ВВЕДЕНИЕ
Хронические гастриты – группа хронических заболеваний, которые морфологически характеризуются воспалительными и дистрофическими процессами в слизистой оболочке желудка (СОЖ).
Согласно классификации хронических гастритов (Сидней, 1990/Хьюстон, 1994), выделяют основные три их типа: неатрофический, атрофический и особые формы [1]. Под атрофией СОЖ понимают утрату специализированных желудочных желез с замещением их метаплазированным эпителием или фиброзной тканью. Основными причинами формирования атрофии СОЖ выступают аутоиммунный процесс и инфекция Helicobacter pylori (НР) [1].
На атрофический гастрит, ассоциированный с НР, приходится 90% среди всех форм гастритов. Атрофия СОЖ является следствием повреждения зон пролиферации и/или деструкции желез посредством как прямого бактериального воздействия, так и воспалительного ответа организма хозяина. В ответ на внедрение НР происходит миграция нейтрофилов в зону внедрения, активация в них процесса перекисного окисления липидов (ПОЛ), увеличение продукции активных форм кислорода, провоспалительных цитокинов (фактора некроза опухоли-альфа, интерлейкинов 8 и 12), повреждение ДНК клеток и формирование кишечной метаплазии. Для данного типа гастрита характерны выраженная воспалительная реакция, более высокая степень кишечной метаплазии. Процесс затрагивает в основном антральный отдел и малую кривизну желудка [2, 3].
Аутоиммунный атрофический гастрит составляет 5% среди всех форм гастритов. Образующиеся при этом заболевании антитела приводят к атрофии железистого эпителия. Аутоиммунные реакции вызывают постепенное разрушение железистых эпителиоцитов с сохранением стволовых клеток. Фовеолярный эпителий при аутоиммунном атрофическом гастрите изменяется минимально, воспаление незначительное. Поражаются в основном тело и дно желудка с формированием гипо-, ахлоргидрии. Для этой формы гастрита типично образование антител к париетальным клеткам желудка и внутреннему фактору Кастла [4–7].
Возможно сочетание аутоиммунного и хеликобактерного гастрита. НР может индуцировать аутоиммунный процесс в желудке посредством механизмов молекулярной мимикрии и/или эпитопного распространения. Было обнаружено, что субъединица уреазы HР и субъединица желудочной АТФ-азы имеют гомологичное строение [8]. НР-ассоциированный аутоиммунный гастрит характеризуется метапластической атрофией тела желудка, нормальным или с умеренным воспалением антральным отделом, текущей или анамнестической инфекцией HР, а также наличием аутоантител [9].
Диагностика аутоиммунного гастрита (АИГ) представляет определенные трудности. Демонстрацией сочетания АИГ с гематологическими и эндокринными проявлениями может служить нижеприведенное клиническое наблюдение.
ОПИСАНИЕ КЛИНИЧЕСКОГО СЛУЧАЯ
Пациентка С., 38 лет, обратилась к терапевту с жалобами на выраженную общую слабость, головокружение, быструю чрезмерную утомляемость, сухость кожи, парестезии в нижних конечностях, ухудшение памяти. Эти симптомы беспокоили в течение месяца к моменту обращения.
Данные осмотра: нормостеническое телосложение, индекс массы тела (ИМТ) 23,4 кг/м2. Кожа бледная, склеры иктеричные. В легких дыхание везикулярное. Тоны сердца ритмичные, частота сердечных сокращений (ЧСС) 70/мин. Артериальное давление (АД) 120 на 80 мм рт.ст. Живот обычной формы, при пальпации мягкий, безболезненный. Печень по Курлову: 9 × 8 × 7 см, край эластичный, селезенка не пальпировалась.
Данные общего анализа крови: гемоглобин – 95 г/л; эритроциты – 3,2 × 1012/л; средний объем эритроцита (MCV) – 102,5 fl; среднее содержание гемоглобина в эритроците (MCH) – 34,6 pg; ретикулоциты – 1,2%; лейкоциты – 3,6 × 109/л; тромбоциты – 124 × 109/л. Лейкоцитарная формула: сегментоядерные нейтрофилы – 54%; лимфоциты – 32%; эозинофилы – 3%; моноциты – 11%. Скорость оседания эритроцитов (СОЭ) – 28 мм/ч.
