ВВЕДЕНИЕ
Инфекции мочевыводящих путей (ИМВП) являются одними из самых распространенных бактериальных заболеваний во всем мире и наиболее частой причиной назначения антибиотиков и госпитализаций, связанных с инфекционным процессом. При этом необоснованное назначение антибактериальной терапии пациентам с ИМВП вносит вклад в селекцию резистентных штаммов возбудителей [1].
ИМВП – ведущие внутрибольничные инфекции, на долю которых приходится до 30–40% всех нозокомиальных патологий [2]. В то же время внебольничные ИМВП во всем мире поражают более 150 млн человек в год и представляют собой серьезную проблему здравоохранения, связанную с высокими экономическими затратами [3].
Результаты многоцентрового международного исследования «Дармис-2018» показали, что 89,2% изолятов, выделенных из мочи пациентов с внебольничными ИМВП, относились к семейству Enterobacteriaceae. Ведущими его представителями были Escherichia coli (69,4%) и Klebsiella pneumoniae (11,5%), в отношении которых максимальной активностью характеризовались фосфомицин и нитрофурантоин. Доля изолятов E. faecalis среди возбудителей ИМВП составляет 4,76%, при этом доля чувствительных к ампициллину штаммов – 98%, а к ципрофлоксацину – 70% [4]. Lu C.L. et al. в своей работе in vitro показали, что 99% штаммов E. faecalis демонстрировали чувствительность к фосфомицину, включая штаммы, резистентные к ванкомицину [5]. Внекишечные E. coli являются факультативными патогенами: они обитают в кишечнике в качестве комменсалов и принадлежат к нормальной микробиоте человека, в то время как вне кишечника в основном связаны с неонатальным менингитом и ИМВП [6]. Генотипически уропатогены характеризуются наличием широкого спектра факторов вирулентности, участвующих в клеточной адгезии, клеточной инвазии, метаболизме железа и уклонении от иммунной системы, а также способностью мигрировать из мочевого пузыря в почки и в кровоток, что приводит к бактериемии и диссеминации бактерий в другие локусы организма-хозяина [7].
Существуют различные взгляды относительно того, влияет ли наличие у пациента клинических проявлений ИМВП или бессимптомной бактериурии на возможность развития глубокой инфекции в области хирургического вмешательства после эндопротезирования. В 1970-х гг. появились первые описания перипротезной инфекции вследствие гематогенного распространения возбудителей ИМВП [8, 9]. Peng L. et al. установили, что наличие бактериурии у пациента значительно повышает риск развития поверхностной раневой инфекции и повторной госпитализации после эндопротезирования [10]. David T.S. и Vrahas M.S. по результатам своего аналитического обзора научных публикаций сделали заключение о необходимости проведения у пациентов 8–10-дневного курса лечения пероральными антибиотиками при бессимптомной бактериурии и выделении из мочи бактерий >103 КОЕ/мл после тотального эндопротезирования суставов [11]. В случаях же наличия клинических симптомов ИМВП или обструкции мочевыводящих путей (МВП) авторы считают целесообразным отсрочить ортопедическую операцию. Напротив, Gallegos Salazar J. et al. полагают, что выполнение санации МВП у пациентов с бессимптомной бактериурией не снижает риск послеоперационных осложнений при проведении неурологических операций [12].
Российское общество урологов в своих рекомендациях от 2016 г. регламентирует проведение эмпирической антибактериальной терапии при ИМВП фосфомицином или нитрофурантоином [13]. При неэффективности этих препаратов рекомендовано микробиологическое исследование мочи; клинически значимой считают бактериурию более 105 КОЕ/мл [14]. Это приобретает особую актуальность в условиях повсеместного роста устойчивости уропатогенов, в частности представителей семейства Enterobacteriaceae, к цефалоспоринам и фторхинолонам [15–17].
