ВВЕДЕНИЕ
Глифлозины представляют собой класс фармакологически активных соединений, способных к селективному обратимому ингибированию трансмембранных гликопротеинов – натрий-глюкозных котранспортеров (sodium/glucose cotransporter, SGLT; рис. 1). SGLT относятся к семейству транспортеров растворенных веществ 5 (solute carrier family 5, SLC5) и включают две изоформы, идентичные приблизительно на 58% [1].
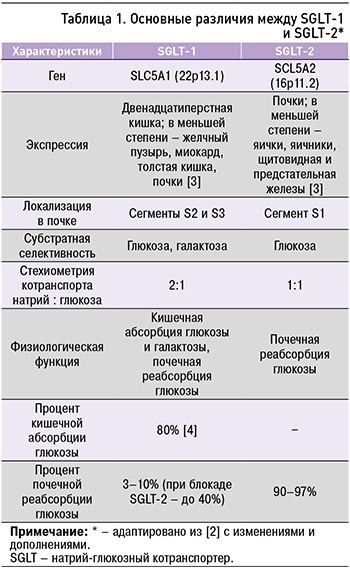
SGLT типа 1 экспрессируются в дистальной части извитого канальца почки и опосредуют реабсорбцию (3–10% в физиологических условиях и до 40% при блокаде SGLT типа 2) глюкозы из первичной мочи. SGLT типа 2 осуществляют натрий-зависимую реабсорбцию 90–97% глюкозы в проксимальных почечных канальцах [2]. Основные различия между двумя типами SGLT приведены в таблице 1.
История глифлозинов началась в 1835 г. с выделением из коры яблони структурного родоначальника группы – халкона флоризина – и последовавшего за этим открытия его способности вызывать глюкозурию [5]. На сегодняшний день известны следующие подгруппы глифлозинов.
1. Селективные ингибиторы SGLT-1: GSK1614235; KGA-2727; мизаглифлозин.
2. Селективные ингибиторы SGLT-2: бексаглифлозин; дапаглифлозин; ипраглифлозин; канаглифлозин; лусеоглифлозин; ремоглифлозин; серглифлозин; тофоглифлозин; эмпаглифлозин; энавоглифлозин; эртуглифлозин.
3. Двойные ингибиторы SGLT-1/2: LX2761; ликоглифлозин; сотаглифлозин.
В общем случае глифлозины являются С-гликозидами β-D-глюкозы с замещенной аномерной гидроксильной группой (рис. 2), однако среди них встречаются соединения с несколько отличающейся структурой как гликона, так и агликона [6].
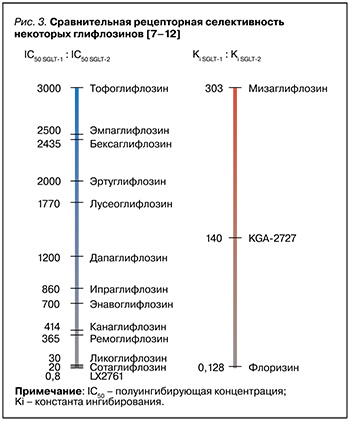
Имеющиеся на сегодня данные о селективности некоторых представителей группы глифлозинов отражены на рисунке 3.
Общие закономерности фармакокинетики глифлозинов подразумевают сравнительно высокую биодоступность при приеме внутрь, высокую степень связывания с белками и печеночный метаболизм с образованием фармакологически неактивных глюкуронидов и окисленных форм. В реакциях метаболизма принимают незначительное участие CYP1A1, CYP1A2, CYP2A6, CYP2C9, CYP2D6 и CYP3A4 [13]. Эмпаглифлозин, канаглифлозин и эртуглифлозин являются субстратами гликопротеина P [14–16].