Данные биохимического анализа крови: билирубин общий – 32 мкмоль/л (норма – до 20,5 ед/л); прямая фракция – 8 мкмоль/л (норма – до 5,1 ед/л); креатинин – 72 мкмоль/л (норма – до 97,0 мкмоль,л); мочевина – 5,2 ммоль/л (норма – до 8,3 ммоль/л); аланинаминотрансфераза (АЛТ) – 32 Ед/л (норма – до 34 Ед/л), аспартатаминотрансфераза (АСТ) – 35 Ед/л (норма – до 40 Ед/л); лактатдегидрогеназа (ЛДГ) – 865 Ед/л (норма – до 250 Ед/л); общий белок – 72 г/л (норма – 65–85 г/л); сывороточное железо – 5,9 мкмоль/л (норма – 6,6–26 мкмоль,л); ферритин – 14 нг/мл (норма – 13–150 нг/мл); витамин В12 – 40 пг/мл (норма – 197–771 пг/мл).
Наличие у пациента макроцитарной гиперхромной анемии с легкой лейкопенией и тромбоцитопенией в сочетании с синдромом гемолиза, который проявлялся иктеричностью склер, повышением уровня ЛДГ, непрямого билирубина и существенным снижением концентрации витамина В12 и железодефицитом, дали основание диагностировать хроническую витамин В12 и железодефицитную анемию легкой степени тяжести. Была проведена терапия цианкобаламином и пероральными препаратами сульфата железа, после которой отмечалась положительная гематологическая динамика: в общем анализе крови гемоглобин повысился до 123 г/л, эритроциты – до 4,8 × 1012/л, ретикулоциты – до 2,5%, лейкоциты – до 4,6 × 109/л, тромбоциты – до 152 × 109/л. MCV составили 89,5 fl, MCH – 32,6 pg. Общий билирубин снизился до 18 мкмоль/л, ЛДГ – до 125 Ед/л.
Для установления причины дефицита витамина В12 пациентке было выполнено эндоскопическое исследование верхних отделов желудочно-кишечного тракта (ЖКТ), по результатам которого сделано заключение о наличии атрофической гастропатии: пищевод свободно проходим, просвет не изменен, слизистая розового цвета, кардиальный жом на расстоянии 40 см от резцов, смыкается полностью, Z-линия четкая. Желудок обычных размеров и формы, натощак содержит небольшое количество желчи, слизь, пену. Перистальтика прослеживается во всех отделах. Складки извитые, продольные, эластичные, воздухом расправляются достаточно. Слизистая незначительно отечная, бледная, в антральном отделе пестрая. Привратник зияет, округлый. Луковица двенадцатиперстной кишки округлой формы. Слизистая оболочка розовая, бархатистая. Постбульбарный отдел не изменен. В просвете желчь.
При морфологическом исследовании гастробиоптатов из 5 стандартных точек желудка был выявлен хронический атрофический мультифокальный активный гастрит с выраженной кишечной метаплазией (более 60%), выраженной степенью активности воспалительного процесса в теле желудка, III стадии по системе OLGA.
Исследование антигена HР в кале дало отрицательный результат. В крови были обнаружены антитела к париетальным клеткам желудка IgG + IgА + IgM в титре 1:1280 (норма менее 1:40).
По прошествии 3 мес у пациентки появились раздражительность, нервозность, учащенное сердцебиение и дрожь в руках, которые она связала со стрессовой ситуацией. Однако было установлено, что месяц назад она перенесла COVID-19 в легкой форме.
При осмотре было обнаружено увеличение щитовидной железы до I степени (ВОЗ); ткань железы однородная, подвижная, безболезненная. Определялся мелко размашистый тремор кистей рук. Тоны сердца ясные, ритмичные, ЧСС в покое – 98/мин, АД – 120/80 мм рт.ст. Физикальных отклонений со стороны других органов и систем не обнаружено.
