ВВЕДЕНИЕ
Контроль безопасности применения лекарственных препаратов является стратегической целью национальной безопасности в области здравоохранения и охраны здоровья нации. Своевременное выявление и регистрация нежелательных реакций (НР) – важная составляющая в реализации этой стратегии. Правилами надлежащей практики фармакологического надзора Евразийского экономического союза регламентируется пострегистрационная оценка профиля безопасности и эффективности всех лекарственных препаратов, поступивших в гражданский оборот [1].
Согласно ч. 3 ст. 64 Федерального закона от 12.04.2010 № 61-ФЗ «Об обращении лекарственных средств» субъекты обращения лекарственных препаратов (медицинские организации) обязаны сообщать об их НР [2]. Врач любой специальности, выявивший НР при использовании лекарственного средства, обязан не только ее купировать, но и зарегистрировать в медицинской документации в соответствии с действующим законодательством. Все спонтанные сообщения о НР сводятся в единую базу данных автоматизированной информационной системы Росздравнадзора «Фармаконадзор 2.0». База пополняется не только врачами медицинских организаций, но и уполномоченными по фармаконадзору фармацевтических компаний.
Для чего необходимо сообщать о НР?
Полученные данные подвергаются анализу, составляются периодические отчеты по безопасности лекарственных препаратов, что в конечном итоге может послужить основанием для внесения новой информации в инструкции по медицинскому применению, а также для проведения других мероприятий по управлению рисками, связанными с применением препарата. Кроме того, по заключению Федеральной службы по надзору в сфере здравоохранения Минздравом России могут инициироваться доклинические и/или клинические исследования лекарственных препаратов, приостанавливаться их применение или отменяться государственная регистрация [1].
Несмотря на простоту и доступность предоставления информации, в региональные центры мониторинга безопасности лекарственных средств по всей стране или напрямую в автоматизированную информационную систему Росздравнадзора «Фармаконадзор 2.0» от врачей поступает недостаточное количество таких сообщений. Чувашский региональный центр мониторинга безопасности лекарственных средств, функционирующий с 01.09.2008, не является здесь исключением: на 01.01.2022 им было получено 2980 спонтанных сообщений о НР.
Опрос 360 врачей из 41 медицинской организации Чувашской Республики, проведенный нами в 2017 г., показал, что медицинские работники осведомлены о необходимости заполнения извещений о нежелательной реакции или отсутствии терапевтического эффекта лекарственного препарата согласно действующим нормативным документам. Однако свою низкую активность при регистрации НР в первичной медицинской документации врачи объясняют загруженностью бумажной работой, сомнениями в наличии НР, недооценке важности проблемы, боязнью показаться некомпетентным при оформлении извещения [3, 4]. Предварительные результаты повторного опроса, начатого нами в 2022 г., свидетельствуют о том, что основные причины низкой активности врачей остаются прежними. Однако имеются и положительные моменты. В частности, анализ результатов анкетирования 49 врачей Республиканского онкологического диспансера продемонстрировал, что если в 2017 г. только 15% респондентов, столкнувшихся в своей практике с НР, подавали извещение, то в 2022 г. были зафиксированы абсолютно все случаи выявленных НР. Каждый второй опрошенный врач хотя бы один раз подавал извещение о подозреваемой НР.
Ежегодно в рамках реализации национальной программы «Борьба с онкологическими заболеваниями» в России регистрируется и вносится в перечень жизненно необходимых и важнейших лекарственных препаратов ряд противоопухолевых средств, преимущественно для проведения таргетной терапии и иммунотерапии. Противоопухолевые лекарственные препараты (ПЛП) относятся к средствам, имеющих достаточно широкий спектр НР: это гепато-, нефро-, гемато-, кардиотоксичность, фебрильная нейтропения, аллергические и кожные реакции и др.
