ВВЕДЕНИЕ
Эпидемия ожирения, в патогенетической основе которого лежит инсулинорезистентность (ИР), является серьезной социально-экономической проблемой. Для решения этой проблемы важны ранняя диагностика, профилактика и лечение составляющих метаболического синдрома (МС): ожирения, дислипидемии, артериальной гипертензии (АГ) и сахарного диабета (СД) 2-го типа. В странах с высоким уровнем жизни население перешло к высококалорийному питанию в сочетании со снижением расхода мышечной энергии, результатом чего стало формирование в генетической памяти механизма ИР и развитие МС.
В настоящее время человек постоянно сталкивается с воздействием новых факторов среды, ранее никогда не встречавшихся на протяжении всей его эволюции, а также испытывает большие «нагрузки» социального и экологического характера [1]. Увеличение мутагенной «нагрузки», миграции населения привело к изменению генетической структуры популяций [1].
Цель исследования – изучить влияние полиморфизмов генов на развитие метаболических нарушений среди молодых жителей Севера.
МАТЕРИАЛ И МЕТОДЫ
Проведено проспективное когортное исследование на базе БУ ХМАО–Югра «Федоровская городская больница», филиала больницы в д. Русскинская, БУ ХМАО-Югра «Сургутская городская клиническая поликлиника № 1». Обследованы 883 молодых человека, длительно проживающих в условиях, приравненных к Крайнему Северу (средняя длительность проживания – 27,9±0,005 лет) за период 2015–2020 гг.: 749 с МС и 134 человека без проявлений. Дизайн исследования представлен в таблице 1.

Критерием МС считалось наличие трех из пяти метаболических нарушений: абдоминального ожирения (АО) в виде повышенной окружности талии (ОТ) (в норме у женщин – 80 см, у мужчин – 94 см), АГ (уровень артериального давления >135 и 90 мм рт.ст.), гипертриглицеридемии (≥1,7 ммоль/л), снижения уровня липопротеидов высокой плотности (3,0 ммоль/л), гипергликемии натощак (уровень глюкозы плазмы натощак ≥6,1 ммоль/л) [2].
Молекулярно-генетическое исследование выполнялось в НИИ терапии и профилактической медицины – филиале Федерального исследовательского центра Института цитологии и генетики Сибирского отделения РАН. Геномную ДНК выделяли из венозной крови методом фенол-хлороформной экстракции. Полиморфизм генов тестировали с помощью полимеразной цепной реакции с полиморфизмом длин рестрикционных фрагментов (ПЦР с ПДРФ) [2]. Были изучены следующие полиморфизмы генов: rs1378942 гена CSK, rs1801133 (С677Т) гена MTHFR, гена ITGA2B, rs7903146 гена TCF7L2, rs1799752 гена АСЕ. Однонуклеотидный полиморфизм (SNP) rs1378942 гена CSK локализован на 15q24.1 хромосоме и кодирует изофермент цитохром P450-зависимой монооксигеназы, тирозинкиназы, играет роль в регуляции клеточного роста и дифференцировки нормальных клеток [2–4]. Функция фермента метилентетрагидрофолатредуктазы MTHFR (расположен на хромосоме 1p36.3) связана с нарушениями обмена гомоцистеина, обладающим атерогенным действием. Была изучена частота полиморфизма rs1801133 (С677Т) гена MTHFR [2, 5, 6]. Ген ITGA2B (локализован на хромосоме 17q21.32) – мембранный белок, димерный интегрин, состоящий из альфа цепи αIIb и бета цепи β3, экспрессируется на поверхности тромбоцитов, являясь рецептором фибриногена [2, 7, 8]. Полиморфизм rs7903146 гена TCF7L2 (хромосома 10q25.3) регулирует секрецию проглюкагона, влияющего на секрецию инсулина и на созревание бета-клеток поджелудочной железы из стволовых клеток [2, 9–11]. Регуляция кровяного давления и баланса электролитов, катализ расщепления ангиотензина I до активного ангиотензина II осуществляется с помощью ангиотензинпревращающего фермента (АПФ), который кодируется геном ACE, расположенным на хромосоме 17q23. Нами был изучена частота полиморфизма rs1799752 гена АСЕ [2, 12].