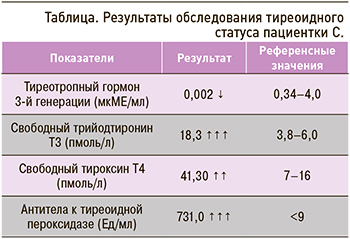
При исследовании тиреоидного статуса было выявлено снижение уровня тиреотропного гормона (ТТГ), повышение содержания свободных фракций трийодтиронина, тироксина и антител к тиреоидной пероксидазе (табл.).
При ультразвуковом исследовании были обнаружены увеличение объема щитовидной железы до 21 см3, диффузно-неоднородная структура и снижение эхогенности ткани железы.
Таким образом, у пациентки С. были выявлены манифестный тиреотоксикоз и синдром зоба. Для верификации диагноза были определены антитела к рецептору ТТГ, которые оказались повышенными до 3,2 МЕ/л (норма – до 1,75 МЕ/л); это позволило диагностировать диффузный токсический зоб I степени (ВОЗ), манифестный тиреотоксикоз.
Пациентке была проведена терапия тиамазолом (таблетки 10 мг 3 раза/сут) и бисопрололом (таблетки 5 мг 1 раз/сут), что привело к нормализации гормональных показателей и купированию клинической симптоматики.
ОБСУЖДЕНИЕ: КОММЕНТАРИЙ ГЕМАТОЛОГА
Согласно определению Всемирной организации здравоохранения (ВОЗ), анемией называют клинико-гематологический синдром, характеризующийся снижением уровня гемоглобина и в большинстве случаев гемоглобина и эритроцитов в единице объема. По критериям ВОЗ, анемия у мужчин диагностируется при уровне Hb ниже 130 г/л, у женщин — ниже 120 г/л, у беременных — ниже 110 г/л.
B12-дефицитная анемия является макроцитарной, к отличительным морфологическим признакам которой относятся гиперхромия эритроцитов и мегалобластный эритропоэз [10]. Учение о В12-дефицитной анемии берет свое начало в 1872 г., когда Biermer М.А. предложил термин «пернициозная» анемия, учитывая ее неизлечимость на тот момент. В 30-х гг. XX в. Castle W.В. начал лечить такую анемию, вводя в рацион больных говядину, обработанную желудочным соком. Это послужило основой для гипотезы о внутреннем и внешнем факторах, которые дают положительный клинический эффект. В 1948 г. витамин В12 был выделен в чистом виде, и на сегодня злокачественный характер витамин В12-дефицитной анемии является только историческим фактом [11].
В настоящее время отмечается рост заболеваемости витамин В12-дефицитной анемией. Эта патология характерна чаще всего для лиц пожилого возраста, но в ряде случаев диагностируется и у молодых [12]. Основным звеном патогенеза такой анемии выступает нарушение процессов синтеза РНК и ДНК в гемопоэтических клетках костного мозга, в которых участвует витамин В12.
Основную долю витамина В12 организм получает из продуктов животного происхождения: печени, мяса, яиц, рыбы, молока. Незначительное его количество синтезируется микрофлорой кишечника. В желудке В12 подвергается протеолизу и связывается с внутренним фактором, который представляет собой гликопротеин, синтезируемый париетальными клетками тела и дна желудка. В дистальном отделе подвздошной кишки комплекс витамин В12-гастромукопротеид всасывается при условиях нейтрального рН и в присутствии ионов кальция. Далее путь витамина В12 идет в портальный кровоток, где он связывается с белками плазмы, которая содержит его в виде трех коэнзимов: метилкобаламина, аденозилкобаламина и гидроксикобаламина. В процессах гемопоэза участвует метилкобаламин. Из него синтезируется тимидин, входящий в состав ДНК. В результате ряда реакций при дефиците витамина В12 происходит накопление метилмалоновой кислоты, которая в повышенных концентрациях обладает выраженным цитотоксическим, в частности нейротропным действием [13].
