В последние годы отмечается увеличение в фармакологическом арсенале высокоактивных лекарственных средств (ЛС), а также препаратов с принципиально новыми механизмами действия (моноклональные антитела, препараты генной, тканевой терапии и т.д.) [1, 2]. Параллельно с этим возрастает и количество случаев лекарственного поражения желудочно-кишечного тракта (ЖКТ).
Так, по данным Fernandes D.C.R. et al. [3], до 5% всех случаев обращений в лечебно-профилактические учреждения за медицинской помощью и 40% всех случаев развития нежелательных реакций (НР) обусловлены именно лекарственным поражением ЖКТ [3]. По информации Агентства по контролю за продуктами питания и лекарственными средствами США (United States Food and drug administration, US FDA), до 10% всех случаев НР – это осложнения фармакотерапии со стороны ЖКТ [4], а количество госпитализаций может достигать 20–40% [5–7]. При этом симптоматика лекарственных поражений ЖКТ может мимикрировать под различные патологические состояния, например синдром раздраженного кишечника или воспалительные заболевания кишечника, поэтому их часто не диагностируют [8].
Выделяют несколько патофизиологических механизмов лекарственно-индуцированного поражения ЖКТ:
- поражение ЖКТ как следствие механизма действия ЛС. Например, препараты с антихолинергической активностью (трициклические антидепрессанты, атропин, хлорпромазин и др.) могут снижать тонус нижнего сфинктера пищевода и тем самым способствовать забросу кислого содержимого желудка в пищевод, что приводит к изжоге и эзофагитам. Также антихолинергические ЛС угнетают моторику гладкой мускулатуры и вследствие этого могут приводить к дисфагии и констипации [3];
- нарушение целостности слизистой ЖКТ. Примером такого механизма поражения ЖКТ может служить гастропатия, индуцированная ингибированием синтеза простагландинов и циклооксигеназы нестероидными противовоспалительными средствами (НПВС) [3];
- прямое повреждающее действие ЛС на слизистую ЖКТ. Подобный механизм характерен для кислых (аскорбиновая кислота, железа сульфат и др.) или гиперосмолярных ЛС (препараты калия), бисфосфонатов, а также цитотоксических препаратов (даунорубицин, цитарабин, 5-фторурацил, метотрексат, винкристин и др.) [3, 9–10];
- изменения микробиоты кишечника. Такой тип поражения ассоциирован, как правило, с приемом антибактериальных средств (например, цефалоспоринов) и может проявляться псевдомембранозным колитом и инфекцией Clostridium difficile [3].
ЛЕКАРСТВЕННО-ИНДУЦИРОВАННОЕ ПОРАЖЕНИЕ РОТОВОЙ ПОЛОСТИ
Среди всех отделов ЖКТ поражения полости рта встречаются относительно редко и обычно ассоциированы с применением лучевой терапии области головы и шеи или приемом цитотоксических ЛС [3]. Воспаление слизистой оболочки ротовой полости у таких пациентов, хотя и редко, но может переходить и на другие отделы ЖКТ [3].
Другой группой ЛС, которая может вызывать поражение ротовой полости, являются ингаляционные глюкокортикостероиды (иГКС). На фоне их применения могут появляться следующие клинические симптомы: ксеростомия, изъязвление слизистой оболочки полости рта, зубной кариес, кандидоз, псевдомембранозные изменения, галитоз, гингивит и периодонтит, изменения вкуса [11]. Перечень других ЛС, которые потенциально могут вызывать лекарственно-индуцированные поражения полости рта, представлен в сводной таблице.
