Остеоартрит (ОА) остается наиболее распространенным хроническим заболеванием суставов, источником хронической боли и инвалидизации. При этом лечение ОА до сих пор недостаточно эффективно и часто сопровождается появлением нежелательных побочных эффектов лекарственной терапии. В настоящее время нет ни одного препарата, который бы достоверно задерживал прогрессирование болезни [1].
ОА может поражать мелкие, средние и крупные суставы, хотя с точки зрения болевого синдрома чаще всего страдает коленный сустав: у каждого восьмого человека старше 60 лет имеются признаки симптоматического гонартроза [2]. Согласно данным на 2013 г., симптоматическим ОА тазобедренного или коленного сустава страдали 242 млн человек [3]. Заболевание относится к возраст зависимым, т.е. связанным со старением. В настоящее время ОА – одна из основных причин нарушения подвижности и инвалидности у пожилого населения. Продолжительность жизни увеличивается, и в скором времени впервые в истории человечества число людей в возрасте 65 лет и старше превысит количество детей в возрасте до 5 лет. По расчетам, число людей в возрасте 60 лет и старше увеличится вдвое к 2050 г. и более чем в три раза – к 2100 г. [4]. Поскольку ОА главным образом связан со старением, его распространенность будет неуклонно нарастать, и ожидается, что он станет основной причиной инвалидности среди населения в целом к 2030 г. [5].
БОЛЬ ПРИ ОСТЕОАРТРИТЕ: ИСТОЧНИКИ И МЕХАНИЗМЫ
Боль – ведущая жалоба пациентов с ОА. Обычно она локализуется в области пораженного сустава, хотя может и передаваться за его пределы: например, при ОА тазобедренного сустава боль может ощущаться в области бедра/колена, она усиливается при нагрузке на сустав и проходить или уменьшаться в покое. Симптомы ОА по мере прогрессирования заболевания нарастают. В то же время известно, что наблюдается слабая корреляция между тяжестью заболевания по данным рентгенологических исследований и болевым синдромом. Бессимптомный рентгенологический ОА часто обнаруживается в суставах пальцев кистей и стоп, а также позвоночника, хотя может встречаться и в более крупных суставах [6]. По данным Национального обследования здоровья и питания (NHANES 1), боль в коленном суставе испытывают менее 50% пациентов с рентгенографическими признаками ОА [7]. Причина этого несоответствия остается неясной, хотя отчасти его можно объяснить низкой чувствительностью обычной рентгенографии к структурным изменениям, происходящим при ОА.
Определение источников и механизмов боли при ОА имеет важное значение в подборе методов лечения, нацеленных на уменьшение симптомов и улучшение функции суставов.
Структурные изменения при ОА прежде всего включают уменьшение объема суставного хряща. Этот характерный патологический признак на рентгенограммах выглядит как уменьшение суставного пространства. Одновременно с потерей хряща и разрушением сустава идут процессы его восстановления с образованием новой костной ткани, а также развитием субхондрального склероза и остеофитов (патологическое ремоделирование кости) (рис. 1).

При ОА в патологический процесс вовлекается весь сустав: связки, мениски, синовиум и суставная капсула. При визуализации сустава с помощью МРТ на субхондральной границе выявляют аномальную структуру костной ткани с кистами и поражение костного мозга. Последнее определяется в виде гиперинтенсивных областей, хорошо видных на изображениях в Т2-взвешенном режиме с подавлением жира или протонно-взвешенных МР изображениях [9].
Ноцицепция в суставе осуществляется чувствительными к болевым стимулам нервными окончаниями, которые расположены в тканях сустава: надкостнице и субхондральной кости, мягких тканях, связках, менисках и синовиуме. Хотя уменьшение объема хряща выступает важной структурной особенностью ОА, этот процесс не приводит к боли, так как собственно хрящ не имеет болевых окончаний и, следовательно, не может быть прямым источником боли при легкой или умеренной тяжести заболевания. Этот факт подтверждается исследованиями in vivo: так, показано, что при выполнении артроскопии с анестезией только мягких тканей вокруг сустава введение внутрисуставных зондов не вызывало болевого ответа при воздействии на ткани хряща. При ОА тяжелой степени в остеохондральном соединении происходит нейроваскулярная инвазия, т.е. сосуды и нервы прорастают в хрящ, что способствует возникновению боли. Кроме того, при легкой и умеренной степени тяжести ОА хрящ может фагоцитироваться клетками, выстилающими синовий, что вызывает воспалительные реакции и боль вследствие синовита [10].