Причинами дефицита витамина В12 могут быть:
1. Длительное недостаточное поступление с пищей (у вегетарианцев) – через 2–4 года полного отсутствия в рационе пищи животного происхождения.
2. Заболевания ЖКТ (тотальная гастрэктомия, рак желудка, полипы желудка и кишечника, энтериты, резекция тонкой кишки, хронический алкоголизм, дивертикулы кишечника, глистная инвазия, врожденная недостаточность внутреннего фактора, аутоиммунные процессы в слизистой оболочке ЖКТ) [14].
Достаточно часто в сыворотке крови пациентов с В12-дефицитной анемией обнаруживаются различные антитела: к антигенам цитоплазмы париетальных клеток желудка, к внутреннему фактору и белкам-носителям. Наличие гематологических отклонений у пациентов с высоким титром антител к париетальным клеткам было исследовано в 2016 г. Е.А. Лосик с соавт. Наличие макроцитарной и гиперхромной анемии было выявлено только у 4 из 71 респондентов (5,6%), причем все они были старше 50 лет. У большинства пациентов единственным проявлением дефицита В12 было увеличение среднего объема эритроцитов [15].
Клинически В12-дефицитная анемия достаточно долго может протекать скрыто. Биохимически дефицит витамина В12 диагностируется при снижении его концентрации в сыворотке крови менее 140 пг/мл [10]. Латентный дефицит В12 выявляется значительно чаще, чем В12-дефицитная анемия [16].
Выделяют группы риска, которым целесообразно проводить скрининг на доклинический дефицит витамина В12 по аналогии с латентным дефицитом железа: пожилые люди старше 75 лет, вегетарианцы, пациенты с резецированным желудком, кишечником, воспалительными заболеваниями кишечника, заболеваниями ЖКТ с синдромом мальабсорбции, принимающие метформин более 3 мес и ингибиторы протонной помпы более 12 мес [17].
Для В12-дефицитной анемии характерны типичные проявления анемического синдрома: слабость, утомляемость, головные боли, шум в ушах, головокружение, одышка при незначительной физической нагрузке, сонливость, сердцебиение, перебои в работе сердца. Кожа у таких пациентов чаще имеет желтушный оттенок за счет развивающегося в ряде случаев гемолиза. Глубокий дефицит витамина В12 приводит к ряду неврологических нарушений, что обусловлено поражением белого вещества задних и боковых рогов спинного мозга и дегенерацией периферических нервов. Синдром фуникулярного миелоза проявляется онемением кончиков пальцев, пощипыванием языка, нарушением координации походки, вплоть до развития атаксии. Нарушаются чувствительность, устойчивость в пробе Ромберга, развиваются гиперрефлексия, клонус стоп, эмоциональная неуравновешенность, агрессивность.
Гематологически витамин В12-дефицитная анемия проявляется снижением гемоглобина различной степени выраженности, гиперхромией и макроцитозом, повышением эритроцитарных индексов (MCV, MCH), анизоцитозом, пойкилоцитозом. Макроциты содержат остатки ядер (тельца Жолли, кольца Кебота), базофильную пунктацию (остатки рибосом). В результате дефицита В12 синтез ДНК нарушается не только в клетках эритроидного ряда, но и в лейкоцитах, тромбоцитах, что может сопровождаться лейко- и тромбоцитопенией. В костном мозге выявляется мегалобластический тип кроветворения [14].
Биохимически может выявляться синдром внутриклеточного гемолиза, который сопровождается желтушностью кожи, иктеричностью склер, повышением общего билирубина за счет непрямой фракции, лактатдегидрогеназы (ЛДГ) и снижением гаптоглобина, иногда увеличением селезенки.
Чаще всего диагностика дефицита витамина В12 не вызывает трудностей. Уже на момент сбора анамнеза и расшифровки общего анализа крови может быть выставлен правильный диагноз. После определения характера анемии необходимо провести диагностику ее причины. Для этого рекомендуется в первую очередь обследование ЖКТ.