По классификации Всемирной организации здравоохранения (ВОЗ), все НР делят на несколько типов. Тип А – дозозависимые НР: токсичность, связанная с передозировкой или взаимодействием лекарственных средств, вторичные НР. Тип В – НР независимые от дозы: НР неизвестного механизма, лекарственная непереносимость, идиосинкразии, гиперчувствительность (иммунологическая), псевдоаллергические реакции (неиммунологические). К типу С относят НР, развивающиеся вследствие длительной терапии: толерантность, зависимость, синдром отмены, кумулятивные эффекты, эффекты подавления выработки гормонов. Тип D – это так называемые отсроченные НР: канцерогенность, эмбриотоксичность, мутагенность, нарушение репродуктивной функции. НР типа E не зависят от дозы – это синдром отмены, реакции, возникающие при прекращении приема лекарства. Наконец, непредсказуемая неэффективность лечения относится к НР типа F [5].
Для определения степени достоверности причинно-следственной связи возникновения НР с приемом лекарственного препарата предлагается использовать классификацию и критерии, разработанные ВОЗ. По этой классификации выделяют 6 степеней достоверности связи «лекарство – НР»: определенная, вероятная, возможная, сомнительная, условная, неклассифицируемая. Кроме того, рекомендуется проводить определение степени достоверности причинно-следственной связи «лекарство – НР» с помощью алгоритма Наранжо. Он состоит из 10 вопросов, ответы на которые оцениваются в баллах. Сумма 9 и более баллов характеризуют определенную связь, 5–8 баллов – вероятную, 1–4 балла – возможную. Результат 0 и менее баллов свидетельствует о сомнительной связи [6].
Цель исследования – изучить структуру НР при применении ПЛП по Чувашской Республике.
МАТЕРИАЛ И МЕТОДЫ
В основу работы был положен ретроспективный анализ 2980 спонтанных сообщений о НР, поступивших за период с 01.09.2008 по 01.01.2022 в Чувашский региональный центр мониторинга безопасности лекарственных средств. База «Фармаконадзор 2.0» пополнялась не только врачами медицинских организаций региона, но и уполномоченными по фармаконадзору фармацевтических компаний. Регистрации подлежали случаи, регламентированные Порядком осуществления фармаконадзора [7].
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
За истекший период было зарегистрировано 79 спонтанных сообщений о НР при применении ПЛП, что составляет 2,65% от общего числа всех зарегистрированных извещений. Начиная с 2016 г. число сообщений увеличивается (табл. 1), что связано как с некоторым повышением активности врачей, так и с более широким применением ПЛП.

Зафиксировано 38 НР при использовании ингибиторов ароматазы (анастрозол, эксеместан), 5 – при применении моноклональных антител (цетуксимаб, трастузумаб, трастузумаб эмтанзин и комбинация трастузумаба с пертузумабом), по 3 случая НР на антиэстрогены (тамоксифен) и таксаны в комбинации с препаратами платины (паклитаксел и карбоплатин). В 13% случаев НР развились при применении комбинаций ПЛП.
Чаще всего документировалась кожная токсичность в виде зудящих дерматозов различной степени выраженности (83%). Кардиоваскулярная токсичность, составляющая 8% от всех извещений о НР при применении ПЛП, была представлена в виде тахикардии, артериальной гипертензии или снижения контроля за артериальным давлением, болями в прекардиальной области, возникновением внутрижелудочковой блокады. В двух извещениях сообщалось о развитии инфаркта миокарда (однако в обоих случаях причинно-следственная связь относилась к категории сомнительной).
Реже фиксировалась гематологическая токсичность (2%) в виде лейкопении, в 3% извещений сообщалось о фебрильной нейтропении. По 2% сообщений пришлось на анафилактическую реакцию и гепатотоксичность. Не было зарегистрировано случаев нефротоксичности, иммуноопосредованных НР, экстравазаций ПЛП. Полный спектр НР, зарегистрированных при применении ПЛП за изучаемый период, представлен в таблице 2.

Как видно из таблицы 2, за истекший период было 5 сообщений о смерти, 4 из них от уполномоченных по фармаконадзору фармацевтических компаний. Извещения оформлялись по медицинским документам, предоставленным родственниками умерших пациентов. Хотя причинно-следственная связь в этих случаях представляется сомнительной, а смерть наступила от прогрессирования основного заболевания, уполномоченный по фармаконадзору фармацевтической компании, на горячую линию которых поступило обращение от родственников умершего пациента, обязаны оформить извещение о НР.