АНТИДИАБЕТИЧЕСКАЯ, КАРДИОИ НЕФРОПРОТЕКТИВНАЯ АКТИВНОСТЬ ГЛИФЛОЗИНОВ
Ингибиторы SGLT-2 разрабатывались как новый класс антидиабетических средств с оригинальным механизмом действия – угнетением почечной реабсорбции глюкозы и снижением ее сывороточной концентрации [17]. В 2012–2014 гг. для клинического применения при сахарном диабете 2-го типа (СД 2) Европейским медицинским агентством (EMA) и Управлением по контролю качества пищевых продуктов и лекарственных средств (FDA) были одобрены дапаглифлозин, канаглифлозин и эмпаглифлозин [18–23]. В то время стандартной проблемой при появлении на рынке новых антидиабетических средств были опасения их неблагоприятных кардиоваскулярных эффектов, особенно развития или утяжеления сердечной недостаточности [24–28]. Поэтому сердечно-сосудистые события стали предметом особенного внимания и при проведении рандомизированных клинических исследований (РКИ) с глифлозинами [29]. Однако итоги уже первого крупного исследования – EMPA-REG OUTCOME (2015) – показали, что такой ингибитор SGLT-2, как эмпаглифлозин не просто безопасен для больных СД 2 высокого сердечно-сосудистого риска, но и кардинально улучшает их кардиоваскулярный и «почечный» прогноз, снижая сердечно-сосудистую и общую смертность [30, 31]. Результаты КИ с канаглифлозином (CANVAS program, CREDENCE), дапаглифлозином (DECLARE-TIMI 58), а позднее и с сотаглифлозином (SOLOIST-WHF, SCORED) в целом подтвердили данные, полученные в EMPAREG OUTCOME, что позволило говорить о классспецифическом эффекте ингибиторов SGLT-2 [32– 36]. Общим для всех изученных глифлозинов оказалось снижение числа госпитализаций по поводу сердечной недостаточности и замедление прогрессирования дисфункции почек у больных СД 2.
Между тем от внимания исследователей не ускользнула диспропорция между «скромным» сахароснижающим эффектом ингибиторов SGLT2 и «драматическим» улучшением исходов, а также отсутствие прямой связи между этими характеристиками. Довольно скоро была сформулирована и в дальнейшем частично экспериментально подтверждена гипотеза об универсальном кардиоренопротективном действии глифлозинов, выходящем за рамки лечения нарушений углеводного обмена [37–43].
Результаты РКИ не заставили себя долго ждать.
В 2019 г. триумфально завершилось исследование DAPA-HF, доказавшее способность дапаглифлозина улучшать прогноз и снижать смертность больных хронической сердечной недостаточностью (ХСН) с низкой фракцией выброса, среди которых больше половины составляли пациенты без нарушений углеводного обмена [44]. В следующем году сходные результаты у близкой по составу группы обследуемых были получены и в РКИ EMPEROR-Reduced, где применялся эмпаглифлозин [45]. Согласно анализу результатов этих исследований, у больных ХСН с низкой фракцией выброса столь же впечатляюще улучшились и «почечные» исходы. Как показали недавно завершенные РКИ EMPEROR-Preserved (2021) и DELIVER (2022), кардиопротективное действие ингибиторов SGLT-2 эмпаглифлозина и дапаглифлозина распространяется и на больных ХСН с сохраненной фракцией выброса независимо от наличия или отсутствия СД [46, 47]. У этих пациентов снизился риск обострения ХСН либо смерти от сердечно-сосудистых причин в сравнении с плацебо.
Не менее убедительными оказались и результаты исследования ренопротективного потенциала глифлозинов. Так, по итогам РКИ DAPA-CKD ингибитор SGLT-2 дапаглифлозин в сравнении с плацебо снижал комбинированный риск стойкого снижения расчетной скорости клубочковой фильтрации не менее чем на 50%, развития терминальной стадии заболевания почек или смерти от почечных или сердечно-сосудистых причин у больных хронической болезнью почек (ХБП) независимо от состояния углеводного обмена и функции почек [48]. Кроме того, лечение дапаглифлозином было ассоциировано со снижением кардиоваскулярной смертности и госпитализаций по поводу ХСН, а также смертности от всех причин. Назначение эмпаглифлозина больным ХБП, в свою очередь, приводило к улучшению комбинированного исхода – риска прогрессирования заболевания почек или смерти от сердечно-сосудистых причин как у пациентов с СД, так и без него [49].