Ранее в нашем центре (Национальный медицинский исследовательский центр травматологии и ортопедии им. Р.Р. Вредена) пациентам с клинико-лабораторными проявлениями ИМВП назначали 5-дневный курс антибактериальной терапии норфлоксацином. С 2018 г. был внедрен протокол по рутинному применению фосфомицина перед плановым эндопротезированием тазобедренного (ТБС) или коленного суставов (КС) у пациентов с установленной лейкоцитурией >6 клеток в поле зрения. Выбор обоснован широким спектром действия этого препарата в отношении потенциальных возбудителей ИМВП и более коротким курсом терапии (однократный прием фосфомицина против 5-дневного курсом норфлоксацина). По данным отдельных фармакоэкономических исследований, затраты на лечение ИМВП фосфомицином у пациентов в амбулаторных условиях сопоставимы с расходами на применение других антибиотиков (триметоприма, нитрофурантоина, фторхинолонов) [18, 19], однако при стационарном лечении однократное введение этого препарата снижает продолжительность терапии и, как следствие, затраты на пребывание больного в стационаре.
Цель исследования – на основании анализа динамики спектра и чувствительности к фосфомицину и фторхинолонам ведущих микроорганизмов, выделенных из мочи пациентов, поступивших для планового эндопротезирования ТБС или КС, провести клинико-экономическую оценку внедрения локального протокола санации МВП.
МАТЕРИАЛ И МЕТОДЫ
Выполнен ретроспективный анализ результатов микробиологических исследований мочи пациентов, поступивших для планового эндопротезирования ТБС или КС с 2018 по 2020 г., с использованием программы микробиологического мониторинга «Микроб-2». К ведущим возбудителям относили микроорганизмы, доля которых в видовой структуре превышала 5%.
Согласно утвержденному локальному протоколу по ведению пациентов, с 2018 г. при выявлении лейкоцитурии >6 клеток в поле зрения, вне зависимости от наличия клинических признаков ИМВП, проводили бактериологический анализ мочи. В исследование включались все положительные результаты без учета полученного титра бактерий. Выделение клинических изолятов выполняли стандартными методиками в соответствии с международными стандартами микробиологических исследований (Standards for microbiology investigations, UK SMI). Видовую идентификацию осуществляли на панелях Microlatest (Erba Lachema) с помощью iEMS Reader MF (Labsistems, Финляндия). Антибиотикочувствительность культур изучали диффузионными методами (диски и тест-полоски Oxoid, Англия), а также методом серийных разведений с помощью автоматического анализатора VITeK 2 compact (BioMerieux, Франция) в соответствии с требованиями EUCAST (2016–2020, v.6–v.10) [20]. Наличие продукции ESBL грамнегативными штаммами определяли методом «двойных дисков» на агаре Мюллера–Хинтон, а также на среде CHROMagar ESBL.
Для определения наиболее экономически эффективного подхода к санации МВП перед плановым эндопротезированием ТБС или КС было выполнено фармакоэкономическое моделирование согласно утвержденному локальному протоколу. Для расчета стоимости учитывали только прямые медицинские затраты:
- стоимость антибиотика;
- стоимость койко-дня;
- стоимость анализов: общий анализ двукратный (первичный и контрольный) и микробиологическое исследование мочи.
Курс терапии антибактериальными препаратами состоял из однократного приема 3,0 фосфомицина или 5-дневного применения фторхинолонов – 400 мг норфлоксацина или 500 мг ципрофлоксацина в таблетках 2 раза/сут (всего 10 таблеток).
Для расчета необходимого количества койко-дней учитывали, что в соответствии с локальным протоколом контрольный общий анализ мочи выполняют через 72 ч после приема фосфомицина (3 койко-дня) и сразу после окончания приема фторхинолонов (5 койко-дней). При нормальных показателях общего анализа мочи пациенту выполняют плановое оперативное вмешательство. В случае сохраняющейся лейкоцитурии после курса терапии фторхинолонами и выделении к этому моменту из мочи микроорганизма, чувствительного к фосфомицину, проводится второй курс антибактериальной терапии фосфомицином с продлением госпитализации на 3 дня. В случае же идентификации возбудителя, резистентного к фосфомицину, пациента выписывают для проведения санации МВП под контролем профильного специалиста.
Стоимость койко-дня определена согласно Генеральному тарифному соглашению г. Санкт-Петербурга за 2022 г. (1970 руб.), лабораторных анализов – по прейскуранту платных услуг нашего Центра: общий анализ мочи – 350 руб., микробиологическое исследование мочи – 600 руб. Затраты на лекарственные средства взяты в соответствии с их закупочной стоимостью за 2022 г.