Полученные результаты были статистически обработаны с помощью пакета программ SPSS 16.0. На первом этапе определялись частоты генотипов и аллелей изучаемых полиморфизмов в группе пациентов с МС и в контрольной группе, затем оценивалось соответствие частот генотипов равновесию Харди–Вайнберга в контрольной группе (по критерию χ²). У обоих изучаемых полиморфизмах распределение частоты генотипов соответствовало равновесию Харди–Вайнберга. Относительный риск МС по конкретному аллелю или генотипу вычислялся как отношение шансов (ОШ) с использованием точного двухстороннего критерия Фишера и критерия хи-квадрат по Пирсону. В качестве уровня значимости использовали p <0,05 [2].
У всех пациентов было получено информированное согласие на участие в исследование.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
По результатам антропометрических исследований у пациентов с МС в этнических группах средние значения массы тела и ИМТ не имели выраженных различий. Средние показатели объема талии как основного критерия МС имели гендерные и этнические отличия.
Среди мужчин с МС, имеющих I и II степени ожирения, средние значения ОТ не превышали 104 см. В группе мужчин – некоренных жителей Севера с МС, страдающих III степенью ожирения, средние величины ОТ (136,34±0,002 см; доверительный интервал (ДИ): 103–154 см) на 23,7% превосходили рекомендуемые показатели. Среди мужчин – коренных жителей Севера, имеющих МС, пациентов с III степенью ожирения нами выявлено не было.
Среди всех обследованных женщин средние значения ОТ при I степени ожирения превышали показатели, рекомендуемых в качестве критериев диагностики МС на 13,2%, при II степени – на 22,9%, при III степени – на 35,6 %. У мужчин-хантов средние значения ОТ при I и II степени ожирения были ниже рекомендуемых клинических критериев МС (102 см) и меньше на 16,7 и 12,8% по сравнению с некоренными мужчинами с МС соответственно.
Среднее значение ОТ среди городских мужчин с МС при ожирении I степени превышало данный параметр у сельских мужчин с МС на 11,8%, при ожирении II степени – на 7,3%, при III степени – на 7,5%. Среди городских и сельских жительниц с МС значимые различия по средним значениям ОТ не наблюдались. Средние величины ИМТ у сельских жительниц с МС при III степени ожирения превышали таковые у городских жительниц на 23,6%. В рамках гендерного сравнения антропометрических данных было установлено, что при наличии МС среднее значение ИМТ у городских жительниц было на 10,4% выше, чем у мужчин, живущих в городе; у сельских жительниц этот параметр на 32,4% превышал таковой у мужчин, проживающих в той же местности.
При анализе показателей углеводного обмена у всех молодых людей с МС, включенных в исследование, было выявлено 238 случаев гипергликемии (31,8%), 103 случая гиперинсулинемии (13,8%). Среди некоренных жителей гипергликемия встречалась у 34,0% человек, гиперинсулинемия – у 13,9%, что превышало распространенность этих нарушений в группе коренных жителей на 9,3 и 2,7% соответственно. У жителей города и села значимых отличий в частоте гипергликемии отмечено не было.
Гипергликемия и гиперинсулинемия чаще регистрировались у мужчин, чем у женщин – на 1,6 и 2,8% соответственно. При этом некоренные жители-мужчины имели гипергликемию в 39,2%, а гиперинсулинемию в 16,7% случаях, что превышало аналогичные параметры у некоренных жительниц на 8,0 и 4,3% соответственно. У мужчин, проживающих в городе, гипергликемия и гиперинсулинемия встречались чаще, чем у сельских молодых людей, на 5,3 и 1,8% соответственно. Среди коренных жительниц Севера гипергликемия наблюдалась на 16,8% чаще, чем среди коренных жителей-мужчин. Мужчины-ханты имели гипергликемию в 3,2 и гиперинсулинемию в 1,7 раз реже, чем некоренные мужчины – жители региона.
Распространенность гипохолестеринемии липопротеидов высокой плотности (ЛВП) среди всех обследованных молодых людей составила 18,7% (165 человек), среди пациентов с МС – 19,4% (n=146). В женской выборке эта разновидность дислипидемии встречалась на 5,3% чаще, чем в мужской: 19,5 против 14,2%. Среди пациентов с МС частота гипохолестеринемии ЛВП у женщин также была выше, у мужчин: 18,6 против 13,9%.