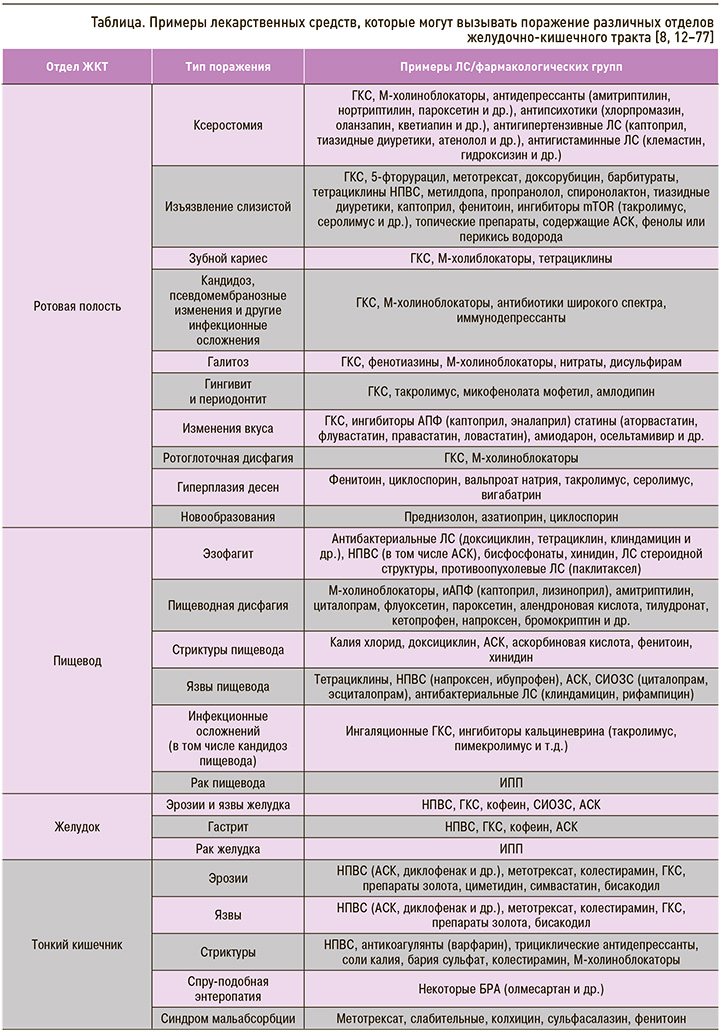
С целью профилактики рекомендуется регулярное проведение гигиены полости рта, а также профилактические осмотры у врача. Метод коррекции лекарственно-индуцированных осложнений зависит напрямую от типа поражения и включает отмену или уменьшение дозы (при невозможности отмены) препарата, вызвавшего поражение, санацию полости рта, нанесение топических средств, ускоряющих заживление тканей или обладающих дубящими эффектами, и при необходимости хирургическую коррекцию. В случае возникновения орофарингеальной дисфагии рекомендована модификация консистенции пищи, консультация логопеда, подбор способа кормления (назогастральный/назодуоденальный зонд, наложение гастростомы и т.д.) [8, 12–57].
ЛЕКАРСТВЕННО-ИНДУЦИРОВАННОЕ ПОРАЖЕНИЕ ПИЩЕВОДА
К симптомам поражения слизистой оболочки пищевода (эзофагита), вызванного приемом ЛС, относятся боль за грудиной, ощущения препятствия прохождению пищи по пищеводу (дисфагия), боль при глотании или в эпигастральной области. Характерной локализацией лекарственного эзофагита являются анатомические сужения пищевода (как правило, в средней трети пищевода в области левого предсердия) за счет увеличения времени контакта слизистой пищевода и ЛС.
Выделяют несколько причин развития лекарственно-индуцированного поражения слизистой пищевода. Это химический ожог слизистой вследствие приема кислых (аскорбиновая кислота, железа сульфат) или гиперосмолярных (препараты калия) ЛС и заброс кислого содержимого желудка в пищевод из-за атонии нижнего сфинктера пищевода (при приеме нитратов, ЛС с антихолинергической активностью, метилксантинов, бензодиазепинов. трициклических антидепрессантов, верапамила и ряда других ЛС).
В результате негативного воздействия ЛС у пациентов могут развиться эзофагиты, пищеводная дисфагия, язвы и стриктуры пищевода, а при длительно существующем процессе – рак пищевода (см. табл.).
