ВВЕДЕНИЕ
Известно, что развитие острого почечного повреждения (ОПП) у пациентов с инфарктом миокарда (ИМ) ухудшает прогноз и течение заболевания, увеличивает риск возникновения летального исхода в десятки раз [1–3]. Примечательно, что это осложнение развивается не у каждого пациента с ИМ, а лишь в 20% случаев [4] и происходит в условиях реализации всех существующих стратегий нефропротекции [5]. В настоящее время нам известен целый ряд гемодинамических и нейрогуморальных механизмов, которые потенциально могут быть вовлечены во взаимодействие между сердцем и почками [2, 4]. При этом успехи применения противовоспалительной терапии у лиц с высокими сердечно-сосудистыми рисками, в том числе у пациентов с наличием хронической ишемической болезни сердца и высоким уровнем С-реактивного белка [6], позволяют предположить, что изучение динамики и взаимодействия клеток, участвующих в процессах регенерации и воспаления и лежащих в основе реализации иммунологических механизмов, также является новым перспективным направлением.
Ряд экспериментальных данных указывает на активное участие клеток врожденного иммунитета в кардиоренальных взаимоотношениях при ИМ [7, 8]. Непрерывная симпатическая стимуляция клеток собирательных канальцев почек в условиях ишемии способствует тому, что макрофаги (МФ) почек выделяют в кровоток ряд молекул, включая гранулоцитарно-макрофагальный колониестимулирующий фактор (GM-CSF), способных индуцировать поляризацию в сердце воспалительных МФ М1 типа в противовоспалительные МФ М2 типа [8]. Данная активация паракринного пути передачи сигналов ведет к развитию адаптивной гипертрофии миокарда и фиброзу сердца [9]. Нами уже получен ряд данных об особенностях макрофагального состава ткани почек и миокарда, их взаимодействиях между собой и влиянии на прогноз у пациентов с фатальным исходом ИМ [10–12]. Однако, каким именно образом развитие ОПП влияет на изменение макрофагального состава миокарда и почек и, возможно, на течение и прогноз заболевания у лиц с фатальным исходом ИМ, не известно. Существует высокая доля вероятности того, что одним из возможных механизмов развития ОПП у лиц с ИМ служит определенное взаимодействие между клетками врожденного иммунитета. Интерес вызывает и определение конкретных фенотипов клеток макрофагального ряда почек и сердца, на изменение которых влияет как наличие острого ишемического повреждения тканей миокарда, так и формирование ОПП. Углубленное изучение функции и динамики этих типов клеток, их взаимосвязей между собой и с клиническими данными, вероятно, сможет в будущем повлиять на прогноз и исход ИМ путем таргетного воздействия на эти клеточные субпопуляции.
Цель нашего исследования − оценка особенностей макрофагального состава почек и ее взаимосвязи с развитием неблагоприятного исхода у пациентов с фатальным ИМ в зависимости от наличия ОПП.
МАТЕРИАЛ И МЕТОДЫ
Материалом для нашей работы послужили фрагменты почек и миокарда (зона инфаркта) пациентов (n=28), умерших от ИМ 1 типа.
Критериями исключения являлись ИМ II–V типа, онкологические заболевания, инфекционные осложнения (сепсис, пневмония), клапанные пороки, требующие хирургического вмешательства.
Средний срок наступления летального исхода составлял 2 сут (1; 30). Исследование было одобрено Комитетом по биомедицинской этике НИИ кардиологии Томского НИМЦ (протокол № 128) и проводилось в соответствии с принципами Хельсинкской декларации. Патологоанатомическое вскрытие осуществлялось согласно приказу Минздрава России от 06.06.2013 № 354н. Подписание информированного согласия пациентов не осуществлялось, что не противоречило правилам проведения исследования согласно Хельсинкской декларации («информированное согласие», п. 32).
Аутопсия проводилась в течение 24 ч после наступления факта смерти. Забранный материал фиксировали в течение суток в 10% забуференном формалине, после чего выполняли стандартную гистологическую проводку и заливку в парафин в аппарате Thermo Scientific Excelsior ES (США). Затем из парафиновых блоков с помощью ротационного микротома Thermo Scientific HM355S (США) были выполнены микротомные срезы почки и сердца. С каждого блока было сделано по 10 срезов для фрагментов почки и по 20 срезов для фрагментов миокарда, после чего материал был нанесен на стекла с L-полилизиновым покрытием, по два среза на одно стекло.