Лечение анемии проводится согласно клиническим рекомендациям 2021 г. Всем пациентам с установленным диагнозом В12-дефицитной анемии вводится цианокобаламин в дозе 100–200 мкг/сут через день; в случае присоединения нарушений функции нервной системы – 400–500 мкг/сут в первую неделю ежедневно, далее с интервалами между введениями до 5–7 дней. В случае развития лекарственного аллергического дерматита прмиенение цианокобаламина сочетают с глюкокортикоидами. При повторных введениях препарата удается избежать развития нежелательных аллергических реакций путем уменьшения его дозы до 100–200 мг/сут, что не снижает эффективность терапии.
Дефицит В12 может сочетаться с латентным дефицитом железа. Активизация эритропоэза введением цианокобаламина может усугубить железодефицит. Поэтому диагностика состояния статуса железа обязательна для пациентов с дефицитом В12 с целью своевременной его коррекции.
Длительность терапии цианкоболамином определяется тяжестью В12-дефицитной анемии. После регресса анемии, лейкопении, тромбоцитопении и всех морфологических аномалий эритроцитов курс лечения продолжается еще 10–14 дней с целью создания «запасов» витамина В12 в печени.
Вследствие нарушения синтеза ДНК дистрофия проявляется практически во всех органах и системах, что усугубляет гипоксию при В12-дефицитной анемии, которая субъективно плохо переносится пациентами. Поэтому вопрос назначения заместительной терапии эритроцитсодержащими компонентами крови решается индивидуально. Пациенты пожилого и старческого возраста часто нуждаются в проведении гемокомпонентной терапии даже при умеренном снижении гемоглобина (до 75–85 г/л) [10].
ОБСУЖДЕНИЕ: КОММЕНТАРИЙ ЭНДОКРИНОЛОГА
Для верификации диагноза у пациентки с манифестным тиреотоксикозом и диффузным увеличением щитовидной железы были исследованы антитела к рецептору ТТГ, которые оказались повышенными. Эти антитела специфичны для диффузного токсического зоба (ДТЗ).
ДТЗ – это аутоиммунное заболевание, характеризующееся тиреотоксикозом и диффузным увеличением щитовидной железы. 80–85% случаев синдрома тиреотоксикоза во всем мире вызвано этим заболеванием. Чаще им страдают женщины молодого возраста. ДТЗ относится к мультифакторным заболеваниям: в его развитии имеют значение наследственность, инфекция, стресс, инсоляция и курение.
Патогенез ДТЗ в первую очередь связан с нарушением иммунорегуляции в сочетании с органической дисфункцией, являющейся следствием антигенспецифической атаки, дополняемой недостаточной супрессией и, следовательно, активацией лимфоцитов, действие которых направлено на антигены тироцитов. Другая сторона патогенеза ДТЗ – нарушение иммунорегуляции в сочетании с выработкой различных цитокинов (например, интерферона-γ), воздействующих на клетки-мишени с близкого расстояния [18, 19].
Запускать выработку аутоантител, которые вступают в перекрестную реакцию с аутоантигеном, после чего развивается иммунный ответ на соответствующие структуры клеток щитовидной железы, способен вирусный или микробный антиген, обладающий сходством с аутоантигеном по механизму молекулярной мимикрии. Аутоиммунные заболевания щитовидной железы, вероятно, провоцируются каким-то внешним фактором, например инфекцией, и этот фактор запускает экспрессию тироцитами HLA-DR, которая и приводит к развитию аутоиммунной патологии. Вместе с тем сторонники данной теории признают и дополнительную необходимость в нарушении функционирования иммунной системы. Вследствие цитокиновой стимуляции или комплементной атаки тиреоидные клетки могут продуцировать иммуноактивные молекулы (простагландин E2, интерлейкины 6 и 8), что дополнительно усиливает тироцитно-иммуноцитную сигнализацию [19, 20].