Пациент, получающий терапию ПЛП, курируется не только онкологом, но находится еще и под наблюдением терапевта или врача общей практики (семейного врача) в прикрепленной медицинской организации. В обязанности этих специалистов входят оформление рецептов на ПЛП в рамках льготного лекарственного обеспечения, мониторинг безопасности проводимой терапии, профилактика и коррекция развития НР при их применении и своевременная подача извещения в случае возникновения НР.
Ежегодно на открытом интернет-портале Российского общества клинической онкологии обновляются и публикуются в свободном доступе рекомендации по обеспечению безопасности лекарственной противоопухолевой терапии, сопроводительной и поддерживающей терапии, с основными положениями которых должны быть знакомы врачи всех специальностей, курирующих пациентов онкологического профиля [8]. В частности, определен перечень минимальных лабораторных и инструментальных обследований перед началом противоопухолевой лекарственной терапии. В дальнейшем при необходимости химиотерапии или таргетной терапии не более чем за 5 дней до проведения очередного ее курса пациенту должен быть выполнен клинический анализ крови (гемоглобин, эритроциты, лейкоциты с подсчетом лейкоцитарной формулы, нейтрофилы, тромбоциты). Не ранее чем за 7 дней до очередного курса терапии выполняется биохимический анализ крови (глюкоза, общий билирубин, печеночные трансаминазы, общий белок, креатинин). Если схема лечения предполагает еженедельное введение ПЛП, то биохимический анализ крови может выполняться каждые 3–4 нед, а остальные анализы – по клиническим показаниям. При использовании препаратов с минимальной токсичностью, таких как антиэстрогены, ингибиторы ароматазы, агонисты гонадотропин-рилизинг-гормона, общий и биохимический анализы контролируют реже – до 1 раза в 2 мес. Согласно последней версии практических рекомендаций, рутинно выполнять контрольный общеклинический анализ крови ранее чем через 7 дней после начала курса цитостатической терапии, если отсутствуют клинические показания, нецелесообразно. В процессе химиотерапии и /или таргетной терапии 1 раз в 3–4 мес показано проведение электрокардиографии [9].
За изучаемый период подавляющее большинство НР (71%) при применении ПЛП выявлено на амбулаторном этапе именно врачами-терапевтами. Во всех случаях это были пациенты, находящиеся на льготном лекарственном обеспечении. Терапевт, прежде чем выписать рецепт на ПЛП, назначает минимальный объем клинических, лабораторных и инструментальных исследований, оценивает полученные результаты и соотносит их с получаемым пациентом лечением.
Гепатотоксичность – одно из проявлений органной цитотоксичности ПЛП. С гепатотоксическими реакциями сталкиваются не только онкологи, но и прежде всего терапевты и/или врачи общей практики (семейные врачи). Именно эти специалисты наблюдают пациента в перерывах между курсами химиотерапии.
В соответствии с существующими клиническими рекомендациями обширную доказательную базу для коррекции гепатотоксичности на фоне использования ПЛП имеет S-аденозилметионин [10–13]. Необходимо помнить, что биодоступность этого лекарственного средства зависит от пути введения: так, при парентеральном применении S-аденозилметионина она составляет 95%, в то время как при приеме внутрь не превышает 5%. В связи с этим рекомендуется назначение S-аденозилметионина в дозе 800 мг/сут парентерально в течение 2 нед с последующим переходом на пероральный прием по 800–1600 мг/сут. Общая длительность терапии зависит от динамики биохимических показателей и может варьировать от 3 до 6 мес. Эффективность терапии S-аденозилметионином необходимо оценивать по степени снижения уровня печеночных аминотрансфераз [11].
При холестатическом/смешанном варианте лекарственного поражения печени возможно совместное назначение S-аденозилметионина с препаратами урсодезоксихолевой кислоты [10–13].