 Анатомо-физиологическое обоснование боли при ОА получено на данных МРТ-исследований больных ОА с и без болевого синдрома. Показано, что боль ассоциируется с рядом факторов: поражением костного мозга, синовиальным утолщением (синовитом), выпотом в колене и периартикулярными изменениями, включающими анзериновый бурсит или бурсит «гусиной лапки» (встречается редко) и некоторыми другими структурными нарушениями [11].
Анатомо-физиологическое обоснование боли при ОА получено на данных МРТ-исследований больных ОА с и без болевого синдрома. Показано, что боль ассоциируется с рядом факторов: поражением костного мозга, синовиальным утолщением (синовитом), выпотом в колене и периартикулярными изменениями, включающими анзериновый бурсит или бурсит «гусиной лапки» (встречается редко) и некоторыми другими структурными нарушениями [11].
Поражение костного мозга (ПКМ) представляет собой плохо очерченные изменения ниже субхондральной кости (рис. 2), которые наблюдаются на МРТ почти у 80% людей с симптоматическим ОА. Патоморфологическая картина ПКМ характеризуется жировым некрозом, локализованным фиброзом костного мозга и микроразрушениями губчатой кости, которые связаны с ее активным ремоделированием и восстановлением. Эти нарушения практически всегда обусловлены травмой кости в областях с высокой локальной нагрузкой [12].
ПКМ чаще наблюдаются у пациентов с ОА и болевым синдромом. Так, в большом обсервационном исследовании среди людей с рентгенографическим ОА и болью при проведении МРТ у 36% было выявлено выраженное ПКМ в коленях, тогда как ОА без болевого синдрома сопровождался ПКМ только у 2% (р <0,001) [14]. Результаты ряда проспективных исследований ОА показывают, что колебания размеров ПКМ коррелируют с изменением боли в колене, так что ПКМ, вполне вероятно, может являться причиной боли. Например, в многоцентровом исследовании ОА с серийными МРТ было выявлено, что появление ПКМ или его увеличение тесно связано с впервые возникшей болью в коленных суставах [15]; далее [16] в течение 30-месячного наблюдения выяснилось, что уменьшение объема ПКМ достоверно связано с уменьшением болевого синдрома. В дополнение к этому есть наблюдения, указывающие на то, что целевые вмешательства по снижению нагрузки на коленный сустав связаны со снижением выраженности ПКМ и боли в коленном суставе [10]. Приведенные данные очевидно свидетельствуют, что ПКМ выступает причиной боли при ОА. Правда, механизм, с помощью которого ПКМ вызывает боль, неизвестен. Возможно, что боли при ОА могут быть обусловлены возникновением субхондральных микротрещин.
Синовит при артроскопии наблюдается примерно у 50% пациентов с болевым ОА коленных суставов, а при проведении МР-исследовании выявляется еще чаще [17]. Сравнение данных МРТ, гистологических и артроскопических характеристик синовиальной оболочки у лиц с симптоматическим ОА коленного сустава [18] показывает высокую корреляцию между степенью утолщения синовиальной оболочки на МРТ и макроскопической оценкой синовита при артроскопии (r=0,58), а также инфильтрацией воспалительных клеток под поверхностные слои синовии (r=0,46). На рисунке 3 представлено изображение МРТ с парамагнитным контрастным усилением, где определяется выраженный синовит и выпот в виде гипоинтенсивности МР сигнала.