Кандидоз пищевода является одной из наиболее частых причин диспепсии (изжоги) или одинофагии (субъективного ощущения боли во время глотания и прохождения пищи по пищеводу) у пациентов, находящихся на терапии ГКС, антибиотиками или иммуносупрессантами [58].
Для профилактики поражения пищевода, вызванного контактом его слизистой оболочки с ЛС, рекомендуется запивать препарат полным стаканом воды (200–250 мл) и 30 мин после этого не принимать горизонтальное положение, так как в противном случае увеличивается время контакта препарата и слизистой пищевода, а следовательно, и риск химического поражения тканей [59–61].
Для профилактики эзофагита вследствие заброса кислого содержимого из желудка (рефлюкса) рекомендуется спать с приподнятым головным концом, а также принимать ЛС из группы ингибиторов протонной помпы (ИПП) [65]. Эффективность профилактики и лечения эзофагита (в том числе тяжелых форм) с помощью ИПП может достигать 80–100% [62]. Однако следует помнить о том, что и сами ИПП могут вызывать серьезные НР, в том числе со стороны пищевода, например рак (см. табл.).
ЛЕКАРСТВЕННО-ИНДУЦИРОВАННОЕ ПОРАЖЕНИЕ ЖЕЛУДКА
Лекарственно-индуцированное поражение желудка – достаточно распространенное осложнение фармакотерапии, которое может проявляться в форме воспаления (гастрита), эрозий, язв, злокачественных новообразований и т.д. (см. табл.). Так, у 50% больных, принимающих НПВС, наблюдается эрозивное поражение слизистой оболочки желудка и двенадцатиперстной кишки, а у 25% – пептические язвы [63]. При этом указанные НР могут осложняться кровотечениями (риск кровотечений у пациентов, принимающих НПВС, выше в 3–5 раз в сравнении с пациентами, не принимающими эти ЛС) и прободениями (увеличение риска в 6 раз), которые, в свою очередь, могут приводить к развитию летального исхода (возрастание риска в 8 раз) [63]. Конечно, с развитием медицинских технологий смертность от кровотечений и перфораций снизилась с 11,6 до 7,4%, но у лиц, принимающих АСК (даже в низких дозах) и НПВС, этот показатель, наоборот, увеличился с 14,7 до 20,9% [63].
Для обозначения поражения желудка на фоне приема НПВС существует специальный терамин «НПВС-индуцированная гастропатия». Ввиду широкой распространенности и актуальности этой проблемы для врачей различных специальностей созданы и постоянно обновляются клинические рекомендации по ее диагностике, лечению и профилактике. Например, в нашей стране разработаны клинические рекомендации Российской гастроэнтерологической ассоциации по диагностике и лечению эрозивно-язвенных поражений желудка и двенадцатиперстной кишки, вызванных НПВС (2014) [63], а также рекомендации по профилактике и лечению эзофаго-гастро-энтеро-колопатий, индуцированных НПВС, подготовленные Российским научным медицинским обществом терапевтов (РНМОТ) [64].
Клинически лекарственно-индуцированное поражение желудка проявляется болями в эпигастральной области, тошнотой, диспепсией, рвотой кровью и меленой (при тяжелом поражении), хотя может протекать и бессимптомно [63, 64].
Механизмы поражения желудка лекарственными препаратами включают:
- химический ожог слизистой вследствие приема кислых (аскорбиновая кислота, железа сульфата) или гиперосмолярных (препараты калия) ЛС;
- снижение защитной функции слизистой оболочки желудка вследствие угнетения синтеза простагландинов, например, за счет ингибирования циклооксигеназы (ЦОГ) НПВС, ухудшения ее кровоснабжения, снижения продукции желудочной слизи и бикарбонатов, усиления выделения соляной кислоты и пепсиногена, активации апоптоза и десквамации эпителиальных клеток, усиления моторики желудка, повышения синтеза свободных радикалов, фактора некроза опухоли [63].