Макрофагальную инфильтрацию почек и миокарда оценивали два независимых эксперта с помощью иммуногистохимического исследования, проводимого на автоматическом иммуностейнере Leica Bond-Max (Германия). Для иммунофенотипирования МФ использовались мышиные моноклональные антитела к общему маркеру МФ CD68 (Cell Marque, разведение 1:500), антитела к маркеру М2 МФ CD163 (Cell Marque, разведение 1:50) и CD206 (Santa Cruz, разведение 1:100), а также дополнительные антитела к маркеру М2 МФ, синтезированные в лаборатории врожденного иммунитета и иммунологической толерантности (Университет Гейдельберга) к стабилину-1 (разведение 1:1000).
В целях визуализации исследованных маркеров применялся набор реагентов для детекции Bond на основе Полимера – Bond Polymer Refine Detection (Великобритания). Иммуногистохимическое окрашивание проводилось по стандартному протоколу [12]. Подсчет клеток в почках и миокарде производился в 10 случайных полях зрения (объектив 40×) на микроскопе Axio Imager M2, Carl Zeiss (Германия), в светлом поле.
Далее пациентов разделили на 2 группы в соответствии с наличием у них ОПП: 1-я группа − ОПП+ (n=10); 2-я группа − ОПП- (n=18). ОПП определяли согласно существующим в клинической практике критериям патоморфологического исследования почек [13]. Критериями морфологического подтверждения ОПП служили наличие истончения тубулярного эпителия преимущественно проксимальных почечных канальцев с утратой щеточной каемки, потеря структурности апикального контура цитоплазмы с усилением ее эозинофильного окрашивания, появление в ядрах эпителиоцитов конденсированного хроматина, повышение базофилии ядер с их пикнозом, кариолизис. При этом просветы канальцев были заполнены десквамированными некротизированными эпителиальными клетками канальцев, массами фибрина и гиалиновыми цилиндрами. Описанные изменения эпителия, прежде всего проксимальных почечных канальцев, интерпретировались как проявления тубулонекроза, являющегося основным морфологическим признаком ОПП.
В таблице 1 представлены клинико-анамнестические данные больных, включенных в исследование. Стоит отметить, что по клинико-анамнестическим данным исследуемые группы были сопоставимы; отличия были обнаружены только между уровнем креатинина при поступлении в стационар.
Для статистической обработки полученных данных использовалось программное обеспечение STATISTICA 12.0. Нормальность количественных данных проверялась по критерию Шапиро–Уилка. Возраст пациентов и уровень креатинина, скорость клубочковой фильтрации при поступлении в стационар описывались средним значением (М) и стандартным отклонением (SD), остальные количественные показатели, не имевшие нормального распределения, – медианой (Ме) и интерквартильным интервалом (Q1; Q3). Категориальные показатели описывались частотами и процентами. Для сравнения количественных показателей в независимых группах применялся критерий Манна–Уитни, для сравнения категориальных признаков – критерий Пирсона (χ2) и точный критерий Фишера. Множественное сравнение между количеством клеток в группах выполнялось с помощью критерия Краскела–Уоллиса. Корреляционные связи между количеством клеток и клинико-анамнестическими данными выявлялись посредством коэффициента корреляции Спирмена. Значение r (коэффициент ранговой корреляции) от 0,4 до 0,7 показало умеренную корреляцию. Проверка статистических гипотез проводилась по уровню значимости р=0,05.
