АНЦА-ассоциированные васкулиты (ААВ) относятся к системным аутоиммунным заболеваниям, которые характеризуются некротизирующим малоиммунным воспалением стенок преимущественно мелких сосудов и циркуляцией антител к цитоплазме нейтрофилов (АНЦА), направленных против миелопероксидазы (МПО-АНЦА) или протеиназы-3 (Пр3-АНЦА) [1]. Этиологический фактор, приводящий к развитию заболевания, не установлен, однако в различных исследованиях обсуждается роль генетической предрасположенности, инфекционных заболеваний, применения некоторых лекарственных препаратов, воздействия факторов внешней среды [2–6].
В основе патогенеза ААВ лежит комплексное нарушение врожденного и приобретенного иммунитета, а именно прайминг нейтрофилов и дальнейшая их активация в результате взаимодействия с АНЦА, активация системы комплемента преимущественно по альтернативному пути и нарушение регуляции Т-лимфоцитов. Все это в результате приводит к повреждению сосудистого эндотелия в различных органах и тканях.
Частота поражения органов и систем при данном заболевании зависит от его нозологической формы и типа циркулирующих АНЦА [7]. К наиболее частым органам-мишеням, определяющим тяжесть болезни и прогноз пациента, относятся легкие и почки. В отсутствие раннего начала лечения прогрессирующее течение ААВ влечет за собой быструю утрату функций вовлеченных органов, что оказывает существенное негативное влияние на выживаемость пациентов [8–10].
Этиотропная терапия ААВ в настоящее время не разработана. Патогенетическое лечение представлено комбинациями различных иммуносупрессивных препаратов. Целью лечения является купирование патологического иммунного ответа и длительное поддержание ремиссии заболевания, позволяющей сохранить качество жизни пациента. Исходя из этого в лечении ААВ выделяют две фазы, требующие различных подходов, – индукцию ремиссии и противорецидивную терапию. Кроме того, методы терапии ААВ зависят от тяжести клинических проявлений в дебюте заболевания.
За последнее десятилетие опубликованы данные ряда исследований, позволяющие оптимизировать существующие подходы к лечению ААВ и профилактике осложнений заболевания и проводимой терапии. Необходимо отметить, что в большинство исследований включались больные гранулематозом с полиангиитом и микроскопическим полиангиитом, а наличие у пациента диагноза эозинофильного гранулематоза с полиангиитом часто служило критерием исключения.
Далее в статье приводятся современные принципы лечения ААВ на основании клинических рекомендаций Европейского общества ревматологов и Европейской ассоциации нефрологов [11].
ИНДУКЦИЯ РЕМИССИИ ПРИ ЖИЗНЕУГРОЖАЮЩИХ ОСЛОЖНЕНИЯХ
В качестве индукционной терапии при тяжелом течении ААВ рекомендуется применять комбинацию глюкокортикостероидов и циклофосфамида или ритуксимаба [11].
Сочетанная терапия высокими дозами глюкокортикостероидов (ГКС) и циклофосфамида (ЦФ) применяется для индукции ремиссии ААВ с 1970- х гг. [12]. В прошедшие десятилетия наиболее используемой схемой индукционной терапии был прием ЦФ перорально в течение как минимум 1 года и далее при каждом рецидиве ААВ. Однако длительный пероральный прием ЦФ ассоциирован с развитием отсроченных нежелательных явлений, в первую очередь геморрагического цистита, рака мочевого пузыря, миелодисплазии, а также нарушений репродуктивной функции [13, 14]. Это создало предпосылки для поиска стратегий, позволяющих уменьшить кумулятивную дозу ЦФ, – препарат стали вводить внутривенно в режиме пульс-терапии.