Приведенные данные свидетельствует об универсальных кардиоренопротективных свойствах ингибиторов SGLT-2, в первую очередь об их способности препятствовать прогрессированию ХСН и ХБП у широкого круга больных, а не только у лиц с СД. Однако признание этого факта, уже нашедшее отражение в современных рекомендациях по лечению СД, сердечной недостаточности и ХБП, поднимает вопрос о механизмах действия глифлозинов, в том числе их возможных плейотропных эффектах. И здесь открывается огромное поле для гипотез и дискуссий, которое не может быть охвачено рамками настоящей работы. Особое внимание привлекают метаболические и противовоспалительные эффекты глифлозинов, которые могут вносить немалый вклад в конечную эффективность этих лекарств и, кроме того, увеличивать число потенциальных мишеней терапии. В частности, широкие перспективы применения глифлозины могут иметь при неалкогольной жировой болезни печени (НАЖБП) – состоянии, тесно связанном с СД и диабетической нефропатией и уже поэтому вызывающем соблазн «убить двух зайцев одним выстрелом», если перефразировать оригинальное заглавие обзора японских исследователей, посвященного лечению НАЖБП [50].
ГЕПАТОПРОТЕКТОРНАЯ АКТИВНОСТЬ ГЛИФЛОЗИНОВ
Гепатотропная активность глифлозинов реализуется различными путями. Так, блокада SGLT-1 сопровождается угнетением постпрандиального всасывания глюкозы и галактозы в тонкой кишке, повышением сывороточных уровней инкретинов глюкагоноподобного пептида-1 (glucagon-like peptide 1, GLP-1), глюкозозависимого инсулинотропного пептида (glucose-dependent insulinotropic peptide, GIP) и пептида YY (peptide YY, PYY), а также вносит определенный вклад в подавление канальцевой реабсорбции глюкозы [51].
GLP-1 – пептидный гормон, секретируемый L-клетками слизистой оболочки кишечника в ответ на поступление пищи в желудочно-кишечный тракт (ЖКТ) [52]. Рецепторы GLP-1 обнаруживаются в поджелудочной железе, белой жировой ткани, печени, головном мозге, скелетных мышцах, почках и легких [53]. GLP-1 стимулирует глюкозозависимую секрецию инсулина β-клетками, подавляет секрецию глюкагона, оказывает анорексигенный эффект и угнетает перистальтику ЖКТ, повышает чувствительность периферических тканей к действию инсулина и замедляет липогенез [54, 55]. Активация рецепторов GLP-1, расположенных на мембранах гепатоцитов, приводит к угнетению продукции профиброгенного FGF21 и снижению скорости глюконеогенеза и интенсификации β-окисления высших жирных кислот. Кроме этого, GLP-1 и его миметики оказывают умеренное противовоспалительное действие за счет косвенного уменьшения продукции C-реактивного белка, провоспалительных цитокинов (включая интерлейкин 6, фактор некроза опухоли-альфа) и хемокинов [55].
Пептид YY (пептид тирозин-тирозин, аноректический пептид) преимущественно секретируется L-клетками дистальных отделов кишечника. Этот инкретин подавляет желудочную и экзокринную панкреатическую секрецию, повышает чувствительность периферических тканей к инсулину, стимулирует энергетический обмен и принимает участие в работе центральных механизмов постпрандиального чувства насыщения [56]. Одно из клинических исследований позволило установить, что улучшение функции печени на фоне терапии дапаглифлозином было ассоциировано со снижением сывороточных уровней дипептидилпептидазы-4, расщепляющей GLP-1 и другие инкретины [57].