Согласно последней версии клинических рекомендаций Российского общества клинической онкологии (RUSSCO) по коррекции гепатотоксичности, индуцированной противоопухолевой терапией (2021), Общества специалистов поддерживающей терапии в онкологии (RASSC), а также клиническим рекомендациям по лекарственному поражению печени ведущих медицинских профессиональных сообществ России для профилактики и лечения лекарственных поражений печени, в онкологической практике рекомендуется к применению препарат состава инозин + меглюмин + метионин + никотинамид + янтарная кислота (Ремаксол) в дозе 400–800 мл/сут внутривенно капельно в течение 3–12 дней при различных типах поражения печени [10–13]. Для профилактики гепатотоксичности препарат вводят 400 мл внутривенно капельно 1 раз/сут в количестве не менее 4 инфузий; для лечения – 400 мл внутривенно капельно 2 раза/сут не менее 4 дней после каждого курса химиотерапии [11]. В научной литературе опубликованы данные, доказывающие эффективность и безопасность применения препарата в онкологии. В частности, профессор О.М. Конопацкова с соавт. оценивали эффективность Ремаксола как средства терапии поддержки при лечении ПЛП у больных раком молочной железы (n=400), колоректальным раком (n=175), раком мочевого пузыря (n=89), меланомой кожи (n=86) [14, 15].
В клинических рекомендациях Общества специалистов поддерживающей терапии в онкологии (RASSC) определены лекарственные средства, при назначении которых требуется профилактическое применение препарата состава инозин + меглюмин + метионин + никотинамид + янтарная кислота (Ремаксол). К ним относят доцетаксел, эрлотиниб, гемцитабин, иматиниб, иринотекан, паклитаксел, сорафениб, топотекан, винорелбин [12].
Своевременное выявление и лечение НР может позволить не изменять или вносить минимальные коррективы в схемы лечения онкологического заболевания.
В качестве иллюстрации вышесказанного приводим два клинических наблюдения.
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ № 1
Пациентка Н., 55 лет. Проживает за пределами Чувашской Республики. Диагноз «рак правой молочной железы люминальный В, HER2-негативный вариант, Т1N3M0, радикальная резекция правой молочной железы с одномоментной пластикой широчайшей мышцей спины от 04.2020».
Данные анамнеза: в послеоперационном периоде получила 4 курса адъювантной полихимиотерапии по схеме АС (доксирубицин + циклофосфамид), затем 4 курса доцетаксела, 11 сеансов лучевой терапии. После завершения лучевой терапии с декабря 2020 г. принимает анастрозол.
В июне 2021 г. пациентка стала отмечать слабость. Поскольку в это время она находилась на территории Чувашии, обратилась терапевту по месту пребывания. В биохимическом анализе крови было выявлено повышение уровня аланинаминотрансферазы (АЛТ) до 76 Ед/л, аспартатаминотрансферазы (АСТ) – до 68 Ед/л, общего билирубина – до 24 мкмоль/л. Маркеры холестаза (щелочная фосфатаза, гаммаглутамилтраспептидаза) находились в пределах референсных значений.
С учетом алгоритма диагностики данное состояние было расценено как лекарственное поражение печени, гепатоцеллюлярный вариант, легкой степени тяжести [11–13].
В соответствии с протоколом RUSSCO пациентке в условиях дневного стационара было проведено лечение препаратом состава инозин + меглюмин + метионин + никотинамид + янтарная кислота (Ремаксол) 400 мл внутривенно капельно, всего 4 инфузии. На фоне проведенной терапии улучшилось самочувствие, нормализовались биохимические анализы, лечение основного заболевания продолжалось в прежнем объеме.
В приведенном случае НР относится к типу А, причинно-следственная связь расценивается как возможная, оценка по шкале Наранжо – 4 балла.
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ № 2
Пациентка П., 66 лет. Диагноз «рак левого яичника (папиллярная аденокарционома)».
Данные анамнеза: в ноябре 2015 г. пациентке была выполнена тотальная гистерэктомия с опухолью левого яичника, придатками, селективной подвздошной лимфодиссекцией, резекция большого сальника и клиновидная резекция печени. Поле операции она получила 6 курсов адьювантной химиотерапии (паклитаксел, карбоплатин). С октября 2016 г. проведено 6 курсов химиотерапии (паклитаксел + бевацизумаб), далее продолжение таргетной терапии бевацизумабом. По данным компьютерной томографии процесс стабилизировался, однако в связи с гематологической токсичностью III степени было решено приостановить дальнейшее проведение химиотерапии. В ходе динамического наблюдения в феврале 2018 г. было документировано прогрессирование онкологического процесса. В течение 2018 г. было проведено 7 курсов химиотерапии паклитакселом + карбоплатином, 3 курса цисплатином + паклитакселом (последний – 23.07.2019).