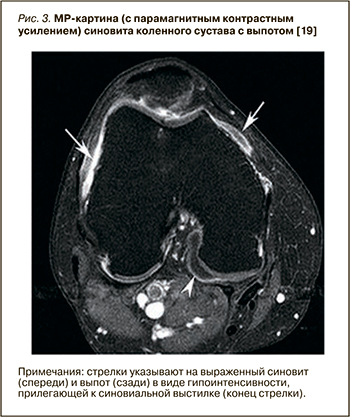 Проведено множество исследований с использованием МРТ, показывающих связь наличия и выраженности синовита и болевого синдрома при ОА коленного сустава [20, 21]. Для выявления синовита предпочтительно МР-исследование с применением контрастного усиления, поскольку контрастное вещество улучшает внешний вид синовиальной оболочки без акцента на других тканях. Так, МРТ с гадолиниевым усилением показало, что синовит более распространен у пациентов с болью в коленном суставе, причем риск возникновения умеренной и сильной боли в суставе при наличии синовита достоверно возрастает. Даже у людей без очевидных рентгенографических признаков ОА, а только с небольшой потерей хряща на МРТ, указывающей на раннюю стадию заболевания, при наличии обширного синовита заметно увеличивался риск появления боли [22]. В открытом исследовании O’Neill T.W. et al. 120 пациентам было проведено сканирование с контрастным усилением до и после внутрисуставной инъекции глюкокортикостероидов [23]. Через 2 нед у пациентов с уменьшением болевого синдрома было отмечено уменьшение выраженности синовита. Кроме того, у пациентов с рецидивом боли в течение 6 мес увеличился объем синовиальной ткани.
Проведено множество исследований с использованием МРТ, показывающих связь наличия и выраженности синовита и болевого синдрома при ОА коленного сустава [20, 21]. Для выявления синовита предпочтительно МР-исследование с применением контрастного усиления, поскольку контрастное вещество улучшает внешний вид синовиальной оболочки без акцента на других тканях. Так, МРТ с гадолиниевым усилением показало, что синовит более распространен у пациентов с болью в коленном суставе, причем риск возникновения умеренной и сильной боли в суставе при наличии синовита достоверно возрастает. Даже у людей без очевидных рентгенографических признаков ОА, а только с небольшой потерей хряща на МРТ, указывающей на раннюю стадию заболевания, при наличии обширного синовита заметно увеличивался риск появления боли [22]. В открытом исследовании O’Neill T.W. et al. 120 пациентам было проведено сканирование с контрастным усилением до и после внутрисуставной инъекции глюкокортикостероидов [23]. Через 2 нед у пациентов с уменьшением болевого синдрома было отмечено уменьшение выраженности синовита. Кроме того, у пациентов с рецидивом боли в течение 6 мес увеличился объем синовиальной ткани.
Точный механизм, с помощью которого синовит может опосредовать боль, неизвестен. Предполагается, что воздействие может осуществляться через выработку цитокинов/медиаторов воспаления, которые сенсибилизируют или активируют чувствительные нервы, или путем механической стимуляции ноцицепторов утолщенным синовием и/или выпотом.
ДРУГИЕ СТРУКТУРНЫЕ ИЗМЕНЕНИЯ, ВЛИЯЮЩИЕ НА ВОЗНИКНОВЕНИЕ БОЛИ ПРИ ОСТЕОАРТРИТЕ
Хрящ не имеет болевых волокон, поэтому его дегенерация не влияет напрямую на возникновение боли при ОА [24]. Однако выраженное разрушение хряща, вовлекающее субхондральную костную пластинку, связано с болью у пациентов с ОА коленного сустава [25]. Также показана связь боли с процессом дегенерации костей (уплощением или истончением кортикального слоя [26]) и патологией мениска [27].
Еще один интересный аспект изучения боли при ОА – значение нейрональных путей и обработки болевых импульсов. Ноцицепторы в суставе при ОА могут стимулироваться различными воздействиями: физическими, механическими или химическими, включая медиаторы воспаления, такие как брадикинины и простагландины E2. Существует несколько типов рецепторов, которые стимулируют дезадаптивные процессы в тканях (ASICs – протон-активируемые ионные каналы, TRP-рецепторы). Больше всего изучено семейство рецепторов транзиентного рецепторного потенциала (TRP), которые обладают трансмембранными доменами, при стимуляции функционирующими как ионные каналы [28].
Потенциал действия, генерируемый при стимуляции ноцицепторов периферических нервных окончаний, передается через немиелинизированные С-волокна и миелинизированные Аδ-волокна. Благодаря более быстрой проводимости волокна типа Aδ отвечают за передачу/ощущение острой боли, тогда как волокна типа C реагируют на множественные сигналы и отвечают за ощущение более диффузной жгучей боли [29].
Афферентные болевые волокна передают болевые сигналы от сустава к спинному мозгу, где происходит синаптическая обработка сигнала. Затем импульс по восходящим путям передается к таламусу и далее к соматосенсорной коре. Нисходящие волокна в спинном мозге помогают модулировать болевые ощущения.