В крупном рандомизированном исследовании CYCLOPS показана сопоставимая эффективность двух схем терапии: внутривенного введения ЦФ в режиме пульс-терапии в дозе 15 мг/кг (3 введения каждые 2 нед, затем каждые 3 нед до достижения ремиссии) или перорального приема ЦФ в дозе 2 мг/кг/сут до достижения стойкой ремиссии [15]. После 9 мес индукционной терапии доля пациентов, достигших ремиссии (88,1 и 87,7% соответственно), общая и почечная выживаемость были сопоставимы в данных группах. Однако, вследствие того что внутривенное введение позволяет снизить кумулятивную дозу ЦФ, такой режим введения был ассоциирован с достоверно более низким риском развития лейкопении. При длительном (> 4 лет) проспективном наблюдении исследуемой выборки обнаружено, что риск обострений выше в группе пациентов, получавших внутривенное введение ЦФ, по сравнению с получавшими препарат перорально. Тем не менее различий общей выживаемости между группами выявлено не было, а вероятность развития осложнений оказалась значимо ниже в группе больных, которым проводились инфузии ЦФ [16].
Вместо ЦФ для индукции ремиссии ААВ возможно применение ритуксимаба (РТ), эффективность которого при этом заболевании установлена в двух рандомизированных контролируемых исследованиях – RAVE и RITUXVAS [17, 18]. В обеих работах продемонстрирована сопоставимая эффективность применения РТ в дозе 375 мг/ м2 № 4 с интервалом 7 дней и ЦФ в стандартной дозе (внутривенно или перорально) с целью индукции ремиссии ААВ. При этом в протоколе RAVE показана более высокая эффективность РТ в сравнении с ЦФ при рецидивах заболевания. Кроме того, в отличие от ЦФ, РТ не оказывает негативного эффекта на репродуктивную функцию, поэтому его применение целесообразно рассматривать в первую очередь у пациентов более молодого возраста.
У больных, у которых в дебюте заболевания развивается диффузное легочное кровотечение и/или тяжелое поражение почек (повышением уровня креатинина >500 мкмоль/л), в том числе диализ-зависимая почечная недостаточность, обсуждается возможность проведения плазмообмена [19, 20]. В исследовании MEPEX выполнение плазмафереза в комбинации со стандартной индукционной терапией ЦФ и ГКС у пациентов с АНЦА-ассоциированным гломерулонефритом и повышением уровня креатинина >500 мкмоль/л показало высокую эффективность в отношении почечной и общей выживаемости на сроках 3 мес и 1 год [21]. При этом, по данным проспективного наблюдения пациентов в течение в среднем 4 лет, применение плазмообмена не привело к снижению почечной и общей выживаемости [22].
Наиболее крупное на настоящий момент рандомизированное клиническое исследование PEXIVAS было посвящено изучению эффективности плазмообмена у пациентов с ААВ и снижением расчетной скоростью клубочковой фильтрации (рСКФ) менее 50 мл/мин/1,73 м2 или диффузным альвеолярным кровотечением [23]. Все пациенты (n=704) получали лечение ЦФ или РТ и были распределены на 4 группы: 1) лечение плазмообменом в сочетании с ГКС в стандартной дозе (1 мг/кг); 2) лечение плазмообменом и ГКС в уменьшенной дозе (50% от стандартной дозы); 3) лечение ГКС в стандартной дозе без плазмообмена; 4) лечение ГКС в уменьшенной дозе без плазмообмена. Частота развития терминальной почечной недостаточности или летального исхода оказалась сопоставимой во всех группах. В пользу использования плазмообмена при АНЦА-ассоциированных васкулита свидетельствуют результаты недавнего метаанализа, который продемонстрировал значимое снижение риска развития терминальной почечной недостаточности в краткосрочной перспективе (3 мес от начала заболевания) у пациентов, получавших плазмообмен [54]. В то же время результаты исследования PEXIVAS дают дополнительные основания для разумного снижения интенсивности и продолжительности терапии ГКС. Таким образом, плазмообмен, по-видимому, не должен входить в стандартный протокол лечения ААВ, однако возможно его персонализированное применение у пациентов с тяжелым поражением почек или диффузным альвеолярным кровотечением в качестве дополнительного инструмента при крайне тяжелом течении заболевания.