При сравнении этнических групп частота гипохолестеринемии ЛВП как среди некоренных, так и коренных жителей Севера была практически идентична: 17,7 и 17,8% соответственно. Однако среди коренных жителей-мужчин данные изменения липидного спектра встречались чаще, чем среди некоренных: 16,1 против 13,5%. Среди женщин некоренные жительницы Севера имели гипохолестеринемию ЛВП в 20,0% случаев, коренные – в 18,4%.
В выборке пациентов с МС встречаемость гипохолестеринемии ЛВП среди мужчин – некоренных жителей Севера была ниже, чем среди коренных: 13,0 против 16,7%. У женщин с МС достоверных отличий между некоренными и коренными жительницами Севера по распространенности гипохолестеринемии ЛВП найдено не было (18,8 и 18,2% соответственно, p <0,001).
Частота гипертриглицеридемии среди всех обследованных составила 66,6% (588 человек), при этом среди пациентов с МС – 78,1%, лиц без МС – в 2,2%. У здоровых обследованных молодых людей 3 сельских жителя (2 мужчин и 1 женщина) имели повышенные уровни триглицеридов (ТГ), среднее значение которых составило 1,9±0,001 ммоль/л. Среди некоренных жителей Севера с МС гипертриглицеридемия встречалась чаще на 4,6%, чем среди коренных: 79,6% (n=402 человека) против 75,0% (n=183). При гендерном сравнении эта форма дислипидемии была более распространена у женского населения (на 3,4%), чем среди мужского, при этом она преимущественно имело место у некоренных сельских жительниц (80,8%). Средние значения ТГ в сыворотке крови во всех сравниваемых группах были идентичны и составили 2,75±0,02 ммоль/л у большинства обследованных пациентов (n=421; 56,2%). Пограничные значения ТГ среди всех обследованных пациентов с МС встречались в 22,7% случаев, среди некоренных жителей Севера – в 20%, коренных – в 22,3%.
АГ была выявлена у 14,1% (n=106) пациентов с МС (11,8 % мужчин и 15,3% женщин). При этом среди городских жителей она отмечалась в 12,3%, сельских – в 17,0%, коренных жителей Севера – в 12,3% случаев. В контрольной группе доля пациентов с АГ составила 8,2% (n=11), при этом среди женщин этот показатель равнялся 4,5%, мужчин – 11,5%. У городских жителей без МС повышение артериального давления (АД) выше 130/90 мм рт. ст. встречалось в 5,3%, при этом у мужчин – в 10% случаев. Среди сельских жителей без МС АГ имела место у 6,5% человек – с одинаковой частотой как среди женщин, так и мужчин. Среди коренных жителей Севера без МС повышенным АД страдали 5 человек (13,2%) – 2 мужчин и 3 женщины.
Были изучены ассоциации полиморфизмов rs1378942 гена CSK, rs1801133 (С677Т) гена MTHFR, гена TCF7L2, rs7903146 гена ITGA2B, rs1799752 гена АСЕ с компонентами МС у обследованных коренных и некоренных жителей. В общей выборке обследованных нами лиц среди изучаемых полиморфных локусов чаще встречались локусы генов АСЕ и CSK (рис. 1).

Частота гетерозиготного генотипа ID полиморфизма rs1799752 гена АСЕ составила 51,5%, что свидетельствует о склонности большинства обследованных пациентов к повышенному уровню АПФ. При этом данный вариант генотипа наиболее часто встречался у коренных жителей (52,8% случаев). Высокий уровень АПФ в общей когорте наблюдался у 21,6% носителей гомозиготного генотипа DD с равной долей распределения между коренными и некоренными жителями Севера (табл. 2).
Носительство аллеля Т полиморфного локуса rs1378942 гена CSK свидетельствует о высоком риске развития у пациентов сердечно-сосудистых заболеваний. В общей когорте гетерозиготный генотип GT гена CSK составил 50,7%, с большей встречаемостью среди некоренных сельских жителей (55,2%). Гомозиготный вариант TT гена CSK был также достаточно высок и равнялся 29,5% в общей когорте; при этом он был больше распространен среди коренных жителей (32,7%; p=0,4989). Носительство мутантного аллеля I гена ITGA2B в общей когорте встречалось в 62,8% случаев; наиболее часто он обнаруживался у коренных жителей (64,7%; p=0,5392) и чаще проявлялся в гетерозиготном варианте ID (45,0%, p=0,7597). Гомозиготный генотип II гена ITGA2B также был достаточно распространенным – 40,2% (p=0,7597), с наибольшей встречаемостью у коренных жителей (41,7%).