В качестве фактора, оказывающего повреждающее действие на щитовидную железу, могут выступать вирусы. Wei L. et al. (2003) пытались найти любое потенциально возможное повреждение собственно ткани щитовидной железы, вызванное SARS-CoV, в образцах, полученных при аутопсии 5 больных, в сравнении с 10 пациентами, умершими от других причин с неповрежденной щитовидной железой. Сверхэкспрессия некоторых неструктурированных белков SARS-CoV способна индуцировать апоптоз. Исследователи предположили, что именно непосредственное поражение железы приводит к снижению продукции тиреоидных гормонов. Было показано, что фолликулярный эпителий повреждался и слоями перемещался внутрь фолликула. Было выявлено множество подвергшихся апоптозу клеток, структура самого фолликула была деформирована: фолликулы становились расширенными или, наоборот, сплющенными, отсутствовали кальцитонин-позитивные клетки [19, 21].
В настоящее время вирусные поражения щитовидной железы рассматриваются чаще всего в контексте триггера подострого тиреоидита, «молчащего тиреоидита», иммуногенного тиреотоксикоза или гипотиреоза. Прямые доказательства присутствия вирусов в тканях щитовидной железы получены для ретровирусов и вируса паротита при подостром тиреоидите, ретровирусов (Т-лимфотропного вируса человека 1 типа, вируса пенистости человека, ВИЧ) при болезни Грейвса, энтеровирусов, вирусов краснухи, паротита, герпесвирусов и парвовирусов при аутоиммунном тиреоидите [22].
В исследование Lui D.T.W. et al. (2021) был включен 191 пациент с COVID-19 без предшествовавшей тиреоидной патологии (средний возраст 53,5±17,2 года, 51,8% мужчин). 84,3% участника перенесли COVID-19 легкой степени, 12,6% – средней и 3,1% – тяжелой степени. Исследователи пришли к выводу, что SARS-CoV-2 может оказывать прямое влияние на функцию щитовидной железы, так как около 15% пациентов с COVID-19 легкой и средней степени тяжести имели дисфункцию этого органа [23].
В другом зарубежном исследовании (Lania A. et al., 2020) приняли участие 287 пациентов (193 мужчин, средний возраст 66 лет, возрастной диапазон 27–92 года), госпитализированных с COVID-19 в обычные отделения. У них была проведена оценка функциональных тестов щитовидной железы и значений сывороточного интерлейкина 6. У 58 пациентов (20,2%) был обнаружен тиреотоксикоз (явный в 31 случае), у 15 (5,2%) диагностирован гипотиреоз (явный только в 2 случаях), 214 пациентов (74,6%) имели нормальную функцию щитовидной железы. В многомерном анализе тиреотоксикоз оказался значимо связанным с более высоким уровнем интерлейкина 6 (отношение шансов 3,25; 95% доверительный интервал: 1,97–5,36; p <0,001), его зависимость от возраста пациентов установлена не была (p=0,09) [24].
На сегодняшний день нет данных о непосредственном поражении щитовидной железы вирусом SARS-CoV-2, тем не менее результаты имеющихся исследований и клинических наблюдений указывают на потенциальное влияние коронавирусных инфекций, в частности SARS-CoV и SARS-CoV-2, на гипоталамо-гипофизарно-тиреоидную ось с развитием различной патологии или изменениями содержания тиреоидных гормонов [25].
В представленном клиническом наблюдении не исключается триггерная роль вируса SARS-CoV-2 в развитии ДТЗ у пациентки с имеющейся иммунной дисфункцией, проявлявшейся аутоиммунным гастритом и пернициозной анемией.