ИНДУКЦИЯ РЕМИССИИ У ПАЦИЕНТОВ БЕЗ ЖИЗНЕУГРОЖАЮЩИХ ПРОЯВЛЕНИЙ
В случае среднетяжелого течения ААВ в качестве индукционной терапии рекомендуется применять комбинацию ГКС и метотрексата или микофенолата мофетила [11].
В качестве альтернативной схемы индукционной терапии допустимо применение метотрексата (МТ) перорально в дозе 20–25 мг/нед у пациентов с локализованным и ранним системным ААВ при отсутствии вовлечения почек. В рандомизированном исследовании продемонстрирована сопоставимая эффективность перорального приема этого препарата в дозе 20–25 мг/нед и перорального приема ЦФ в дозе 2 мг/кг/сут у пациентов с ранней системной формой ААВ [24]. Критерием исключения из протокола было наличие тяжелых проявлений ААВ, таких как легочное кровотечение, церебральный васкулит, мононевриты, псевдоопухоль орбиты, миокардит и повреждение почек. Вместе с тем при динамическом наблюдении обнаружено, что риск обострений был выше в группе пациентов, получавших МТ в сравнении с пациентами, применявших ЦФ [25].
Недавно опубликованы результаты двух рандомизированных исследований, в которых сравнивали эффективности микофенолата мофетила (ММФ) и ЦФ для индукции ремиссии при ААВ без жизнеугрожающих проявлений [26, 27]. В обоих продемонстрирована сопоставимая эффективность приема ММФ перорально в дозе 2 г/сут и лечения ЦФ (перорально в дозе 2 мг/кг/сут или внутривенно в дозе 15 мг/кг). Однако в исследовании MYCYC частота развития рецидивов ААВ оказалась выше у пациентов, получавших ММФ, при этом риск обострений заболевания был значимо выше у пациентов с Пр3-АНЦА [27]. Таким образом, еще одной альтернативной схемой лечения у пациентов со среднетяжелым течением ААВ без жизнеугрожающих проявлений заболевания (преимущественно у больных с МПО-АНЦА) может служить применение ММФ в дозе 2 г/сут.
ЛЕЧЕНИЕ ОБОСТРЕНИЙ АНЦА-АССОЦИИРОВАННЫХ ВАСКУЛИТОВ
При развитии рецидивов ААВ с жизнеугрожающими осложнениями рекомендуется применять схемы индукционной терапии – комбинацию ГКС и ЦФ или РТ [11].
В большинстве проводимых исследований больных не подразделяли не тех, у кого ААВ диагностирован впервые, и пациентов с обострением заболевания. В наиболее крупном рандомизированном контролируемом исследовании RAVE продемонстрирована более высокая эффективность РТ в сравнении с ЦФ у пациентов с обострениями ААВ [17]. Кроме того, показано, что в группе больных с Пр3-АНЦА использование РТ ассоциировано с более высокой вероятностью достижения ремиссии через 6 мес от начала индукционного курса, чем применение ЦФ (отношение шансов 2,11; 95% доверительный интервал: 1,04–4,30), в том числе в моделях с поправкой на пол, возраст и характер течения заболевания [28]. В исследовании RITAZAREM, в котором участвовали 188 пациентов с рецидивами гранулематоза с полиангиитом (ГПА) и микроскопического полиангиита (МПА), было установлено, что индукционная терапия РТ в сочетании с ГКС высоко эффективна для лечения обострений ААВ [55].
Необходимо отметить, что выбор ЦФ для лечения обострений ограничен достижением предельной «безопасной» кумулятивной дозы препарата. В одном из исследований показано, что кумулятивная дозировка ЦФ более 36 г ассоциирована со значительным увеличением частоты развития онкологических заболеваний [13]. Исходя из этого, представляется предпочтительным применение РТ для лечения пациентов с тяжелым обострением ААВ.