Носительство мутантных аллелей T полиморфизма гена ITGA2B и rs1801133 гена MTHFR в общей когорте наблюдалось реже: 22,1 и 28,6% соответственно; чаще они были представлены гетерозиготными вариантами генотипов СТ (см. табл. 2).
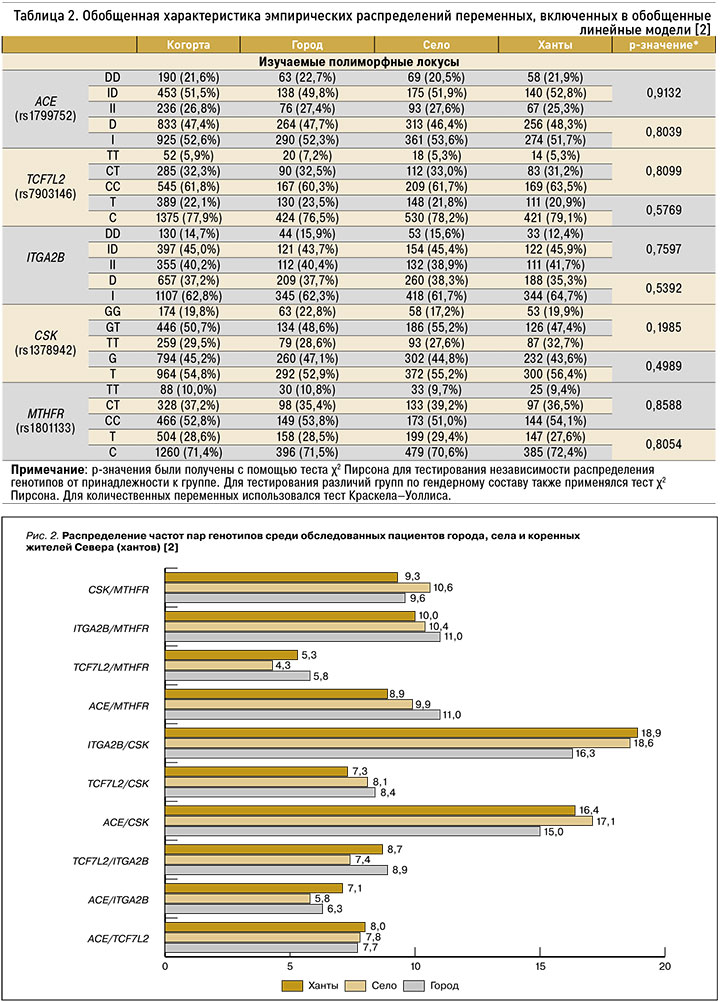
При анализе распределения пар генотипов среди обследованных пациентов с МС в общей когорте наиболее распространенными были сочетания полиморфизма rs7903146 гена ITGA2B и полиморфного локуса rs1378942 гена CSK (18,0%; см. рис. 1). При этом чаще данные сочетания встречались среди коренных (18,9%) и некоренных сельских (18,6%) жителей Севера (рис. 2). Сочетания полиморфизмов rs1799752 гена АСЕ и rs1378942 гена CSK выявлялись в 16,3% случаев (см. рис. 1), с наибольшим распространением среди некоренных жителей села (17,1%; см. рис. 2). Реже встречались варианты с полиморфизмом rs1801133 гена MTHFR и rs7903146 гена TCF7L2 (5,0%) – в равных долях среди коренных и некоренных жителей Севера.
При анализе сочетания компонентов МС были выявлены наиболее часто встречающиеся взаимодействия полиморфизмов генов. Так, у 7 (0,9%) пациентов с МС, у которых имело место абдоминального ожирение в сочетании с гипертриглицеридемией, АГ, гипергликемией и гипохолестеринемией ЛВП, в 57,1% случаев встречалось следующее распределение полиморфизмов генов: гетерозиготные генотипы ID гена ACE, ITGA2B, гомозиготные генотипы СС генов TCF7L2 и MTHFR, гетерозиготный генотип GT гена CSK. Среди 71 пациента (9,5%) с МС при сочетании абдоминального ожирения и гипертриглицеридемии, гипергликемии и гипохолестеринемии ЛВП наблюдались те же варианты сочетания полиморфизмов генов в 47,9% случаях. У 183 пациентов (24,4%) с сочетанием абдоминального ожирения, гипертриглицеридемии и гипергликемии данное взаимодействие полиморфизма генов было обнаружено в 50,3% случаев. Наиболее распространена гипертриглицеридемия у пациентов с абдоминальным ожирением (548 человек, 73,2%) при носительстве той же комбинации полиморфизмов генов (табл. 3).