В связи с травмой, воспалением или другими ноцицептивными стимулами порог для местного возбуждения и передачи сигналов может снижаться, что приводит к увеличению чувствительности периферических ноцицепторов. Это явление называется «периферическая сенсибилизация». Оно объясняет возникновение у некоторых людей с ОА феноменов гипералгезии (или гиперчувствительности) и аллодинии (боли в ответ на неболевые раздражители).
Фактор роста нервов (NGF) играет важную роль в формировании воспалительных болей за счет повышения чувствительности ноцицепторов [30]. На животной модели артрита показано, что системное введение ослабленного анти-NGF уменьшает прорастание нервных волокон (спрутинг) и боль при артрите [31]. В исследованиях подтверждено, что блокада фактора роста нервов уменьшает боль при ОА коленного и тазобедренного суставов [32], и это указывает на причинную связь между болью NGF и ОА.
Подобно периферической сенсибилизации, болевые пути центральной нервной системы, в том числе управляющие нисходящим торможением, находятся под влиянием постоянной стимуляции ганглиев дорсального корешка воспалительными цитокинами, которая возникает в суставе и приводит к центральной сенсибилизации. Показано, что не только периферическая, но и центральная сенсибилизация участвуют в формировании боли при ОА [33, 34]. Сенсибилизация, как правило, возникает не сразу, а по мере развития заболевания, что объясняет постепенное усиление болевого синдрома. Она может также объяснить имеющуюся в ряде случаев низкую результативность стандартного обезболивающего лечения и, напротив, хорошую эффективность препаратов центрального действия, таких как дулоксетин [35].
Таким образом, патофизиологический статус каждого компонента в синовиальном суставе связан с дегенерацией сустава и соответствующим восприятием боли. Местный гомеостаз внутри сустава может быть нарушен различными факторами, такими как старение, травмы и генетическая предрасположенность. Хроническое воспаление слабой степени в суставе не только способствует ускоренному повреждению хряща и синовиту, но также делает сустав чувствительным к периферической и в некоторых случаях центральной сенсибилизации [36]. Поэтому терапия боли при ОА должна включать два класса препаратов: действующих как на центральную, так и периферическую сенсибилизацию, поскольку оба эти механизма приводят к одному конечному результату – появлению боли у пациента с ОА.
ФАРМАКОТЕРАПИЯ ОСТЕОАРТРИТА И РОЛЬ НПВП
Медикаментозная терапия ОА в клинической практике ведется сразу по нескольким направлениям: уменьшение болей, снятие воспаления, попытки замедления дальнейшей дегенерации хрящевой ткани. Препараты, применяемые для этих целей, принято называть симптом-модифицирующими (SMOADS). Их делят на две основные группы:
- быстро действующие средства: анальгетики, нестероидные противовоспалительные средства (НПВП), внутрисуставные глюкокортикоиды, опиоиды и др.;
- медленно действующие средства, или SYSADOA (Symptomatic Slow Acting Drugs for Osteoarthritis): глюкозамин, хондроитин, гиалуроновая кислота, модуляторы цитокинов.
Что касается первой группы, то в ней препаратами выбора считаются парацетамол и НПВП. Внутрисуставные глюкокортикоиды и опиоиды применяются в ситуациях, когда препараты первой линии не эффективны.
НПВП уже более столетия служат базовой терапией ОА и, несмотря на потенциальные риски, связанные с их длительным применением, остаются основой эффективной стратегии обезболивания.
В 2018 г. была представлена обновленная версия рекомендаций по применению НПВП в клинической практике, основанная на метаанализах большого числа клинических и эпидемиологических исследований и учитывающая мнения ведущих мировых экспертов [37]. Вот ключевые положения этих рекомендаций:
- все НПВП в средних и максимальных терапевтических дозах при длительном применении имеют равный обезболивающий потенциал (уровень доказательности (УД) 1a);
- эффективность НПВП зависит от дозы: использование более высоких дозировок позволяет обеспечить более выраженное обезболивающее и противовоспалительное действие (УД 1b);
- применение инъекционных форм НПВП, а также быстро растворимых препаратов для приема внутрь может иметь преимущество в скорости наступления обезболивающего эффекта по сравнению с пероральным приемом (УД 1b). Однако оценка эффективности приема таких лекарственных форм при лечении более 1 дня показала спорные результаты (УД 1b);
- НПВП в средних и максимальных терапевтических дозах более эффективны, чем максимальная терапевтическая доза парацетамола 4 г/сут (УД 1a);
- при лечении хронической боли НПВП в средних и максимальных терапевтических дозах не уступают по эффективности трамадолу (УД 1a);
- при ОА длительное непрерывное использование НПВП обеспечивает лучший контроль симптомов, чем прием НПВП в режиме «по требованию» (УД 1b);
- при ОА длительный прием НПВП в ряде случаев способен уменьшить частоту рецидивов (УД 1b);
- при лечении ОА локальные формы НПВП обладают доказанной обезболивающей и противовоспалительной эффективностью (УД 1a).
