроме того, у пациентов с ВЗК по сравнению с популяцией чаще развивается аутоиммунный панкреатит 2-го типа [103]. В исследовании Klebl F.H. et al. до 40% пациентов с болезнью Крона имели антитела к экзокринным клеткам поджелудочной железы, которые не были связаны с активностью заболевания и, возможно, не повышали риск развития панкреатита, но нарушали экзокринную секрецию железы [104].
Повышение активности амилазы может определяться у 17% пациентов с болезнью Крона и 9% с язвенным колитом, а частота повышения активности липазы при этих заболеваниях составляет до 9 и 7% соответственно [105]. Эти явления могут быть ассоциированы с протяженным и тяжелым обострением ВЗК.
БРОНХОПУЛЬМОНАЛЬНЫЕ ВНЕКИШЕЧНЫЕ ПРОЯВЛЕНИЯ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЙ КИШЕЧНИКА
Поражение бронхов и легких при ВЗК является редким осложнением, при котором может поражаться любой сегмент дыхательной системы. К бронхопульмональным проявлениям ВЗК относятся заболевания дыхательных путей, интерстициальное поражение и гранулематоз легких, имитирующий саркоидоз. Интерстициальные заболевания в основном ассоциированы с язвенным колитом, а гранулематоз – с болезнью Крона [106]. Бронхопульмональные внекишечные проявления могут развиться даже после колэктомии, выполненной в связи с осложнениями язвенного колита. По данным ряда исследований, функциональные легочные тесты при ВЗК часто бывают бессимптомно изменены, и латентно протекающие интерстициальные заболевания легких могут встречаться у 20–55% пациентов с ВЗК [44].
Отдельную группу легочных поражений составляют лекарственно-индуцированные поражения после терапии препаратами 5-АСК и иммуносупрессорами, которые могут протекать как интерстициальное или гранулематозное поражение [100]. Для верификации таких осложнений используются следующие диагностические критерии: анамнез приема лекарственного препарата; изменения в легких, появившиеся после терапии; исключение других причин; улучшение после отмены препарата; возвращение симптомов после возобновления терапии [107].
СОСУДИСТЫЕ ПОРАЖЕНИЯ ПРИ ВОСПАЛИТЕЛЬНЫХ ЗАБОЛЕВАНИЯХ КИШЕЧНИКА
У пациентов с ВЗК существует повышенный риск венозных тромбозов, особенно глубоких вен нижних конечностей, внутренних органов и легочной артерии [108]. В патогенез тромбоэмболий вносят вклад эндотелиальная дисфункция, активация тромбоцитов и нарушение процессов фибринолиза. Общий риск венозных эмболий при ВЗК повышен в 3 раза по сравнению с общей популяцией. Этот риск увеличивают прием некоторых препаратов, например блокаторов JAK-киназ, воспаление в кишке и госпитализации по поводу обострения ВЗК [26].
Тромбоз воротной вены как внекишечное проявление ВЗК возникает очень редко, но может иметь жизнеугрожающие последствия. Для пациентов с ВЗК характерны повышенные риски развития этого состояния: так, согласно исследованиям клиники Мейо, портальный или мезентериальный тромбоз встречался у 1,3% пациентов с ВЗК и имел 50% летальность [109].
Факторами риска тромбоза воротной вены являются молодой возраст, женский пол и анамнез недавней абдоминальной операции [110]. В его патогенезе участвует целый ряд факторов: воспаление, длительная иммобилизация, протяженность поражения кишки, объем хирургического вмешательства, установка центральных катетеров, применение ГКС и курение [108, 111]. Более того, у пациентов с ВЗК изначально имеются нарушения в коагуляционной системе: увеличенное количество тромбоцитов, повышенный уровень факторов V, VIII и фибриногена, сниженный уровень антитромбина III. При этом, даже несмотря на риск развития желудочно-кишечного кровотечения, препаратами выбора для лечения в данной ситуации все равно будут низкомолекулярный гепарин и варфарин [99]. При врожденном состоянии гиперкоагуляции следует рассмотреть возможность пожизненной антикоагулянтной терапии, хотя в других протромбогенных состояниях адекватную защиту обеспечивает 6-месячный курс приема соответствующих препаратов [112].
Сосудистые поражения при ВЗК становятся исходом системного воспалительного ответа и эндотелиальной дисфункции. Появляются новые данные о повышении кардиоваскулярных рисков у пациентов с ВЗК вследствие уплотнения стенок аорты [113], а также увеличения риска острого инфаркта миокарда, сердечной недостаточности [114] и инсульта [115].
ЗАКЛЮЧЕНИЕ
По данным Регистра Северо-западного центра ВЗК, внекишечные проявления наблюдаются у 22,9% пациентов с ВЗК, с большей распространенностью при болезни Крона, чем при язвенном колите (43,5 против 23,2% пациентов соответственно); они включают поражения опорно-двигательного аппарата, кожи, полости рта, глаз, гепатобилиарной системы, периферических сосудов, почек и бронхолегочной системы.
Вовлечение в иммуновоспалительный процесс при ВЗК не только кишечника, но и других органов и систем отражает системный характер аберрантного иммунного ответа и обусловливает возникновение различных внекишечных проявлений, которые, в свою очередь, влияют на прогноз, выбор терапии и качество жизни пациентов. Современная концепция патогенеза внекишечных проявлений ВЗК рассматривает их как результат антиген-специфического воспалительного события, реализующегося при наличии генетических факторов риска и/ или взаимодействии факторов окружающей среды. Особый вклад в патогенез многих внекишечных проявлений при ВЗК вносит нарушение микробиома кишки и повышенная кишечная проницаемость.
Учитывая возможность манифестации ВЗК с внекишечных проявлений, необходимы хорошая осведомленность врачей различных специальностей о «масках» ВЗК и углубленное обследование пациентов из групп высокого риска: больных с олиго-/полиартритом неясной этиологии, анкилозирующим спондилоартритом, сакроилеитом, ПСХ, узловатой эритемой, воспалительными заболеваниями глаз (эписклерит, склерит, увеит). Лечение пациентов с внекишечными проявлениями ВЗК требует мультидисциплинарного подхода с привлечением ревматолога, дерматолога, офтальмолога, сосудистого хирурга, морфолога и включает контроль над активностью воспалительного процесса в кишечнике и специфическое лечение внекишечного проявления. В ряде случаев оптимальный выбор противовоспалительной терапии основного заболевания позволяет купировать внекишечные поражения, однако часть внекишечных проявлений не ассоциирована с активностью заболевания и требует особых подходов к терапии. Кроме того, внекишечные проявления при ВЗК могут быть побочными эффектами проводимой противорецидивной терапии, что требует ее клинического и лабораторного мониторинга врачом с последующей своевременной коррекцией.