Для каждой из стратегий рассчитывались коэффициенты эффективности затрат (CER – cost-effectiveness ratio). Расчет коэффициента проводился с использованием формулы CER = DС/ EF, где DC – прямые медицинские затраты (direct cost), а EF – эффективность метода (effective of methods), т.е. вероятность успешного лечения, исходя из чувствительности к антибактериальному препарату наиболее часто регистрируемых бактерий с учетом доли каждого из них в спектре возбудителей инфекции (доля каждого микроорганизма в спектре умножалась на чувствительность к каждому из антибиотиков, для каждого антибиотика подсчитывался суммарный показатель). При идентичной эффективности сравниваемых стратегий выполняли анализ минимизации затрат (CMA – cost minimization analyze): из стратегии с большей стоимостью вычитали стоимость стратегии с меньшей стоимостью (CMA = DC1 – DC2).
Для сбора данных и полученных результатов проведенного анализа, статистической обработки применяли систему MS Office Excel, 2007 (Microsoft, США). Для статистического анализа полученных данных был использован Z-критерий стандартного нормального распределения для оценки разности между долями.
РЕЗУЛЬТАТЫ
За исследуемый период получено 872 положительных посева мочи и выделено 1266 микроорганизмов (2018 г. – 416, 2019 г. – 498, 2020 г. – 352). Из них в 573 (51,4%) случаях изолирована монокультура, в 48,6% – микробные ассоциации из 2, 3 или 4 патогенов. Снижение количества изолированных культур в 2020 г., по сравнению с 2018 и 2019 гг., по-видимому, обусловлено уменьшением числа госпитализированных пациентов в связи с пандемией новой коронавирусной инфекции (COVID-19).
Всего с 2018 по 2020 г. в результате микробиологического исследования мочи было выделено 24 представителя различных таксонов бактерий, 73,8% из которых составили E. coli, K. pneumoniae и E. faecalis. На рисунке 1 представлена структура основных патогенов, выделенных за 3 года из образцов мочи с бактериурией.
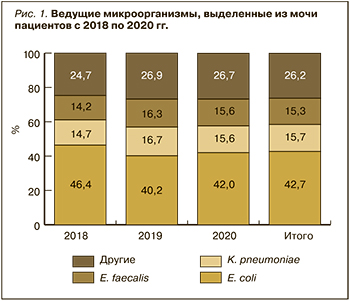
Ведущим патогенном в течение 3 лет остается E. coli, на долю которого в среднем приходится 42,7 %. Еще около 30% составляют K. pneumoniae и E. faecalis. В четверти случаев выделялись другие микроорганизмы. При этом структура ведущих возбудителей на протяжении 3 лет была достаточно стабильной.
Доля штаммов грамотрицательных продуцентов ESBL (Extended-spectrum beta-lactamase) также оставалась стабильной на всем анализируемом отрезке времени (рис. 2).
В среднем около трети всех представителей семейства Enterobacteriaceae являлись продуцентами ESBL. В общей структуре спектра уропатогенов доля E. coli ESBL(-) составила 29,6% (n=375), E. coli ESBL(+) – 13,1% (n=166), K. pneumoniae ESBL(-) и K. pneumoniae ESBL(+) – соответственно 9,8% (n=124) и 5,9% (n=75).
Дальнейший анализ показал отсутствие изменений чувствительности E. coli (p=0,009) и К. pneumoniae (p=0,02) к фосфомицину вне зависимости от наличия продукции ESBL (рис. 3). Фосфомицин был активен в отношении подавляющего большинства штаммов E. coli (>98%), при этом доля чувствительных к нему штаммов К. pneumoniae была в среднем на 20,5% ниже.