Лечение пациентов со среднетяжелыми обострениями ААВ без развития жизнеугрожающих осложнений предусматривает увеличение дозы системных ГКС; в большинстве случаев такой подход позволяет достигнуть ремиссии заболевания, но в то же время характеризуется высокой частотой возникновения рецидивов в достаточно короткие сроки [29]. Ввиду отсутствия крупных исследований, изучающих лечение нетяжелых обострений ААВ, представляется возможным использование схем индукционной терапии для данной выборки пациентов.
ЛЕЧЕНИЕ ПРИ РЕФРАКТЕРНОМ ТЕЧЕНИИ АНЦА-АССОЦИИРОВАННЫХ ВАСКУЛИТОВ
У пациентов с рефрактерным течением заболевания необходимо производить замену терапии ЦФ на РТ и наоборот [11].
Под рефрактерным течением ААВ подразумевают:
1) отсутствие изменения или нарастание активности заболевания в течение 4 нед от начала стандартной терапии;
либо
2) снижение менее чем на 50% активности заболевания, оцененной по индексу BVAS через 6 нед от начала стандартной терапии;
либо
3) персистирование одного большого или трех малых критериев активности в течение 8 нед от начала лечения [30].
В исследованиях продемонстрирована эффективность применения РТ при рефрактерном течении ААВ после стандартной индукционной терапии ЦФ + ГКС [31, 32]. Другая возможная опция терапии при рефрактерном течении заболевания – переход на пероральный прием ЦФ [33].
ПОДДЕРЖАНИЕ РЕМИССИИ
Для поддержания ремиссии рекомендован прием низких доз ГКС в комбинации с азатиоприном, РТ, МТ или ММФ [11].
Десятилетие назад в качестве поддерживающей терапии использовали ЦФ, длительный прием которого ассоциирован с развитием серьезных нежелательных явлений. Это привело к поиску более безопасных режимов поддерживающей терапии [14, 34, 35].
В протоколе CYCAZAREM продемонстрирована сопоставимая эффективность в отношении профилактики обострений при применении азатиоприна (АЗА) в дозе 2 мг/кг/сут в течение 18 мес или ЦФ в стандартной дозе, при этом АЗА обладал лучшим профилем безопасности [36]. Только у небольшой части пациентов лечение этим препаратом может приводить к тяжелой нейтропении [37].
В исследовании MAINRITSAN выполнено сравнение эффективности поддерживающей терапии АЗА в стандартной дозе в течение 22 мес и РТ в виде инфузий по 500 мг в дни 0 и 14, затем через 6, 12 и 18 мес после проведения индукционной терапии ЦФ в виде внутривенных инфузий [38]. К 28 мес наблюдения тяжелые обострения ААВ были зарегистрированы у 29% больных, получавших поддерживающую терапию АЗА и у 5% пациентов, лечившихся РТ, при сопоставимой частоте развития нежелательных явлений в обеих группах. В протоколе RITAZAREM также было показано превосходство РТ над АЗА в предотвращении рецидивов ААВ в течение 20 мес у пациентов, перенесших индукционную терапию РТ [39].
С целью уменьшения объема иммуносупрессивной терапии в исследовании MAINRITSAN2 было сделано сравнение двух схем поддерживающей терапии РТ у пациентов, получавших индукционную терапию ЦФ [40]. Введение РТ в виде инфузий по 500 мг в дни 0 и 14, а также через 6, 12 и 18 мес не имело преимущества перед лечением РТ в режиме «по требованию», при котором препарат вводили только в случае восстановления популяции B-лимфоцитов и/или повышения либо удвоения титра АНЦА. Кроме того, не было выявлено различий в частоте развития нежелательных явлений при использовании двух схем лечения, несмотря на то, что количество инфузий РТ в группе лечения «по требованию» было меньше, чем в группе стандартной терапии.