К прямым эффектам ингибиторов SGLT-2 относят гипогликемизирующий и натрийуретический. Уменьшение гликемии происходит путем увеличения выведения глюкозы с мочой и не зависит от секреторной функции поджелудочной железы и чувствительности инсулинзависимых тканей к действию инсулина. Помимо этого, для этой группы соединений характерно наличие ряда вторичных механизмов действия, в том числе обусловливающих их эффективность в условиях патологий печени [2].
Так, при уменьшении концентраций глюкозы в плазме крови происходит сдвиг равновесия в сторону утилизации липидов, а также уменьшение инсулин/глюкагонового индекса, что вызывает интенсификацию кетогенеза, глюконеогенеза, гликогенолиза и β-окисления высших жирных кислот [58, 59]. Ингибиторы SGLT-2 снижают уровни лептина через подавление его секреции адипоцитами [60, 61], что обусловливает анорексигенный эффект, способствует уменьшению инсулинорезистентности и препятствует активации звездчатых клеток печени, лежащей в основе фиброгенеза [62]. Увеличение сывороточных концентраций адипонектина на фоне терапии ингибиторами SGLT-2 уменьшает гиперинсулинемию, увеличивает чувствительность периферических тканей к действию инсулина и оказывает косвенное противовоспалительное действие путем ингибирования сигнального пути фактора некроза опухоли-альфа (tumour necrosis factor α, TNFα) [63].
Кроме этого, ингибиторы SGLT-2 способны угнетать активность симпатической нервной системы и усиливать влияние блуждающего нерва, что определяет их противовоспалительный эффект за счет подавления активации клеток Купфера [58]. Благодаря своему диуретическому и натрийуретическому действию, ингибиторы SGLT-2 могут оказывать положительное действие при портальной гипертензии и асците на фоне патологий печени [63].
Основные механизмы действия глифлозинов в условиях НАЖБП приведены на рисунке 4.

МЕТОДОЛОГИЯ ПОИСКА ПУБЛИКАЦИЙ О ПРИМЕНЕНИИ ГЛИФЛОЗИНОВ ПРИ НАЖБП
Поиск публикаций, посвященных клиническим исследованиям эффективности глифлозинов при НАЖБП, проводился в августе 2023 г. по базе данных MEDLINE (https://pubmed.ncbi.nlm. nih.gov/) [64] с использованием поискового запроса: sodium-glucose transporter 2 inhibitors [Mesh] OR gliflozin [All Fields] OR empagliflozin [All Fields] OR canagliflozin [All Fields] OR dapagliflozin [All Fields] OR sotagliflozin [All Fields] OR ertugliflozin [All Fields] OR licogliflozin [All Fields] OR ipragliflozin [All Fields] OR remogliflozin [All Fields] OR bexagliflozin [All Fields] OR luseogliflozin [All Fields] OR mizagliflozin [All Fields] OR enavogliflozin [All Fields] OR sergliflozin [All Fields] OR tofogliflozin [All Fields]) AND non-alcoholic fatty liver disease [Mesh] OR non-alcoholic steatohepatitis [All Fields]) AND clinical trial [Publication Type].
Поиск проводимых на момент написания обзора клинических исследований выполнялся в августе 2023 г. по международному реестру клинических исследований Национального института здоровья США (https://www.clinicaltrials. gov/) [65] с использованием поискового запроса, содержавшего наименование препарата и целевой патологии: «NASH – Nonalcoholic steatohepatitis; NAFLD – Nonalcoholic fatty liver disease»; по реестру клинических исследований Евросоюза (https://www.clinicaltrialsregister.eu/ ctr-search/search) [66] с использованием поискового запроса, включавшего наименование препарата + Nonalcoholic fatty liver disease; по реестру клинических исследований РФ ClinLine (https://clinline.ru/reestr-klinicheskihissledovanij.html) [67] с использованием поискового запроса, содержавшего название целевой патологии – «стеатогепатит (жировая болезнь печени)».
Алгоритм работы с источниками приведен на рисунке 5.