РЕЗУЛЬТАТЫ
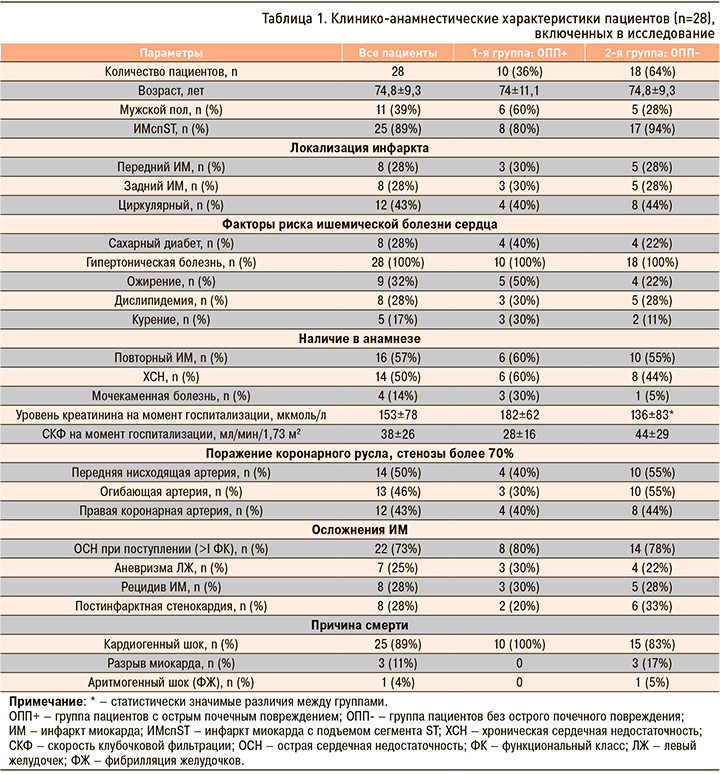
По данным иммуногистохимического исследования нами было обнаружено, что как в общей выборке, так и у лиц из групп ОПП- и ОПП+ среди всех исследуемых клеток в почках преобладают CD163+ клетки (рис. 1, табл. 2).
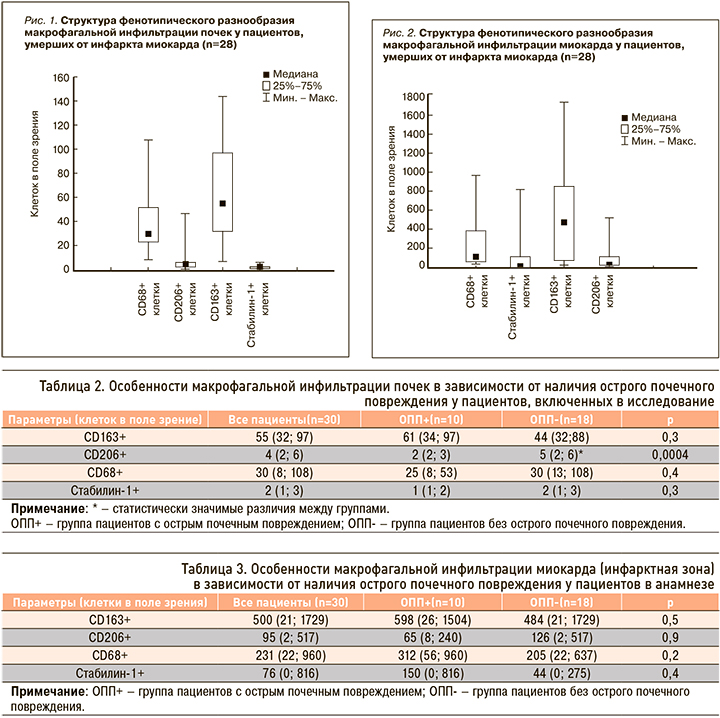
Особенности макрофагальной инфильтрации почек в зависимости от наличия ОПП у пациентов в анамнезе отражены в таблице 2.
Исходя из полученных данных, очевидно, что исследуемый нами макрофагальный состав почек у пациентов с развившимся ОПП аналогичен таковому у пациентов без ОПП, за исключением количества CD206+ клеток.
Нами обнаружено, что в общей выборке, а также у лиц из групп ОПП- и ОПП+ среди всех исследуемых клеток в миокарде преобладают CD163+ и CD68+ клетки (рис. 2).
Межгрупповых отличий по количеству клеток в миокарде нами выявлено не было (табл. 3).
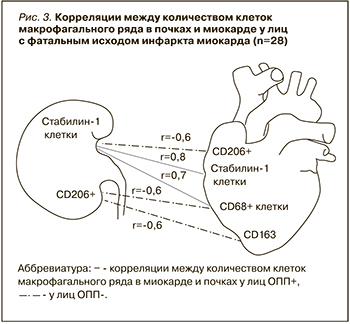
После этого мы оценили наличие корреляций между развитием ОПП у пациентов с ИМ и клинико-анамнестическими данными, а также изменениями макрофагального состава миокарда. Были установлены обратные корреляции между сроком наступления летального исхода и наличием ОПП (r=-0,7; p <0,5), а также между количеством CD206+ клеток в почках и наличием ОПП (r=-0,5; p <0,05). Корреляции между количеством клеток в миокарде и в почках отображены на рисунке 3.