Возможность применения ММФ в качестве противорецидивной терапии изучалась в протоколе IMPROVE [41]. У больных, получавших индукционную терапию ЦФ и ГКС, при дальнейшем противорецидивном лечении ММФ в дозе 2 г/ сут зарегистрирована большая частота обострений, чем при поддерживающим лечении АЗА в дозе 2 мг/ кг/сут. При этом частота нежелательных явлений была сопоставима в этих группах.
Поддерживающая терапия МТ в дозе 20–25 мг/ нед после индукционной терапии ЦФ возможна у пациентов с сохранной функцией почек (уровень сывороточного креатинина <130 мкмоль/л) [42]. При проспективном наблюдении в течение 10 лет больных, включенных в протокол WEGENT, была установлена сопоставимая эффективность противорецидивной терапии АЗА в дозе 2 мг/кг и МТ 25 мг/нед при сравнимой частоте нежелательных реакций [43].
ПРОДОЛЖИТЕЛЬНОСТЬ ПОДДЕРЖИВАЮЩЕЙ ТЕРАПИИ
Поддерживающую терапию ААВ рекомендовано продолжать не менее 24 мес [11].
К настоящему времени опубликовано ограниченное число исследований, позволяющих сделать вывод об оптимальной длительности поддерживающей терапии ААВ. В ретроспективном исследовании сделан вывод, что длительное поддерживающее лечение АЗА или МТ (в течение ≥36 мес) ассоциировано с меньшим риском развития обострений ААВ в сравнении с коротким курсом (18– 36 мес) поддерживающей терапии [44]. В проспективном исследовании REMAIN также показано, что длительный курс противорецидивной терапии АЗА в сочетании с низкими дозами ГКС (48 мес) оказался более эффективным, нежели двухгодичный курс лечения [45].
В протоколе MAINRITSAN3 установлено, что более длительный курс поддерживающего лечения РТ (каждые 6 мес на протяжение >18 мес) ассоциирован с достоверно более высокой безрецидивной выживаемостью (96% за 28 мес наблюдения), чем короткий курс (74% на протяжении <18 мес) [46].
Вопрос о длительности поддерживающего лечения ГКС также остается актуальным ввиду высокого риска развития нежелательных явлений при их продолжительном использовании. Данные метаанализа, включившего 13 работ (8 рандомизированных и 5 обсервационных клинических исследований), свидетельствует о том, что длительное лечение низкими дозами системных ГКС оказалось более эффективно в плане профилактики рецидивов ААВ, чем быстрая отмена препарата в течение 12 мес [47]. Однако в различных рандомизированных контролируемых исследованиях продемонстрировано, что сокращение длительности приема или дозы поддерживающей терапии системными ГКС не приводит к увеличению количества рецидивов заболевания [23, 48].
СОПУТСТВУЮЩАЯ ТЕРАПИЯ
Нежелательные явления проводимой иммуносупрессивной терапии являются одной из ведущих причин летального исхода и стойкой утраты трудоспособности пациентов с ААВ. Контролировать их развитие позволяют определенные лекарственные препараты.
Наиболее значимое осложнение цитостатической терапии ААВ – инфекционные заболевания, в большинстве случаев вызванные Pneumocystis jirovecii [49]. К другим факторам риска развития пневмоцистной пневмонии относят возраст старше 55 лет, снижение уровня лимфоцитов <0,3х 109/л и длительное лечение ГКС в дозе >15–20 мг/сут в пересчете на преднизолон. Несмотря на отсутствие исследований по профилактическому лечению, всем болным ААВ, получающим индукционную терапию ЦФ, РТ или ГКС в высокой дозе, представляется целесообразным назначать сульфаметоксазол/триметоприм (ко-тримоксазол) в дозе 400/80 мг/сут ежедневно (при значимом снижении функции почек – через день).
У пациентов с ААВ широко применяются вакцины, за исключением живых. В разных исследованиях продемонстрирована высокая эффективность вакцинации против гриппа при отсутствии ее влияния на развития рецидивов ААВ [50, 51]. Вместе с тем вакцинация, проведенная во время иммуносупрессивной терапии, может оказаться не эффективной [52].