КЛИНИЧЕСКАЯ ЭФФЕКТИВНОСТЬ ГЛИФЛОЗИНОВ ПРИ НАЖБП
На настоящий момент большинство соединений из группы глифлозинов успели стать объектом клинических исследований, посвященных их эффективности при НАЖБП, как правило, сопутствующей СД 2. Исключение составляют LX2761, бексаглифлозин, мизаглифлозин, сотаглифлозин и энавоглифлозин – их оценка в качестве потенциальных средств терапии НАЖБП пока не проводилась [65–67].
Ингибиторы SGLT-2
Дапаглифлозин
Эффективность дапаглифлозина оценена в 7 проспективных КИ, что делает его одним из наиболее исследованных при НАЖБП глифлозинов.
По данным ретроспективного анализа, у пациентов с НАЖБП и СД 2 препарат оказывал положительное влияние на лобулярное воспаление, баллонную дистрофию гепатоцитов и фибротические изменения в печени, а также уменьшал активность аланинаминотрансферазы (АЛТ), аспартатаминотрансферазы (АСТ) и гамма-глютамилтранспептидазы (ГГТП) [68]. В проспективном КИ II фазы EFFECT-II дапаглифлозин (10 мг/сут в течение 12 нед) значительно уменьшал уровни маркеров повреждения гепатоцитов, включая АЛТ, АСТ, ГГТП, цитокератин-18 (CK-18) и FGF21, а также снижал плотность жировой фракции при НАСГ и СД 2 [69].
Комбинация дапаглифлозин + метформин оказалась более эффективна в отношении нормализации уровня АЛТ и снижения массы тела, чем комбинации метформина с ситаглиптином или линаглиптином у пациентов с НАЖБП на фоне СД 2 [70]. При параллельном сравнении с пиоглитазоном и глимепиридом дапаглифлозин продемонстрировал наиболее выраженное влияние на массу тела и степень висцерального ожирения, а по эффективности влияния на стеатоз печени был сопоставим с пиоглитазоном [71].
При оценке индекса FLI (Fatty Liver Index – индекс стеатоза печени) дапаглифлозин превосходил пиоглитазон [72], а в комбинации с эксенатидом эффективно снижал не только индекс FLI, но и FIB-4 [73]. По антифибротической активности, подтвержденной результатами оценки индекса APRI (Aspartate Aminotransferase to Platelet Ratio Index – индекс отношения АСТ/количество тромбоцитов) у пациентов с НАЖБП, СД 2 и фиброзом печени, дапаглифлозин не уступал пиоглитазону и значительно превосходил ситаглиптин [74]. Согласно данным Shimizu et al., дапаглифлозин эффективен против фиброза печени только на его продвинутых стадиях [75].
По данным небольшого пилотного КИ, дапаглифлозин может обладать терапевтической активностью при НАЖБП даже в отсутствие СД [76]. В настоящее время в пяти КИ 3–4-й фазы оценивается эффективность применения препарата у пациентов с различными стадиями заболевания, в том числе циррозом печени в исходе НАЖБП (NCT05849220), имеющих или не имеющих сопутствующий СД 2 (NCT05849220, NCT03723252, NCT05459701, NCT05308160, NCT05254626). В одном из КИ в качестве препарата сравнения планируется использовать пиоглитазон (NCT05254626).
Ипраглифлозин
Изучению возможностей применения ипраглифлозина при НАЖБП посвящено два завершенных КИ. Этот препарат (50 мг/сут в течение 16 нед) достоверно снижал индекс FLI у пациентов с СД 2 вне зависимости от изменения массы тела и объема подкожного и висцерального жира [77]. По данным ретроспективного исследования, ипраглифлозин уменьшал активность печеночных трансаминаз у пациентов с НАСГ и фиброзом печени, не уступая по эффективности ингибитору DPP4 ситаглиптину [78]. Ипраглифлозин сопоставим с пиоглитазоном в режиме монотерапии по противовоспалительной активности [79] и проявляет синергизм с комбинацией метформин + пиоглитазон, усиливая ее антистеатозное действие [80].