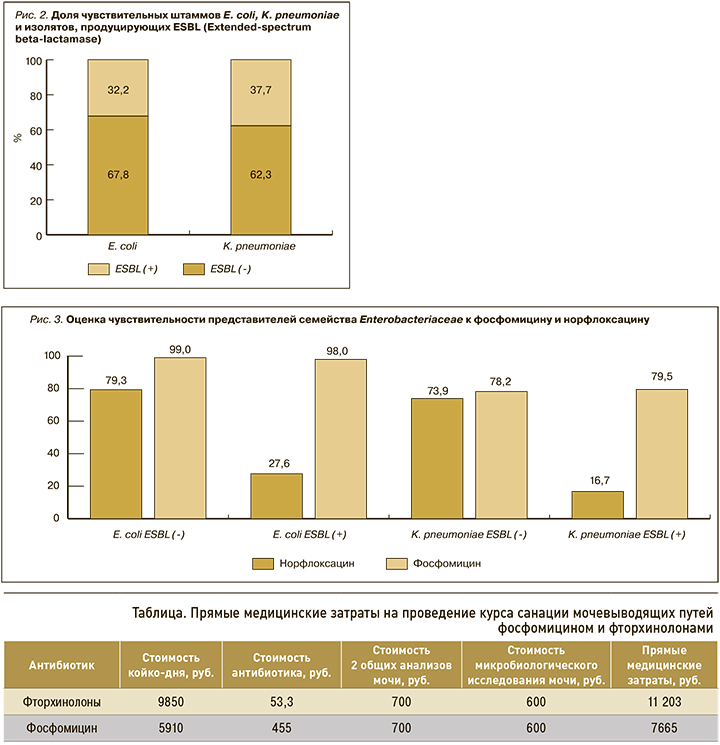
Чувствительность выделенных штаммов к норфлоксацину также была стабильной за исследуемый период. Не было выявлено изменения доли резистентных к норфлоксацину E. coli (p=0,008) и К. pneumoniae (p=0,017). Установлено, что чувствительность к норфлоксацину была ассоциирована с наличием продукции ESBL штаммами E. coli и K. pneumoniae. Среди продуцентов ESBL чувствительность к норфлоксацину была в 2,8 и 4,3 раза ниже, чем у изолятов E. coli и K. pneumoniae без продукции бета-лактамаз расширенного спектра.
За исследуемый период у всех выделенных штаммов E. faecalis (n=195) сохранялась чувствительность к ампициллину. Доля чувствительных E. faecalis к ципрофлоксацину составила 76,47%. Чувствительность этого микроорганизма к фосфомицину не определяли.
Последующий фармакоэкономический анализ продемонстрировал, что большую часть всех медицинских затрат составили расходы на пребывание пациента в стационаре: при санации МВП фторхинолонами – 88%, фосфомицином – 77% (табл.). В связи с этим прямые медицинские затраты при проведении санации МВП фторхинолонами были на 32% выше, чем фосфомицином, несмотря на то что стоимость фторхинолонов в 8,5 раз ниже, чем фосфомицина, а доля затрат на антибиотики составила в общей структуре соответственно 0,4 и 5%. Финансовые расходы на лабораторные анализы при обеих стратегиях были одинаковыми, а их доли составили 12% для фторхинолонов и 17% для фосфомицина (табл. 1).
Эффективность (EF) стратегии санации МВП фосфомицином составила 70%, фторхинолонами – 47%. CER при стратегии с использованием фторхинолонов и фосфомицина отличался в 2,2 раза: в первом случае этот показатель равнялся 238, во втором – 110 (рис. 4).
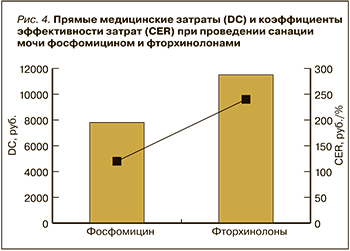
Отдельно были посчитаны затраты на лечение, при котором эмпирически назначали фторхинолоны. Следует подчеркнуть, что такой подход не приводил к нормализации общего анализа мочи, и при проведении микробиологического исследования был получен изолят, резистентный к фторхинолонам и чувствительный к фосфомицину. В этом случае проводился второй курс терапии фосфомицином. Прямые затраты таким образом состояли из затрат на курс фторхинолонами с учетом его эффективности и второй курс фосфомицином (без учета одного общего и биохимического анализа мочи). В результате прямые затраты на такой курс составили 11 203,3 + 6715 = 17 918,3 руб. Соответственно при проведении анализа минимизации затрат было выявлено, что эмпирическое назначение фосфомицина на 10 253,3 руб. более выгодно, чем проведение двойного курса антибактериальной терапии (сначала фторхинолоны, затем фосфомицин).