Как видно из рекомендаций, локальные (топические) НПВП входят в первую линию терапии ОА. Последние обновленные рекомендации по лечению ОА, представленные на конгрессе ESCEO в 2019 г. в Париже, сообщают, что локальные НПВП в целом предпочтительно назначать до пероральных НПВП, так как они обладают действием на боль, сравнимым с эффектом форм для приема внутрь, но благодаря низкой системной абсорбции имеют лучший профиль безопасности [38].
Недостатки НПВП хорошо известны. Прежде всего это ряд побочных эффектов со стороны желудочно-кишечной и сердечно-сосудистой систем, органов кроветворения, почек и печени. В случае терапии ОА безопасность назначаемых средств особенно актуальна, поскольку пациенту необходимо их длительное применение. Так, у пациентов с язвенной болезнью, диабетической или другой нефропатией следует воздержаться от назначения оральных форм НПВП. Поэтому в лечении ОА, особенно при изолированном процессе, используются местные формы НПВП (мази, кремы, гели и др.), которые позволяют воздействовать непосредственно на очаг поражения, сводя к минимуму влияние на другие органы.
Местная лекарственная форма представляет собой смесь активного вещества с основой, способствующей его проникновению через кожу. Нанесенное на поверхность кожи средство играет роль депо, которое обеспечивает постепенное поступление и поддержание терапевтической концентрации препарата в очаге поражения. Считается, что средства локальной терапии в виде геля легче и быстрее проникают через кожу. Кроме того, гелевая форма удобна для применения и более гигиенична.
По данным Кокрановского обзора 2016 г. с анализом 39 исследований (10 631 участник), при наружном применении НПВП для лечения хронической скелетно-мышечной боли преимущественно у пациентов с ОА коленных суставов показана большая эффективность локальных форм диклофенака и кетопрофена по сравнению с плацебо: значительное уменьшение боли отметили около 60% пациентов. При этом сравнительная оценка локальных и пероральных НПВП показала в целом одинаковую их эффективность [39].
Диклофенак натрия – одно из наиболее широко применяемых лекарственных средств, «золотой стандарт» среди неселективных НПВП. Оригинальный (референтный) препарат диклофенака (Вольтарен®) производится в том числе и в формах для местного применения – Вольтарен® Эмульгель (GlaxoSmithKline UK). Концентрация диклофенака диэтиламина в пересчете на диклофенак натрия в эмульгеле составляет 1% и 2% в зависимости от выбранной дозировки препарата. Исследования in vitro продемонстрировали, что эмульгель действует эффективнее, чем липогель и мазь [40]. После нанесения препарата на кожу активное соединение накапливается в региональных мягких тканях, синовиальной оболочке и синовиальной жидкости суставов, при этом концентрация диклофенака в плазме крови примерно в 100 раз ниже, чем после перорального приема сопоставимой дозы диклофенака. Системная абсорбция препарата после 12-часовой аппликации составляет около 6%. Содержание диклофенака в мышцах в зоне аппликации препарата Вольтарен® Эмульгель примерно в 3 раза превышает его уровень в отдаленной мышечной ткани [41].
Вольтарен® Эмульгель обладает выраженным анальгезирующим, противовоспалительным и жаропонижающим действием, а благодаря водно-спиртовой основе проявляет успокаивающий и охлаждающий эффект. Основным, хорошо известным механизмом обезболивающего действия диклофенака служит угнетение циклооксигеназы (ЦОГ), которое приводит к снижению выработки простагландина Е2 (PgE2) и тромбоксана. Вместе с тем существуют и другие, менее известные периферические и центральные механизмы действия диклофенака [42]: это ингибирование ряда медиаторов боли, воспаления и внутриклеточных путей, позволяющее дополнительно воздействовать на хроническую боль. Таким образом, влияние препарата Вольтарен® Эмульгель на воспалительные и болевые процессы довольно многогранно и патогенетически обосновано. Наибольшая его эффективность проявляется при терапии ноцицептивной и хронической центральной боли, характерных для ОА [43].