Длительная (50 мг/сут в течение 72 нед) терапия ипраглифлозином также была ассоциирована с уменьшением выраженности неалкогольного фиброза печени по данным гистологического исследования [81].
В настоящее время к проведению планируются 2 рандомизированных КИ с суммарным включением 160 пациентов с СД 2 и НАЖБП, посвященных сравнению эффективности канаглифлозина и пиоглитазона (NCT05513729, NCT05422092).
Канаглифлозин
Как и дапаглифлозин, канаглифлозин (100 мг/сут, 24–52 нед) оказывал положительное влияние на лобулярное воспаление, баллонную дистрофию гепатоцитов и фибротические изменения в печени и уменьшал активность печеночных ферментов при НАЖБП/фиброзе печени и СД 2 [68, 82]. Канаглифлозин селективно повышал чувствительность гепатоцитов, но не адипоцитов и клеток скелетных мышц к действию инсулина, а также снижал накопление триацилглицеролов в гепатоцитах. При этом эффективность препарата была выше у пациентов с подтвержденным стеатозом печени, чем в группе, имевшей только факторы предрасположенности к его развитию [83]. Антифибротический эффект канаглифлозина наблюдался также в условиях НАЖБП и фиброза печени в отсутствие СД [84] и был более заметен у пациентов с фиброзом 1-й стадии по сравнению со стадиями 2–3 [85].
Лусеоглифлозин
Луcеоглифлозин (2,5 мг/сут) превосходил метформин при оценке снижения выраженности стеатоза у пациентов с СД 2 и НАЖБП [86], а также способствовал уменьшению активности АЛТ, АСТ и ГГТП по сравнению с плацебо [87]. В то же время не было выявлено значимого влияния этого препарата на выраженность фиброза печени по результатам оценки уровней некоторых биомаркеров, индекса FIB-4 и шкалы фиброза при НАЖБП [87].
Ремоглифлозин
В двойном слепом плацебо-контролируемом РКИ, включившем 336 пациентов с НАЖБП на фоне СД 2, ремоглифлозин (ремоглифлозина этабонат; 50–1000 мг 2 раза/сут в течение 12 нед) уменьшал выраженность фиброза печени на 5–17% по результатам оценки с использованием индекса FIB-4 и шкалы фиброза при НАЖБП. У пациентов, получавших это лекарственное средство, также наблюдалось уменьшение активности АЛТ на 32–42% по сравнению с исходным уровнем [88]. Несмотря на положительные результаты, полученные в исследовании, о дальнейшем изучении ремоглифлозина в качестве средства лечения болезней печени неизвестно.
Тофоглифлозин
Эффективность тофоглифлозина при НАЖБП, сопутствующей СД 2, оценивалась в режиме монотерапии в сравнении с глимепиридом и пиоглитазоном, а также в комбинации с последним. Тофоглифлозин продемонстрировал более заметное влияние на гистологические признаки поражения печени, включая воспаление и фиброз, нежели глимепирид [89], а также уменьшал стеатоз печени в равной степени с пиоглитазоном, но при этом значимо превосходил его по снижению массы тела [90]. При использовании комбинированного режима терапии был отмечен значительный синергизм между пиоглитазоном и тофоглифлозином во влиянии на стеатоз, неинвазивные маркеры фиброза печени, а также активность АЛТ [91].
Эмпаглифлозин
Результаты ряда клинических исследований свидетельствуют о наличии у эмпаглифлозина (10 или 25 мг/сут) антистеатозной и антифибротической активности в условиях НАЖБП различных стадий на фоне СД 2 [92, 93]. Относительное снижение содержания липидов в печени при терапии этим глифлозином составляет 4–22% [94, 95] от исходного уровня и не коррелирует с уменьшением концентрации гликолизированного гемоглобина (HbA1c) или массы тела [94]. Снижение активности АЛТ под влиянием эмпаглифлозина также не зависит от указанных факторов и наблюдается в том числе у пациентов с субоптимальным гликемическим контролем [93]; при этом влияние препарата на уровни АСТ менее выражено [92].