Профилактика лекарственного поражения желудка, с одной стороны, заключается в назначении ЛС с наименьшими рисками развития гастропатии, например, селективных ингибиторов ЦОГ-2 (так называемых коксибов – целекоксиба, эторикоксиба и др.) или НПВС, не относящиеся к коксибам, но также преимущественно угнетающих ЦОГ-2 (мелоксикама, этодолака, нимесулида и др.). С другой стороны, профилактика и лечение лекарственно-индуцированных поражений желудка подразумевает назначение таких ЛС, как синтетические аналоги простагландина Е1, блокаторы Н2-рецепторов, ИПП, гастропротекторы (например, ребамипид – препарат Ребагит® производства «ПРО.МЕД.ЦС Прага а.о.»), которые также показаны для терапии язвенной болезни желудка и гастритов, в том числе вызванных приемом гастротоксических лекарств (НПВС, этанола, кислот и др.) [65].
ЛЕКАРСТВЕННОЕ ПОРАЖЕНИЕ КИШЕЧНИКА
В связи с развитием эндоскопических методов диагностики, включая трансназальную гастроскопию, стало возможным проводить точную диагностику состояния слизистой оболочки желудка и выявлять лекарственно-ассоциированые эзофаго-, гастро- и дуоденопатии. Но лишь одна медицинская технология, а именно капсульная эндоскопия, совершила настоящий прорыв в понимании проблемы лекарственно-индуцированных заболеваний ЖКТ и позволила по-другому взглянуть на частоту осложнений фармакотерапии. Так, с ее помощью выяснилось, что тонкий кишечник является одной из наиболее частых локализаций лекарственного поражения ЖКТ (20–40% всех случаев) [3, 7, 66]. Столь высокая частота поражения кишечника объясняется очень «деликатными» взаимоотношением нервной системы этого органа и центральной нервной системы, высокой метаболической активностью клеток, отвечающих за процессы абсорбции и секреции, а также активностью кишечной микробиоты. Не стоит забывать и о таких факторах, как достаточно длительная экспозиция препаратов в просвете кишечника по мере их прохождения по пищеварительному тракту, а также потенциальная рециркуляция ЛС с желчью [3, 7, 66].
Клиническая картина лекарственно-индуцированных поражений кишечника разнообразна: от бессимптомного течения или незначительных проявлений до тяжелой, жизнеугрожающей симптоматики, характерной для перитонита (вследствие перфорации) и ишемического колита [3, 7, 66].
Важно отметить, что лекарственное поражение тонкого кишечника может приводить к развитию осложнений (в том числе потенциально летальных) и ассоциированых патологических состояний, таких как, например, синдром мальабсорбции, мальнутриция, непроходимость, парез, которые также будут требовать коррекции, а следовательно, ухудшать прогноз, увеличивать риск полипрагмазии и новых осложнений и НР («порочный круг») [3].
Лечение зависит от типа поражения (воспаление, эрозии, язвы, стриктуры, спру-подобная энтеропатия и др., табл.) и включает отмену/замену препарата, вызвавшего повреждение тонкого кишечника, симптоматическую терапию и при необходимости проведение хирургического вмешательства.
В профилактических целях рекомендуется использовать препараты с наименьшим риском развития осложнений со стороны ЖКТ и регулярно выполнять эндоскопическое исследование кишечника. Также следует минимизировать длительность курса применения ЛС, обладающих потенциальным энтеротоксическим действием, особенно в случае с лекарственными формами модифицированного высвобождения (например, НПВС, покрытых кишечно-растворимой оболочкой или замедленного высвобождения), поскольку такие формы, помимо прочего, вызывают поражение ЖКТ в том числе за счет более длительной экспозиции в просвете кишечника. Наконец, целесообразно регулярно контролировать состояние микробиоты кишечника, исключить наличие клостридиальной и других кишечных инфекции [3, 67].