ОБСУЖДЕНИЕ: КОММЕНТАРИЙ ГАСТРОЭНТЕРОЛОГА
При иммунологическом исследовании пациентки были получены высокие титры антител IgG+A+M к париетальным клеткам желудка, которые составили 1:1280, и отрицательный результат тестирования на HP, что позволило диагностировать АИГ, III стадию (OLGA). Это заболевание встречается нечасто, в основном у женщин (3:1 относительно мужчин), и протекает бессимптомно. Иногда пациентов могут беспокоить проявления диспепсии, при этом в 60% случаев они соответствуют постпрандиальному дистресс-синдрому. Carabotti М. et al. исследовали зависимость симптомов АИГ от выраженности изменений в желудке и не обнаружили какой-либо связи между гистологической тяжестью повреждения СОЖ и наличием клинических признаков. Распределение легкой, умеренной и тяжелой атрофии тела желудка достоверно не отличалось между группами симптомных и бессимптомных больных. Дискинетические нарушения желудка были достоверно связаны с гипохлоргидрией [26].
Наиболее характерные клинические проявления АИГ – гематологические заболевания. У пациентов формируется железодефицитная анемия, которая связана с ахлоргидрией и нарушением всасывания негемового железа, или В12-дефицитная анемия, обусловленная отсутствием внутреннего фактора Кастла и связанного с этим нарушением всасывания цианокобаламина [27].
АИГ сочетается с другими аутоиммунными заболеваниями и наиболее часто с болезнями щитовидной железы. При АИГ в 3–8 раз чаще, чем в общей популяции, диагностируется аутоиммунный тиреоидит Хашимото. Нарушения фолликулярных клеток щитовидной железы и париетальных клеток желудка обусловлены многофакторной этиологией, возникающей в результате ассоциации между генетической восприимчивостью и рядом факторов окружающей среды. Специфический механизм, приводящий к повреждению тироцитов и/ или париетальных клеток, до сих пор плохо изучен, но этот сходный феномен частично объясняют общим эмбриологическим происхождением слизистых оболочек желудка и фолликулярных клеток щитовидной железы, развивающихся из эндодермы и имеющих некоторые функциональные и морфологические сходства [27].
Сахарный диабет 1-го типа – второе по частоте аутоиммунное заболевание, связанное с АИГ: у соответствующих пациентов он диагностируется в 2 раза чаще, чем в общей популяции. В более редких случаях встречаются витилиго, болезнь Аддисона, целиакия, ревматоидный артрит, псориаз, синдром Шегрена, миастения и другие аутоиммунные болезни [28].
К характерным иммунологическим показателям при АИГ относятся повышенные титры антител к париетальным клеткам желудка и внутреннему фактору Кастла.
Аутоиммунное повреждение клеток желудка, так же как и инфекция НР, вызывает атрофию слизистой оболочки, с выраженностью и распространенностью которой коррелирует риск развития рака желудка, что признано всеми международными сообществами. Хеликобактерный атрофический гастрит повышает риск некардиального рака желудка, а аутоиммунный – нейроэндокринных опухолей [29, 30]. В исследовании Terao Sh. et al. (2020) у больных с АИГ нейроэндокринные опухоли 1-го типа диагностировались в 11,4% случаев, аденокарциномы – в 9,8%, гиперпластические полипы – в 21,1% [31]. Развитие нейроэндокринных опухолей объясняется гиперплазией энтерохромафинных клеток на фоне гиперсекреции гастрина в ответ на ахлоргидрию у пациентов с АИГ. Опухоли 1-го типа встречаются наиболее часто среди всех нейроэндокринных опухолей (70–80%), носят мультицентрический характер с локализацией в области тела и дна желудка, имеют малый размер (менее 1–2 см). В 65% опухоли являются множественными, в 78% – полиповидными, в 5–10% случаев они метастазируют в лимфоузлы, в 2–5% – в печень [28].