ИНЫЕ АСПЕКТЫ ОБЕЗБОЛИВАЮЩЕЙ ТЕРАПИИ ПРИ ОСТЕОАРТРИТЕ
Понимание того, что центральная нервная система вносит значительный вклад в хронификацию боли, привело к увеличению количества исследований препаратов центрального действия для лечения ОА. Так, для лечения костно-мышечной боли одобрено применение ингибитора обратного захвата серотонина и норэпинефрина дулоксетина [28]. В комплексной терапии возможно также использование антиконвульсантов (прегабалина, габапентина).
Существенным моментом терапии болевого синдрома является не только его эффективное купирование, но и предотвращение дальнейшего развития патологического процесса, использование мер, направленных на восстановление структурного и функционального повреждения. Для этих целей применяют препараты группы SYSADOA – хондромодулирующие и/или хондропротективные вещества (предшественники хрящевого матрикса – глюкозамин, хондроитин, гиалуроновая кислота; модуляторы цитокинов – диацереин и ингибиторы металлопротеаз), действие которых развивается гораздо медленнее, чем у быстро действующих симптом-модифицирующих средств. Отметим, что до сих пор существуют разногласия насчет результативности применения SYSADOA, хотя и накоплена достаточная доказательная база, свидетельствующая об их эффективности в отношении долгосрочного лечения и профилактики развития ОА. Кроме того, комбинирование SYSADOA с НПВП позволяет снизить дозу последних и тем самым предотвратить ряд нежелательных побочных эффектов [44].
Несмотря на доступные варианты терапии, пациенты с ОА продолжают страдать от неадекватного обезболивания. Существует большая потребность в разработке новых методов безопасного лечения хронической боли, связанной с ОА.
На сегодня определены три новых крупных направления терапии ОА:
- биологическая терапия моноклональными антителами (анти-NGF – танезумаб и др.);
- воздействие на малые молекулы двух классов: рецепторы, связанные с G-белком (GPCR), и ионные каналы;
- крионевролиз.
Моноклональные антитела (МА) в настоящее время разрабатываются для широкого спектра заболеваний, и ОА с болевым синдромом не является исключением. МА против фактора роста нервов (NGF) считаются наиболее перспективной стратегией терапии ОА с болевым синдромом. Экспрессия нейротрофина или NGF у человека, испытывающего боль, заметно повышена. NGF оказывает глубокое сенсибилизирующее действие на ноцицептивную систему [45]. Разработано несколько гуманизированных МА с высокой специфичностью и сродством к NGF, которые связывают его, тем самым предотвращая соединение с рецептором. Эти антитела включают танезумаб (Pfizer и Eli Lilly), фасинумаб (Regeneron и Teva) и фулранумаб (Janssen и Amgen).
На сегодня наиболее изученным среди анти-NGF МА препаратом является танезумаб (6 законченных и 5 активных клинических исследований), который близок к завершению клинических испытаний III фазы, т.е. к одобрению для применения во врачебной практике. Количество научных исследований по эффективности анти-NGF МА пока не велико, но выводы каждого обзора схожи: по сравнению с плацебо ингибирование NGF приводит к значительному уменьшению боли и улучшению функции. В исследованиях монотерапии танезумаб (внутривенно 5 и 10 мг) статистически значимо превосходил активные компараторы (НПВП, опиаты) [46, 47], при этом лечение низкой дозой препарата (±2,5 мг) имело сравнимую эффективность с более высокой дозировкой, но вызывало значительно меньше побочных эффектов [48]. Тем не менее риск возникновения нежелательных явлений, включая экзацербацию структурных повреждений при ОА, на фоне применения препаратов этой группы сохраняется, поэтому механизмы их действия требуют дальнейшего изучения.
Другие антитела, проходящие клинические исследования на предмет анальгетической эффективности при ОА, связываются с различными мишенями: цитокинами [49], NGF/ TNF (MEDI7352), фактором некроза опухолей (адалимумаб), интерлейкином 1 (IL-1α) и IL-1β (ABT-981) [50], рецептором IL-6 (тоцилизумаб) [51] и др.