ОСОБЫЕ ФОРМЫ ЛЕКАРСТВЕННО-ИНДУЦИРОВАННОГО ПОРАЖЕНИЯ ТОНКОГО КИШЕЧНИКА
Поражение тонкого кишечника (эрозии, язвы, стриктуры, спру-подобную энтеропатию, синдром мальабсорбции и др.) могут вызывать многие группы ЛС. Тонкий кишечник является частым (до 2/3 пациентов) органом-мишенью поражения со стороны НПВС, в том числе ацетилсалициловой кислоты (АСК), особенно в кишечно-растворимой оболочке [68]. Как правило, патология протекает в скрытой форме и возникает на фоне длительного приема этих препаратов. Основным методом ее диагностики служит капсульная эндоскопия [68]. К косвенным клиническим признакам, позволяющим предположить наличие энтеропатии, относятся повышение проницаемости кишечника (у 44–70% пациентов), слабо выраженное воспаление (60–70%), мальабсорбция (40–70%) и, как следствие, этого гипоальбуминемия, железодефицитная анемия (≈30%) и др. [68].
Спру (англ. sprue) – одна из форм энтерита с тенденцией к прогрессированию, клиническими симптомами которой выступают понос, мальабсорбция, мальнутриция, анемия (как правило, фолиево-дефицитная), нарушения деятельности желез внутренней секреции, явления гипокальциемии. В литературе описано несколько случаев развития спру-подобной энтеропатии на фоне применения блокаторов рецепторов ангиотензина II (БРА). При этом наибольшее число сообщений об этой НР ассоциировано с применением олмесартана (около 94%) в течение длительного времени (от нескольких месяцев до нескольких лет) [69]. Отмена олмесартана и/или других препаратов-индукторов в большинстве случаев приводит к улучшению состояния пациента [70–71]. По всей видимости, спру-подобный энтерит все-таки не является класс-специфической НР, а, скорее всего, обусловлен именно особенностями конкретной молекулы [72]. Тем не менее описаны единичные случаи спру-подобной энтеропатии на фоне приема ирбесартана, валсартана, лозартана [70–77].
Точная распространенность данной патологи не известна. В крупном исследовании Basson M. et al., в которое вошли медицинские данные 4 546 680 пациентов, оценивалась частота госпитализации по поводу мальабсорбции вследствие энтеропатии у больных, принимающих олмесартан, другие БРА или ингибиторы АПФ [78]. Было установлено, что частота госпитализации среди лиц, принимающих олмесартан, была в 2,49 раза выше по сравнению с ингибиторами АПФ и в 3,17 раза выше по сравнению с другими БРА. Также авторами отмечено, что риск госпитализации по поводу симптомов энтеропатии у пациентов, принимающих БРА, в 0,78 раз выше, по сравнению с ингибиторами АПФ. По всей видимости, это свидетельствует о том, что среди всех БРА именно прием олмесартана ассоциирован с риском развития энтеропатии [78, 79].
Патогенез спру-подобной энтеропатии точно не известен. Возникновение энтеропатии вследствие длительного приема препарата и отсутствие зависимости от дозы делают маловероятной такую причину, как прямое токсическое действие препарата [74]. В исследовании Narietta E.V. et al. [80] было выявлено, что у пациентов со спру-подобной энтеропатией имеется нарушение регуляции интерлейкина-15 (IL-15) с последующим разрушением белков плотных контактов ZO-1, что, вероятно, обусловливает иммуноопоследованный механизм поражения [74]. Отмечено повышение количества бактериальной флоры у пациентов с олмесартан-индуцированной спру-подобной энтеропатией, однако терапия антибактериальными средствами не приводит к улучшению состояния таких больных [74]. Данное наблюдение может быть важным, поскольку нарушения микробиома кишечника играют значительную роль в патогенезе осложнений, потенциально обусловленных реакциями гиперчувствительности, например, олмесартан-индуцированной спру-подобной энтеропатии.