Для диагностики атрофии СОЖ рекомендуется использовать неинвазивную методику определения уровня пепсиногенов в сыворотке крови. При НР-ассоциированном гастрите чаще регистрируются нормальный уровень пепсиногена I типа, разнонаправленные изменения пепсиногена II типа и снижение гастрина-17, тогда как для АИГ характерно значительное снижение пепсиногена I типа, нормальные цифры пепсиногена II типа и повышение гастрина-17 [32]. Согласно международным рекомендациям, низкие уровни пепсиногена-I в сыворотке и/или низкое соотношение пепсиногенов I и II типа позволяют выявить пациентов с продвинутыми стадиями атрофического гастрита, которым рекомендуется выполнение эндоскопии. При этом эндоскопическое исследование должно проводиться с взятием биопсийных образцов как минимум на двух топографических участках (по малой и большой кривизне, в антральном отделе и в теле желудка), также необходима дополнительная биопсия из угла желудка. Для выявления пациентов с гастритом на поздних стадиях могут использоваться системы гистологического определения стадии заболевания (системы OLGA и OLGIM). III и IV стадии указывают на более высокий риск развития рака желудка [33].
В целях канцеропревенции пациенты с атрофическим гастритом нуждаются в лечении и наблюдении. При НР-ассоциированном гастрите основной терапевтической стратегией служит эрадикационная терапия. Признано, что эрадикация НР лечит хронический неатрофический гастрит, может привести к регрессии атрофического гастрита и снижает риск рака желудка [33]. В исследовании Е.А. Лосик с соавт. показано, что проведение эрадикационной терапии НР у пациентов с антителами к париетальным клеткам желудка тормозит дальнейшее прогрессирование атрофических изменений слизистой оболочки тела желудка и способствует стабилизации уровня пепсиногена I [9].
Вместе с тем собственно лечение АИГ не разработано. В рамках терапии осуществляется коррекция железо- или В12-дефицитной анемии, возможно применение цитопротекторов. Было показано положительное влияние при АИГ ионов висмута, которые способны блокировать свободные кислородные радикалы, ослабляя повреждение клеток желудка [34]. В двух рандомизированных клинических исследованиях с участием 280 пациентов продемонстрировано уменьшение воспаления при применении ребамипида, в одном из них отмечено влияние препарата на кишечную метаплазию и дисплазию низкой степени [35, 36]. Согласно рекомендациям Российской гастроэнтерологической ассоциации (РГА) 2021 г., пациентам с хроническим гастритом, в том числе атрофическим, с целью потенцирования защитных свойств слизистой оболочки возможно рекомендовать терапию висмута трикалия дицитратом или ребамипидом в течение 4–8 нед (уровень убедительности рекомендаций – В, уровень достоверности доказательств – 2) [37].
Дальнейшее наблюдение за пациентами с атрофическим гастритом предусматривает использование высококачественной эндоскопии каждые 3 года при выраженных атрофических изменениях или кишечной метаплазии как в антральном отделе, так и в теле желудка (OLGA/OLGIM III/ IV стадии) и каждые 1–2 года при наличии семейного анамнеза рака желудка, а также при АИГ [33, 37, 38].
ЗАКЛЮЧЕНИЕ
Хронический атрофический гастрит относится к заболеваниям, которые в значительной степени недооцениваются. Исходом его может стать формирование аденокарциномы желудка. В условиях хронического воспаления происходит потеря желез желудка, с метаплазией или без нее, в основном из-за инфекции НР или аутоиммунного процесса. Независимо от этиологии, диагноз атрофического гастрита должен быть подтвержден гистологически. Всех пациентов с атрофическим гастритом необходимо обследовать на наличие инфекции HР, и при ее выявлении проводить эрадикационную терапию.
Менее распространенной причиной атрофии слизистой оболочки выступает аутоиммунный процесс. Диагностика АИГ – довольно сложная задача ввиду отсутствия специфических желудочно-кишечных симптомов. Ахлоргидрия, разрушение париетальных клеток и внутреннего фактора Кастла приводят к дефициту железа и витамина В12, что и определяет клиническую картину АИГ. У таких пациентов часто имеются сопутствующие аутоиммунные расстройства, наиболее распространенными из которых являются аутоиммунные заболевания щитовидной железы; это также необходимо учитывать при диагностике и лечении. Важное клиническое последствие АИГ – формирование нейроэндокринных опухолей желудка, требующее регулярного эндоскопического и морфологического наблюдения.