Малые молекулы, участвующие в механизмах развития хронической боли и задействованные в фармакологических разработках, делятся на два класса: рецепторы, связанные с G-белком (GPCR), и ионные каналы.
GPCR (всего около 800) кодируют приблизительно 4% генома человека. Эти рецепторы широко представлены во всех клетках периферического и центрального болевого пути. В зависимости от G-белка, участвующего в передаче сигналов, GPCR могут оказывать возбуждающее или ингибирующее действие на боль. Примером такого действия служит проалгезирующее влияние брадикинина и обезболивающий эффект морфина. Опасения по поводу безопасности опиоидов побудили разработчиков к созданию новых препаратов этого класса с улучшенными профилями безопасности, а именно препаратов, избирательно воздействующих на каждый из трех опиоидных рецепторов (δ-, κ- или µ-). Не оставлены без внимания и каннабиноидные рецепторы, которые также являются хорошо изученными анальгетическими мишенями.
Ионные каналы выступают основополагающими компонентами болевого пути, поскольку обеспечивают возбудимость нейронов при различных хронических болевых состояниях. В целях изучения влияния на боли при ОА был протестирован ряд веществ, нацеленных на разные ионные каналы. Например, внутрисуставной капсаицин, который обратимо деактивирует свободные окончания болевых волокон в суставе, показал хороший анальгетический эффект. Кроме того, для лечения боли при ОА используются местные производные капсаицина в форме крема [52].
Крионевролиз (также называемый криоаналгезией, криогенной блокадой) — это метод, основанный на локальном замораживании периферического нерва, который вызывает повреждение аксонов и блокирует нервную проводимость. При крионевролизе температуры в диапазоне от -60 до -100 °C вызывают повреждение нерва и влияют на все типы нервных волокон. Повреждения нерва приводят к валлеровской дегенерации аксона, не убивая клеточное тело; как правило, они обратимы в течение недель или месяцев. Кроме того, этот тип замораживания не влечет за собой воспаления и фиброза, что способствует обратимости структурных и функциональных повреждений нерва. Метод используется в дополнение к фармакологическим подходам. Эффективность его показана на результатах недавно опубликованного рандомизированного контролируемого исследования при ОА коленного сустава [53]. Само устройство overa (Myoscience, Fremont, CA, USA) недавно получило разрешение FDA США и выходит на рынок медицинских изделий [54]. Однако необходимы дальнейшие клинические испытания, чтобы сделать четкие выводы о том, способна ли эта технология улучшить клиническую картину пациентов с ОА.
Таким образом, ОА — это гетерогенное заболевание со сложным патогенезом, ведущую роль в котором играет воспаление, вовлекающее в патологический процесс все ткани сустава, в том числе кости, хрящ и синовиальную оболочку. Для понимания основных структурных коррелятов боли у пациентов с симптоматическим ОА используется МР-диагностика: данные наблюдательных и интервенционных исследований с использованием МРТ помогли охарактеризовать вклад особенно синовиального воспаления (синовита) и поражений костного мозга в построении гипотезы патогенеза боли при ОА. Показано, что измененная чувствительность периферических нервов, иннервирующих сустав, а также сенсибилизация ЦНС, может объяснить постоянную боль при ОА.
Применение современных анальгетических препаратов при ОА ограничено по эффективности, переносимости и безопасности, поэтому активно ведется разработка новых лекарственных средств. Наиболее перспективной стратегией терапии ОА с болевым синдромом считаются моноклональные антитела. Два антитела против фактора роста нервов – тануземаб и фасинумаб – находятся в стадии разработки. Также активно тестируются вещества с другими механизмами действия, включая ингибиторы цитокинов, селективные агонисты μ-, δ- и k-опиоидных рецепторов, золедроновая кислота и внутрисуставной капсаицин. В дополнение к фармакологическим подходам внедряются интервенционные стратегии, нацеленные на периферические нервы, такие как крионевролиз, но профиль эффективности и долгосрочное воздействие такого лечения требуют дальнейшего изучения. Персонализированный подход к диагностике и терапии с выявлением причин боли у каждого отдельно взятого пациента может быть полезным для подбора терапии, уменьшения симптоматики ОА и улучшения функционирования пациента.