ЛЕКАРСТВЕННО-ИНДУЦИРОВАННЫЙ КОЛИТ
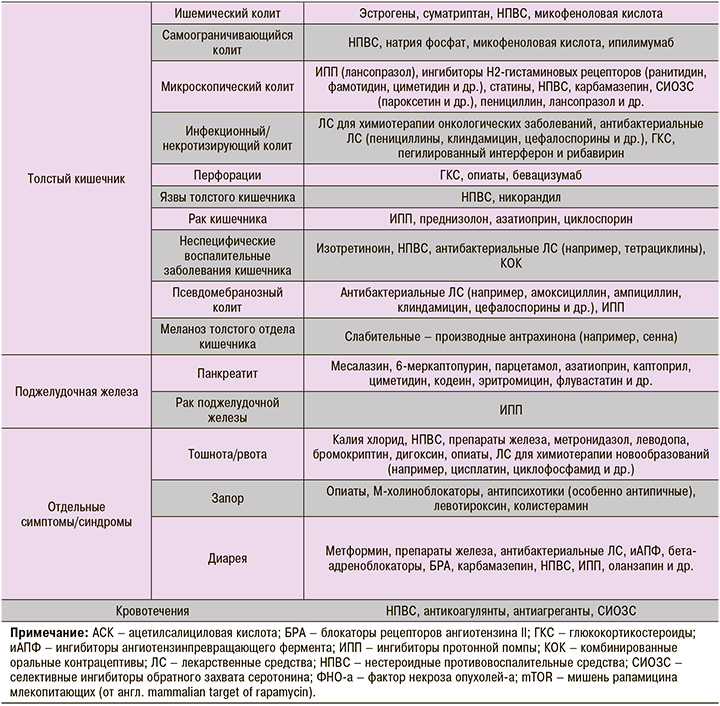
Точная частота развития лекарственно-индуцированного колита не известна. В 10% всех случаев колита причиной поражения толстого кишечника становятся НПВС [81–82].
В основе патогенеза развития лекарственно-индуцированного колита лежит прямое цитотоксическое действие ЛС, нарушение синтеза простагландинов (например, на фоне приема НПВС), иммунологический ответ, изменение микробиоты толстого отдела кишечника [83].
Клиническая картина лекарственно-индуцированного колита не специфична, зависит от типа поражения и может складываться из диареи или запора, наличия крови в стуле, болей по ходу толстого кишечника [81–83]. Необходимо подчеркнуть, что колит может осложняться прободениями стенки кишки, кровотечениями, инфекциями, перитонитом и другими осложнениями [81–83].
Тактика лечения зависит от типа поражения и может включать хирургические методы, антибиотикотерапию и применение симптоматических средств (слабительные или противодиарейные препараты) [81–83].
МИКРОСКОПИЧЕСКИЙ КОЛИТ
Микроскопический колит (МиК) – воспалительное заболевание кишечника, этиология которого не известна. Клиническая картина его складывается из хронической диареи водянистой консистенции, наличия характерных патоморфологических изменений (утолщения субэпителиальной коллагеновой выстилки, увеличение количества интраэпителиальных лимфоцитов – более 20 интраэпителиальных лимфоцитов/100 эпителиоцитов), дегенерации поверхностного эпителия, повышения содержания лимфоцитов и плазмоцитов в собственной пластинке слизистой оболочки при отсутствии макроскопических признаков поражения толстой кишки [84]. Выделяют две формы МиК – коллагенозный (КК) и лимфоцитарный колит (ЛК). Распространенность МиК оценивается в 103 случая (42 – КК, 69 – ЛК) на 100 000 населения, однако некоторыми авторами [85, 86] при этом отмечается тенденция к возрастанию его распространенности и заболеваемости [84].
В ряде исследований выявлена ассоциация развития МиК с приемом лекарственных препаратов [84, 87] – НПВС, ИПП, блокаторов H2-рецепторов, статинов, β-блокаторов, ингибиторов обратного захвата серотонина [87–92]. В исследовании Fernandez-Banares F. et al. [93] обнаружен повышенный риск развития КК у пациентов, принимавших лансопразол (отношение шансов (ОШ) 6,4), низкие дозы АСК (ОШ 3,8), β-блокаторы (ОШ 3,6), а также повышенная вероятность ЛК у больных, лечившихся серталином (ОШ 17,5), омепразолом (ОШ 2,7) и низкими дозами АСК (ОШ 4,7) [93]. Предполагают, что лекарственно-индуцированные формы МиК – результат идиосинкразии [94]. Центральными патогенетическими звеньями развития заболевания считают повреждение эндотелия и/или активацию иммунного ответа [84].