ОБСУЖДЕНИЕ
Самый крупный метаанализ (Gomez-Ochoa S.A. et al.), включавший исходы эндопротезирования крупных суставов у 29 371 человека (35 323 суставов) за 47 лет, показал, что доля пациентов с развитием инфекции области хирургического вмешательства (ИОХВ) выше в группе с бактериурией (2,3 против 1,1%; p <0,001). Кроме того, выделение микроорганизмов из мочи в дооперационном периоде было связано с более высоким риском развития ИОХВ (отношение шансов (ОШ) 2,89; 95% доверительный интервал (ДИ): 1,36–6,17). Вместе с тем авторы установили, что только у 12,7% пациентов возбудитель, выделенный из раны, имел связь с культурой, выделенной из мочи, т.е. имело место гематогенное распространение микроорганизма из локуса в локус [21].
Следует отметить, что большинство исследований, проводимых с целью установить наличие/отсутствие связи между персистированием микроорганизмов в моче и риском развития ИОХВ или перипротезной инфекции (ППИ), сосредоточены на бессимптомной бактериурии (ББУ). На наш взгляд, это неверный подход, так как ББУ – это выделение микроорганизма от пациента без каких-либо клинико-лабораторных признаков инфекции [14]. А рутинный скрининг всех пациентов перед плановым ортопедическим лечением на наличие бессимптомной бактериурии не только клинически не целесообразен, но и экономически не выгоден.
Иначе обстоят дела при наличии клинико-лабораторных признаков ИМВП у пациента. Выполнение плановой ортопедической операции не показано пациентам с активным инфекционным процессом. Так, 96% участников Второй согласительной конференции по скелетно-мышечной инфекции 2018 г. высказались за то, что дооперационная ИМВП повышает риск имплантат-ассоциированной инфекции в дальнейшем, и таким пациентам показано дооперационное проведение санации МВП. При этом 89% участников считает, что рутинный скрининг на бактериурию перед проведением ортопедической операции не показан [22]. Согласно утвержденному в нашем Центре локальному протоколу, бактериологическое исследование мочи выполняли рутинно при наличии в общем анализе мочи лейкоцитурии (>6 лейкоцитов в поле зрения микроскопа при ×400 увеличении), которая является признаком воспаления. Таким образом, проведение санации МВП у этих пациентов было оправдано.
Структура уропатогенов в работах отечественных [4, 23] и зарубежных авторов [7, 24] стабильна. Наиболее частым этиологическим агентом неосложненной ИМВП выступает E. coli [7]. По результатам международного исследования ARESC (Antimicrobial Resistance Epidemiological Survey on Cystitis) E. coli служил причиной инфекционно-воспалительного процесса в 76,7% случаев [24], а по данным международного исследования «Дармис-2018» – в 69,4% [4].
В нашем исследовании E. coli также был наиболее часто изолируемым видом из мочи пациентов с лейкоцитурией перед плановым эндопротезированием крупных суставов нижних конечностей. Однако его доля в структуре возбудителей ИМВП оказалась существенно ниже – 43,7%. При этом, по данным исследования «Дармис-2018», общая частота продукции бета-лактамаз расширенного спектра среди изолятов E. coli на основании фенотипических тестов составляет 22% [4], тогда как в нашем исследовании она была несколько выше – 32,2%. Вероятно, это отражает рост резистентности данного уропатогена.
На втором месте в структуре ведущих возбудителей ИМВП в нашем исследовании была K. pneumoniae – 16%. В исследовании «Дармис-2018» аналогичный показатель равнялся 11,5% [4]. Это подтверждает ведущую роль представителей семейства Enterobacteriaceae в этиологии ИМВП. Обращает на себя внимание тот факт, что из всех изолятов K. pneumoniae 37,7% были продуцентами бета-лактамаз расширенного спектра.
Роль E. faecalis в этиологии развития ИМВП спорная. В исследовании «Дармис-2018» доля этого уропатогена составила 5%, что существенно менее значимо суммарной доли всех энтеробактерий [4]. Однако при развитии осложненной ИМВП этот возбудитель выявляется значительно чаще. Например, С.В. Котов с соавт. установили, что он занимает первое место в структуре уропатогенов (34%), вызывающих осложненные ИМВП [15]. В нашем исследовании на долю E. faecalis пришлось 15%; этот факт заслуживает отдельного внимания, поскольку в исследовании принимали участие по большей части пациенты с неосложненной ИМВП.