Состояние пациентки ухудшалось, появились признаки частичной кишечной непроходимости. В ходе стационарного лечения в Республиканском онкологическом диспансере явления кишечной непроходимости удалось разрешить консервативно, осуществлялась посиндромная терапия.
С 20.09.2019 было принято решение возобновить химиотерапию (гемцитабин + бевацизумаб). Перед повторным курсом в общем анализе крови от 07.10.2019 отмечались лейкопения, анемия легкой степени, тромбоцитопения (табл. 3).
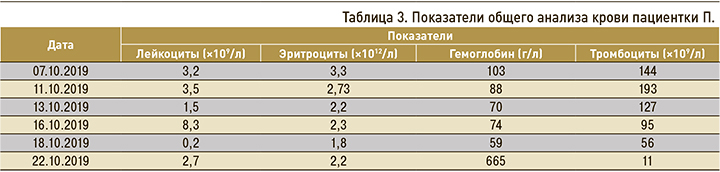
Пациентке была назначена симптоматическая терапия и 09.10.2019 проведена химиотерапия (гемцитабин 1650 мг + бевацизумаб 465 мг), которую пациентка перенесла удовлетворительно. Кроме этого, была начата ферротерапия в сочетании с введением колониестимулирующего фактора (лейкостим), дополнительное питание (фрезубин). Однако по общему анализу крови у пациентки была документирована анемия тяжелой степени, лейкопения 4 степени, тромбоцитопения 3 степени.
В условиях реанимационного отделения продолжалась интенсивная терапия: колониестимулирующие факторы, переливание эритромассы, 5 доз тромбоконцентрата, свежезамороженная плазма, альбумин, осуществлялась анибактериальная и противогрибковая терапия, вводились аминокислоты, выполнялись дезинтоксикация, гастро-, гепато- и нейропротекция, в том числе с применением сукцинатсодержащих препаратов. Для купирования гипертермического синдрома использовали парацетамол.
21.10.2019 у пациентки была зафиксирована клиническая смерть с последующим восстановлением сердечной деятельности. 24.10.2019 после безуспешных реанимационных мероприятий была констатирована биологическая смерть.
На секции обнаружено: канцероматоз брюшины и диафрагмы – на париетальной и висцеральной брюшине, плевральной и брюшной поверхности диафрагмы определяются плотные очаги белесоватого цвета размерами 0,1–0,2 см (гистологически – метастатический рост аденокарциономы). Метастазы в печень – на разрезе множественные опухолевидные образования округлой формы в диаметре до 3,0 см, метастазы в забрюшинные лимфатические узлы (гистологически – метастатический рост аденокарциономы). Кишечные спайки с непроходимостью. Разлитой серозно-геморрагический перитонит, тромбоэмболия мелких ветвей легочной артерии, правостороння пневмония с локализацией в нижней доле, двусторонний гидроторакс, стенозирующий атеросклероз церебральных артерий. Причиной смерти следует считать полиорганную недостаточность.
Выявленная в данном клиническом случае НР относится к типу А, причинно-следственная связь – возможная, оценка по шкале Наранжо – 4 балла.
ЗАКЛЮЧЕНИЕ
Несмотря на увеличение потребления ПЛП, значительного повышения частоты регистраций НР в Чувашской Республике не выявлено, при этом имеются положительные тенденции. Основное количество сообщений о НР при использовании ПЛП подано врачами-терапевтами амбулаторного звена, которые куририруют пациентов онкологического профиля в медицинских организациях по месту жительства. Необходимо осуществлять дальнейшую работу по повышению мотивации врачей не только к своевременному выявлению НР при использовании лекарственных препаратов, в том числе и противоопухолевых, но и к их регистрации в медицинской документации и подаче извещений.