НЕСПЕЦИФИЧЕСКИЕ ВОСПАЛИТЕЛЬНЫЕ ЗАБОЛЕВАНИЯ КИШЕЧНИКА
Неспецифические воспалительные заболевания кишечника (ВЗК) – группа идиопатических хронических воспалительных патологий кишечника, основными представителями которых являются болезнь Крона (БК) и язвенный колит (ЯК) [52].
Точная этиология и патогенез этих заболеваний до сих пор остаются не до конца изученными. Предложен ряд теорий, согласно которым вследствие воздействия различных этиологических факторов (инфекции, генетическая предрасположенность и др.) запускается механизм развития аутоиммунного воспаления в стенке кишечника.
При этом некоторые ЛС также могут вызывать/провоцировать обострение или усугублять ВЗК. Например, первые сообщения о развитии изотретиноин-индуцированных ВЗК датируются 1986 г. [52]. По данным Crockett S.D. et al. [95], применение изотретиноина может быть ассоциировано с риском развития ЯК. Учеными было проведено исследование типа «случай–контроль с использованием базы данных страховых компаний, в которой содержалась информация о 8189 пациентах с ВЗК (3664 БК, 4428 ЯК и 97 ВЗК неуточненного типа), составивших группу наблюдения, и о 21 832 лицах без ВЗК, вошедших в группу контроля. В общей сложности было выявлено 24 пациента в группе наблюдения и 36 в группе контроля, которые получали изотретиноин. Установлено, что пациенты с ЯК получали это ЛС в период от 12 (ОШ=4,36) до 24 мес (ОШ = 2,90) до постановки диагноза, при этом отмечалось увеличение риска развития ВЗК пропорционально дозе препарата (увеличение ОШ на 1,50 с увеличением дозировки на каждые 20 мг) [95].
К другим ЛС, прием которых потенциально может быть ассоциирован с риском развития ВЗК, относят НПВС, комбинированные оральные контрацептивы и антибактериальные ЛС [52]. Так, прием оральных контрацептивов у женщин ассоциируется с увеличением риска развития БК и ЯК (относительный риск от 1,5 до 2,5) [96].
ИШЕМИЧЕСКИЙ КОЛИТ
Ишемический колит (ИК) – воспалительные изменения толстой кишки, обусловленные нарушением кровоснабжения кишечной стенки [97–102]. У лиц пожилого и старческого возраста ИК встречается не менее чем в трети всех случаев воспалительных изменений толстого отдела кишечника [97–101].
Клиническими проявлениями ИК выступают боли в животе, периодические кишечные кровотечения, неустойчивый стул (чередование запоров и поносов), вздутие живота, повышение температуры тела. Выделяют острый и хронический, обратимый и необратимый ИК [102].
Характерными гистологическими паттернами ИК являются уменьшение и расширение крипт, гиалинизированная собственная пластинка слизистой с небольшим количеством воспалительных клеток, фибриновые тромбы в мелких сосудах, наличие поверхностных язв слизистой и в ряде случаев формирование псевдомембран [14].
В большинстве случаев причинами возникновения ИК служат нарушение кровотока вследствие атеросклероза или окклюзии сосудов толстого отдела кишечника, хронические заболевания, сопровождающиеся гипотонией (хроническая сердечная недостаточность, аневризма брюшной части аорты, шок, инсульты, массивные кровотечения и др.), дегидратация, повышение внутрикишечного давления и другие факторы [97–101]. Однако в литературе имеется описание клинических случаев развития лекарственно-индуцированного ИК. Так, c повышенным риском развития ИК ассоциирован прием комбинированных оральных контрацептивов, опиатов и противомигренозных средств (например, суматриптана) [14].