В исследовании, посвященном оценке нормализации морфологических изменений печени под действием эмпаглифлозина при НАСГ, было установлено, что препарат значительно уменьшает стеатоз, фиброз и баллонную дистрофию гепатоцитов, но не оказывает заметного влияния на лобулярное воспаление [95]. По антистеатозной и антифибротической активности эмпаглифлозин превосходит пиоглитазон [96] и метформин [94]. По данным исследования II фазы, эти эффекты эмпаглифлозина наблюдаются и у пациентов с патологией печени, не имеющих СД 2 [97].
Эмпаглифлозин является безусловным лидером среди глифлозинов по числу активных КИ в области патологии печени. На сегодняшний день планируется оценка его эффективности при различных стадиях НАЖБП, включая цирроз печени неалкогольной этиологии, как в режиме монотерапии (6 КИ: NCT04642261, NCT05605158, NCT03867487, NCT05694923, NCT02964715, NCT05946148), так и в комбинации с другими агентами, включая пиоглитазон (NCT04976283, NCT03646292, NCT05942963), пан-PPAR-агонист ланифибранор (NCT05232071), аналог GLP-1 семаглутид (NCT04639414), метформин (NCT05041673) и экспериментальные соединения MET409 (NCT04702490) и BI 685509 (2021-005171-40).
В качестве препаратов сравнения будут выступать как пиоглитазон, так и сравнительно более новые ланифибранор и дулаглутид. Характерной тенденцией является значительное количество КИ с включением пациентов, имеющих НАЖБП без СД 2 (NCT04642261, NCT05605158).
Эртуглифлозин
Эртуглифлозин – один из новейших ингибиторов SGLT-2, продемонстрировавших потенциальное гепатопротекторное действие. По данным post hoc-анализа, в исследованиях серии VERTIS с участием больных СД 2 эртуглифлозин (5 или 15 мг/сут в течение 52 нед) способствовал нормализации активности печеночных трансаминаз, но не влиял на значение индекса фиброза печени FIB-4 [98]. Препарат планируется оценить на предмет эффективности при НАЖБП на фоне СД 2 в обеих дозах в исследовании 4-й фазы с включением 164 пациентов (NCT05644717).
Двойные ингибиторы SGLT-1/2 Ликоглифлозин
В клиническом исследовании IIa фазы (n=107 пациентов), ликоглифлозин (LIK066; 150 мг/сут в течение 12 нед) значимо уменьшал относительное содержание липидов в печени (на 38,7%), активность АЛТ (на 32%), АСТ (на 32%) и ГГТП (на 36%) по сравнению с плацебо. В дозе 30 мг/сут препарат снижал уровни АСТ и ГГТП, но оказывал лишь незначительное влияние на активность АЛТ и выраженность стеатоза печени. У 77 и 49% пациентов, получавших ликоглифлозин в высокой и низкой дозах соответственно, отмечалось развитие диареи, в подавляющем большинстве случаев носившей нетяжелый характер [99].
Эффективность, безопасность и переносимость комбинации ликоглифлозина, агониста фарнезоидных Х-рецепторов тропифексора и их комбинации оцениваются в рандомизированном двойном слепом мультицентровом исследовании ELIVATE, первичной конечной точкой в котором служит разрешение фиброза печени и/или НАСГ (NCT04065841).
Краткая графическая характеристика распределения объектов и времени проведения последних КИ, посвященных эффективности глифлозинов при НАЖБП, отражена на рисунке 6.

ЗАКЛЮЧЕНИЕ
Таким образом, проведенный анализ показывает, что глифлозины обладают потенциалом для эффективного лечения НАЖБП за счет своей способности уменьшать уровни печеночных ферментов, снижать выраженность стеатоза и фиброза печени, улучшать печеночную и системную инсулинорезистентность, снижать массу тела и т.д. Учитывая высокую коморбидность НАЖБП, особое значение имеет способность этой группы препаратов уменьшать кардиологические и нефрологические риски пациентов как на фоне СД 2, так и без него.