Использование ЦФ ассоциировано с высокой опасностью развития у пациентов геморрагического цистита и переходноклеточного рака мочевого пузыря [13, 35]. Для профилактики этих осложнений рекомендовано применение препаратов 2-меркаптоэтансульфоновой кислоты (месны), которые связывают токсичные метаболиты ЦФ и уменьшают риск повреждения слизистой мочевых путей.
Осложнением длительной терапии ГКС выступает остеопороз, частоту развития которого у пациентов с ААВ возможно контролировать при использовании современных стероидосберегающих стратегий. Больным, которым планируется длительная терапия ГКС, рекомендуют (в отсутствие противопоказаний) прием препаратов кальция, витамина D, по индивидуальным показаниям бисфосфонатов.
Наименее изученными остаются подходы к профилактике венозных тромбоэмболических осложнений у пациентов с ААВ, частота которых существенно превышает показатели в общей популяции [53].
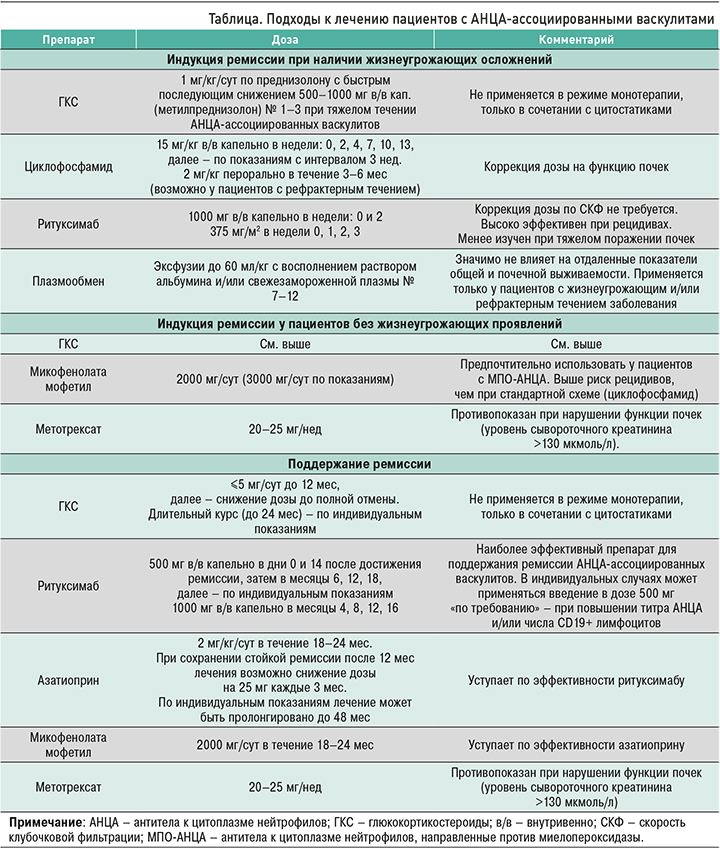
ЗАКЛЮЧЕНИЕ
Опубликованные за последние годы работы позволяют пересмотреть традиционные представления об оптимальных подходах к лечению ААВ. К основным тенденциям можно отнести уменьшение объема и продолжительности индукционной терапии ГКС и цитостатиками, а также возможность подбора схем терапии, основанных на тяжести течения заболевания и серологического профиля пациента. Наиболее общие принципы лечения, применявшиеся в различных исследованиях, представлены в таблице. Многообразие схем терапии, успешно апробированных в рандомизированных клинических исследованиях, с одной стороны, позволяет реализовывать персонализированный подход к ведению пациента, с другой – создает сложную проблему выбора наиболее подходящего препарата. В связи с этим необходимо подчеркнуть важность определения тактики лечения и наблюдения пациентов с ААВ в специализированных экспертных центрах.