Полученные результаты по спектру возбудителей, на наш взгляд, можно объяснить выборкой пациентов, поступающих для эндопротезирования крупных суставов нижних конечностей. Преобладающую долю среди них составляют женщины и лица пожилого возраста, имеющие коморбидный фон. По-видимому, большинство из них имеет хронические заболевания мочевыводящей системы.
Чувствительность штаммов E. coli к фосфомицину остается стабильно высокой. Согласно результатам ARESC и «Дармис-2018», 98% изолятов сохраняют чувствительность к этому антибактериальному препарату [19]. Полученные нами результаты совпадают с опубликованными данными: доля чувствительных к фосфомицину штаммов E. coli составила те же 98%. K. pneumoniae также сохраняет высокую чувствительность к фосфомицину – 88% [24]; в нашем исследовании этот показатель был несколько ниже – 78%. Оценка чувствительности E. faecalis с фосфомицину в нашем исследовании не проводилась. Lu C.L. et al. в своей работе показали, что штаммы E. faecalis сохраняют 99% чувствительность с фосфомицину в исследованиях in vitro [5]. При этом интересен тот факт, что фосфомицин активен в отношении резистентных к ванкомицину штаммов Enterococcus spp.: доля чувствительных штаммов в исследованиях in vitro оценивалась в 95–98% [25, 26].
По данным «Дармис-2018», 60,3% штаммов E. coli, 63% K. pneumoniae и 70% Enterococcus spp. сохраняли чувствительность к фторхинолонам [4]. Аналогичные результаты получены и в нашем исследовании, при этом чувствительность штаммов E. coli и K. pneumoniae к этой группе антибактериальных средств коррелирует с наличием продукции ESBL: доля чувствительных к фторхинолонами штаммов E. coli и K. pneumoniae была в 2,8 и 4,3 раза ниже среди продуцентов ESBL, чем у штаммов без их продукции. Литературных данных, объясняющих этот феномен, найти не удалось.
Высокая устойчивость к фторхинолонам штаммов представителей семейства Enterobacteriaceae является причиной того, что данные препараты не рекомендуют в качестве первой линии терапии лечения ИМВП [13]. Таким образом, учитывая спектр ведущих уропатогенов и их чувствительность к основным антибактериальным средствам, можно сделать вывод, что для санации ИМВП наиболее эффективен фосфомицин. Фторхинолоны допустимо использовать при отсутствии возможности проведения терапии фосфомицином (аллергические реакции в анамнезе, отсутствие препарата на рынке). Эти факты подтверждаются в большом числе отечественных и зарубежных работ [13, 27, 28].
Наличие у пациента бактериурии с лейкоцитурией и необходимость санации МВП с целью снижения риска послеоперационных инфекционных осложнений приводит к продлению сроков госпитализации и, как следствие, к удорожанию лечения. Если затраты на проведение санации МВП различными антибиотиками в амбулаторных условиях в целом сопоставимы [18, 19, 29], то при стационарном лечении ведущим фактором, влияющим на стоимость терапии, становится длительность лечения.
ЗАКЛЮЧЕНИЕ
Впервые в российских условиях было проведено фармакоэкономическое моделирование применения фосфомицина и фторхинолонов для санации МВП перед плановым эндопротезированием ТБС или КС. Прием фосфомицина позволяет на 32% сократить прямые медицинские затраты в сравнении с использованием фторхинолонов, прежде всего за счет сокращения количества койко-дней, затраченных на курс терапии и подтверждение его эффективности. По данным локального микробиологического мониторинга структуры и антибиотикочувствительности уропатогенов и результатам проведенного фармакоэкономического исследования, эффективность применения фосфомицина в 1,5 раза выше, чем фторхинолонов, а коэффициент эффективности затрат (CER) при использовании фторхинолонов в 2,2 раза выше, чем для фосфомицина. В случае необходимости проведения двойного курса антибактериальной терапии (фторхинолоны, при их неэффективности – фосфомицин) затраты возрастают в 2,3 раза по сравнению с фосфомицином при одинаковой конечной эффективности.
Полученные результаты свидетельствуют об экономической целесообразности включения фосфомицина в локальный протокол по ведению пациентов с симптомами ИМВП перед плановым эндопротезированием крупных суставов.