ОБСУЖДЕНИЕ
Актуальность и перспективность изучения места системы врожденного иммунитета в формировании кардиоренальных взаимоотношений, их влияния на течение и прогноз ИМ обусловлена, помимо многообещающих экспериментальных данных [1, 8, 10], наличием определенных клинических успехов в области применения противовоспалительных препаратов у пациентов с высоким сердечно-сосудистым риском [6, 14].
При изучении особенностей макрофагального состава почек и миокарда у лиц с фатальным ИМ нами была выявлена их гетерогенность, присутствовали все типы исследуемых нами клеток. Похожие данные были получены ранее как при анализе макрофагального состава миокарда в условиях ИМ [15], так и биоптатов почек, у лиц с патологией почек воспалительного [16] и аутоиммунного генеза [17]. Среди всех изученных клеток в нашей выборке отмечено преобладание CD163+ и CD68+ клеток как в почках, так и в миокарде, у всех пациентов, независимо от наличия ОПП. Известно, что данный тип клеток характеризуется повышенной экспрессией в условиях острого и хронического воспалительного процесса [18]. Вероятно, высокая концентрация CD163+ клеток у лиц с ИМ также отражает наличие активного воспалительного фона, поддержание которого способствует развитию и прогрессированию как почечной, так и сердечной недостаточности, и ассоциировано с неблагоприятным исходом. Стоит отметить, что исследователи ранее также обнаруживали высокое содержание CD163+ клеток в биоптатах почек у пациентов с волчаночным нефритом [17], ассоциированное с неблагоприятным течением и прогнозом заболевания; подобные данные были характерны и для больных с IgA-нефропатией [19], и для пациентов после трансплантации почки [20]. Высокое содержание клеток этого типа в миокарде в отдаленный постинфарктный период также было связано с неблагоприятным исходом [15]. Открытым остается вопрос, являются ли CD163+ клетки в тканях представителями резидентного пула клеток, присутствующих в тканях с момента эмбриогенеза и участвующих в поддержании гомеостаза, либо же повышение концентрации CD163+ клеток обусловлено привлечением этого типа клеток в очаг повреждения?
Отталкиваясь от наших данных, можно предположить, что пул CD163+ клеток преимущественно является резидентным. В пользу такой гипотезы свидетельствует отсутствие различий по количеству данных клеток между группами ОПП+ и ОПП-. Однако тот факт, что содержание CD163+ и CD68+ клеток в зоне инфаркта миокарда в десятки раз превышает их содержание в почках при ИМ, и более чем в сотни раз, в биоптатах почек у здоровых лиц [19], указывает на активную вовлеченность клеток этого типа в процессы постинфарктной регенерации миокарда и существование пришлого пула данного типа клеток, рекрутированных в очаг некроза миокарда. Однако наиболее вероятным нам представляется участие этого типа клеток в поддержании гомеостаза тканей, иммунологической регуляции и регенерации тканей при повреждении, что и объясняет их высокую концентрацию в тканях [21].
Помимо CD163+ клеток, в исследовании выявлено преобладание в почках и миокарде CD68+ клеток. СD68 – иммуногистохимический маркер обшей популяции МФ, реализующих свою основную функцию, которая заключается в поглощении апоптотических и поврежденных клеток [22]; вероятно, этим обусловлено их высокое содержание и в почках, и в миокарде у лиц с ИМ. Высокая концентрация клеток этого типа может свидетельствовать об их участии в пролонгированной воспалительной реакции, указывать на вовлеченность системы врожденного иммунитета в процессы постинфарктной регенерации как миокарда, так и почек и быть ассоциированной с неблагоприятным прогнозом. Для того чтобы понять конкретную причину высокой концентрации CD163+ и CD68+ клеток, необходимо провести сравнение между содержанием этих клеток у лиц с ИМ и здоровых лиц, что служит объектом нашего дальнейшего исследования. Известно, что в ряде исследований содержание CD68+ клеток коррелировало с наличием протеинурии и с неблагоприятным исходом [23]. Количество же данных клеток в нашей группе было сопоставимым с количеством CD68+ клеток в почках у лиц со сниженной скоростью клубочковой фильтрации и наличием волчаночного нефрита [24].