Идентичное взаимодействие полиморфизмов генов встречалось при сочетании абдоминального ожирения с гипергликемией у 238 человек (31,8%), абдоминального ожирения с гипохолестеринемией ЛВП – у 146 (19,5%), абдоминального ожирения с АГ – у 102 (13,6%).
Наличие мутантных аллелей генов как в гетеро-, так и в гомозиготных генотипах распространенных комбинаций полиморфизмов генов ACE, ITGA2B, TCF7L2, MTHFR, CSK предопределяет генетически детерминированный высокий риск развития метаболических нарушений и сердечно-сосудистых заболеваний у соответствующих пациентов. Влияние внутренних (пол, возврат, принадлежность к этническим группам) и внешних (климат, социокультурные факторы) факторов способствует появлению и распространению новых мутаций или изменению частот аллелей, присутствовавших ранее в генофонде популяции [13].
В результате сочетанного действия нескольких генов реализуются такие их клинические эффекты, как сочетание абдоминального ожирения с нарушениями липидного и углеводного обменов, с АГ. В большинстве случаев при обследовании пациентов с МС (65,1%) мы получили 10 двухлокусных моделей, являющихся детерминантами развития этого синдрома. Выраженные клинические проявления наблюдались при сочетании полиморфизма генов ITGA2B/CSK (в 18,0% случаев) и генов ACE/CSK (16,3%), при этом они преобладали среди коренных жителей и некоренных жителей села.
5 компонентов (0,9%) и 4 компонента (9,5%) МС встречались в меньшей степени и определялись мутантными аллелями гетерозиготных генотипов ID гена ACE, ITGA2B, гомозиготных генотипов СС генов TCF7L2 и MTHFR, гетерозиготного генотипа GT гена CSK. Такое сочетание генотипов формировалось достаточно долго в результате накоплений генетических изменений под влиянием различных факторов. Необремененное жиром, холестерином и углеводами питание на фоне физической активности обеспечивало поглощение и утилизацию питательных веществ. Данных аллелей, адаптивных в прошлом, стало меньше, появились измененные гены и аллели в условиях нарушенного пищевого питания и снижения физической активности, что способствовало развитию предрасположенности к СД, АГ и ожирению.
Для проживания в определенных климатических условиях требуется формирование определенных генетических детерминант в популяции. Жители, долго проживающие и родившиеся в северных условиях, по частотам некоторых аллелей приближаются к частотам, характерным для коренного населения [14]. Учитывая необходимость большего энергетического запаса у коренного населения, мы наблюдали у них частое повышение в сыворотке крови уровня ТГ, которые при избытке накапливаются в клетках жировой ткани. Организм получает ТГ с пищей (чаще мясными продуктами), а также в результате их синтеза печенью из углеводов. Среди обследованных нами пациентов мы наблюдали высокую распространенность гипертриглицеридемии (73,2%) в сочетании с абдоминальным ожирением при частом сочетании следующих полиморфизмов генов: гетерозиготные генотипы ID гена ACE, ITGA2B, гомозиготные генотипы СС генов TCF7L2 и MTHFR, гетерозиготный генотип GT гена CSK.
ЗАКЛЮЧЕНИЕ
Таким образом, клинические проявления МС у обследованных некоренных и коренных молодых жителей Севера обусловлены сложными межгенными взаимодействиями изученных нами однонуклеотидных полиморфизмов генов ACE, TCF7L2, ITGA2B, CSK, MTHFR. Наиболее часто встречаются следующие сочетания мутантных полиморфизмов генов: гетерозиготные генотипы ID гена ACE, ITGA2B, гомозиготные генотипы СС генов TCF7L2 и MTHFR, гетерозиготный генотип GT гена CSK, из которых ген CSK занимает преимущественную роль в развитии МС. Раннее выявление генетических предикторов метаболических нарушений имеет важное клиническое значение в целях своевременной профилактики развития сердечно-сосудистых заболеваний и СД.