ПСЕВДОМЕМБРАНОЗНЫЙ КОЛИТ
Псевдомембранозный колит (ПМК) – разновидность колита, преимущественно вызываемого грамположительной анаэробной бактерией Clostridium difficile (C. difficile) [103]. Характерный признак этой патологии – фибринозные наложения на слизистой оболочке толстой кишки. Как правило, ПМК возникает из-за нарушения кишечного микробиома с его последующей избыточной колонизацией C. difficile и продуцированием ею токсинов (А (TcdA), В (TcdB) и бинарного токсина), которые вызывают воспаление и повреждение толстого отдела кишечника [103].
ПМК является достаточно распространенной с тенденцией к увеличению заболеваемости, что может быть связано с широким применением антибактериальных средств. Так, в США отмечено статистически значимое (р <0,01) увеличение заболеваемости ПМК на 24% (с 34,1 до 42,3 случаев на 100 тыс. населения), при этом наиболее часто им болеют пациенты старше 65 лет (163,18 случаев на 100 тыс. населения) [103].
Основной причиной развития ПМК, как уже было отмечено, служит нерациональное и бесконтрольное применение антибактериальных ЛС, особенно широкого противомикробного спектра (например, амоксициллина, ампициллина, клиндамицина, цефалоспоринов и др.). Также с нарушением кишечного микробиома и риском развития ПМК может быть ассоциировано длительное применение ИПП.
ЛЕКАРСТВЕННО-ИНДУЦИРОВАННЫЙ ПАНКРЕАТИТ
Это достаточно редкое (0,1–2% всех случаев панкреатита) осложнение фармакотерапии [104], которое может быть ассоциировано с применением месалазина, 6-меркаптопурина, парацетамола, азатиоприна, каптоприла, циметидина, кодеина, эритромицина, флувастатина и ряда других ЛС (см. табл.). Клинические проявления панкреатита – интенсивная боль в эпигастральной области, иррадиирующая в спину, или опоясывающего характера, которая сопровождается эпизодами многократной рвоты и напряжением мышц в верхней половине живота [105].
Острый лекарственно-индуцированный панкреатит может осложниться появлением псевдокист поджелудочной железы, панкреонекрозом, инфицированием бактериальной флорой, тромбозом вен селезенки и другими осложнениями [104, 105].
Выделяют несколько патогенетических механизмов развития лекарственного панкреатита: обтурация протока поджелудочной железы, цитотоксическое действие ЛС на клетки поджелудочной железы, накопление в клетках поджелудочной железы токсических метаболитов, реакция гиперчувствительности, лекарственно-индуцированная гипертриглицеридемия и длительно существующая гиперкальциемия, образование отека в области поджелудочной железы и тромбоз артерий поджелудочной железы [104].
Для лечения лекарственно-индуцированного панкреатита следует отменить препарат, ставший причиной его развития, рассмотреть необходимость и возможность назначения зондирования и аспирации желудочного содержимого, местную гипотермию (холод на живот), применение ИПП, анальгетиков и спазмолитиков, провести дезинтоксикационную терапию и коррекцию электролитных нарушений (при необходимости), а также разработать индивидуальный план нутритивной поддержки [105].
Специфической профилактики лекарственно-индуцированного панкреатита не существует. С этой целью возможно рекомендовать лечение и профилактику состояний, которые потенциально могут предрасполагать к его появлению (например, нарушения липидного профиля), отказ от курения и алкоголя, употребления в пищу чрезмерно жирной пищи и т.д., а также регулярный контроль количества амилазы в сыворотке крови.
ЗАКЛЮЧЕНИЕ
Таким образом, в данной статье были рассмотрены основные типы и патофизиологические механизмы поражения ЖКТ на фоне ЛС. Очевидно, что эта проблема крайне актуальна для специалиста практического здравоохранения и требует более глубоко изучения научными сотрудниками ввиду большого количества белых пятен, в частности механизмов развития поражения, факторов риска и подходов к диагностике и профилактике.