Наряду с вышеперечисленными клетками нами были изучены CD206+ клетки, относящиеся к МФ М2 типа. Количество клеток этого типа у лиц из группы ОПП+ было значимо меньшим, чем в группе ОПП-: вероятно, это отражение того факта, что истощение CD206+ клеток может быть ассоциировано как с прогрессированием и/или развитием кардиоренального синдрома, так и с течением самого заболевания. Ранее в исследованиях были получены результаты, отчасти сопоставимые с нашими: так, Li J. et al. (2015) при сравнении количества клеток в биоптате почек у лиц с острым интерстициальным нефритом и у когорты лиц с острым тубулярным некрозом обнаружили, что во втором случае количество CD206+ клеток было меньшим [19]. Тем не менее другие выводы этого исследования противоречат нашим и говорят о высоком содержании СD206+ клеток в зоне ишемии. Наличие взаимосвязи между низким уровнем CD68+ и CD163+ клеток и высоким уровнем CD206+ клеток у лиц без ОПП может свидетельствовать о присутствии взаимодействий между макрофагами оси «сердце–почка» у человека, схожих с теми, которые были обнаружены в экспериментах на грызунах в условиях моделирования ишемии миокарда [8, 9]. Известно, что изменение макрофагальной инфильтрации почек в условиях ишемии, обусловленное истощением резидентных МФ почек М2 типа и рекрутированием МФ с провоспалительным фенотипом в ткани почки, способствует изменению макрофагальной инфильтрации миокарда с поляризацией МФ миокарда в клетки с М2 фенотипом, и наоборот. Вероятно, больший резерв МФ М2 типа может быть необходим для изменения поляризации МФ сердца в ранний период ИМ, что, в свою очередь, может влиять на активность воспалительного ответа в ранний постинфарктный период и быть связанным с развитием смертельных осложнений и наступлением более раннего летального исхода. Количество клеток данного типа в нашей выборке было в 2 раза ниже, чем у лиц с тубулярным некрозом, [20]. Это может свидетельствовать об их активной вовлеченности в межорганные взаимоотношения «сердце–почка» в условиях ишемии.
Кроме того, мы оценивали содержание в почках и миокарде стабилин-1+ клеток, их содержание между группами не отличалось. Интересным представляется изменение соотношения между стабилин-1+ и CD206+ клетками в миокарде в зависимости от наличия ОПП. Несмотря на то что эти клетки относятся к МФ М2 типа [25], их соотношение изменяется. Это может указывать на их разные функции и роль в процессах воспаления и регенерации; возможно, у таких клеток существует определенная тканевая специфичность. Интересно и то, что содержание этого типа клеток в миокарде в сотни раз больше, чем в почке, что, по-видимому, говорит об их минимальной вовлеченности в процессы кардиоренальных взаимоотношений.
Полученные нами данные об особенностях состава макрофагального инфильтрата почек и миокарда у лиц с ИМ в зависимости от наличия ОПП, а также о взаимосвязях между клетками макрофагального ряда миокарда и почек, служат предпосылкой для более глубокого изучения на большей выборке пациентов с ИМ роли и места конкретных клеток макрофагального ряда в неблагоприятном течении кардиоренальных взаимодействий и таргетного влияния на эти клеточные субпопуляции. Для дальнейшего вывода о том, является ли повышенное содержание CD163+ и CD68+ клеток в почках и миокарде проявлением системной воспалительной реакции, характерной для любого течения ИМ, либо же этот процесс служит проявлением неблагоприятного течения ИМ, а также для оценки снижения концентрации CD206+ клеток в почках в качестве возможного предиктора неблагоприятного течения и прогноза ИМ необходимо сопоставление полученных нами данных с результатами исследования тканей, полученных от здоровых лиц.
Ограничение исследования: наше исследование являлось одноцентровым, было проведено на небольшой выборке, что связано с ограниченным количеством времени сбора материала и строгими критериями включения. По этим причинам необходимы дальнейшие исследования.
ЗАКЛЮЧЕНИЕ
Макрофагальный состав почек и миокарда у пациентов с фатальным ИМ, независимо от степени повреждения почек, характеризовался преобладанием CD163+ клеток. ОПП у лиц с фатальным ИМ было ассоциировано с меньшим уровнем CD206+ клеток в почках и скорым наступлением летального исхода.



